Abstract
We have identified the existence of a productive, PKC-α-dependent endocytotic silencing pathway that leads gymnotically-delivered locked nucleic acid (LNA)-gapmer phosphorothioate antisense oligonucleotides (ASOs) into late endosomes. By blocking the maturation of early endosomes to late endosomes, silencing the expression of PKC-α results in the potent reduction of ASO silencing ability in the cell. We have also demonstrated that silencing of gene expression in the cytoplasm is vitiated when PKC-α expression is reduced. Restoring PKC-α expression via a reconstitution experiment reinstates the ability of ASOs to silence. These results advance our understanding of intracellular ASO trafficking and activity following gymnotic delivery, and further demonstrate the existence of two distinct silencing pathways in mammalian cells, one in the cytoplasmic and the other in the nuclear compartment.
Introduction
Antisense oligonucleotides (ASOs) target their complementary sequences on mRNA to produce gene silencing.1 Many of these ASOs contain phosphorothioate (PS) backbones; because of their length and negative charge, it had long been assumed2 that PS ASOs could not penetrate cell membranes. Thus, for the past several decades, numerous encapsulation strategies have been employed to overcome this problem. It is “commonly accepted” that PS ASOs, when encapsulated in transfection vehicles, enter cells via endocytosis.3,4 Evidence exists that under these conditions, they are then transported to the cell nucleus,5,6 where they interact with TCP1-complex proteins, form nuclear “speckled” bodies,5,7 and attract RNase H.8
However, transfection is neither required for the PS ASO delivery nor for ASO function. Gap-mer ASOs that consist of a PS backbone and two or three locked nucleic acid (LNA9) moieties at the 3' and 5' molecular termini have been shown to penetrate cells, and to produce profound, Watson-Crick-dependent gene silencing.10 This process is referred to as gymnosis, as the ASOs are delivered naked (i.e., without any encapsulation or conjugations). Gymnosis has often been confused with the process of “free uptake”, though the two are not identical. In general, “free uptake” is a term employed to note only that PS ASOs become associated and are internalized by cells, but not that they subsequently have an intracellular silencing function, as in gymnosis.10 Gymnosis has been demonstrated in numerous cell types and animal models,11,12,13 and with several other end-capped chemical modifications in addition to LNAs.14,15 In first step of the gymnosis process, naked PS ASOs bind directly to numerous, heparin-binding cell surface proteins16; these proteins, which may differ both by cell type and in their binding affinity for PS ASOs, have been well-characterized in only a very few cases.17,18,19 Internalization then occurs via the combined processes of adsorptive endocytosis and pinocytosis (fluid phase endocytosis).20
The subcellular fate of gymnotically delivered ASOs is being increasingly understood. In a recent work,21 we took note of the observation that gymnotic gene silencing occurs at substantially lower nuclear ASO concentrations than does silencing post-transfection (e.g., with encapsulating reagents such as Lipofectamine). Consistent with this observation, we found that gymnotic silencing can also occur in the cell cytoplasm through an Ago-2-dependent mechanism, though we also demonstrated that Ago-2 does not appear to be the mRNA cleavage enzyme. These, in combination with other results presented in ref. 21, showed that gymnotically delivered antisense LNA PS oligos produce profound cytoplasmic gene silencing. Furthermore, they accomplish this by using some of the components of the endogenous siRNA silencing machinery.21
However, the process by which gymnotically delivered ASOs become associated with the RNA silencing machinery is not understood. In this paper, we demonstrate that protein kinase C (PKC), a family of serine-threonine kinases22,23 that consists of 11 isoforms, is critically important for gene silencing via gymnosis. PKC has previously been identified as having a role in regulating endocytosis24,25; it is also found in caveolae.26 One isoform in particular, PKC-α, has been shown to regulate the sorting of molecules in endosomal compartments.24,27,28 Critically, PKC-α has also been shown to promote maturation of early to late endosomes (also known as multivesicular bodies (MVBs)29).
In this work, we silenced PKC-α by several methods. These included the use of Go6976, a small molecule inhibitor of the classical, Ca++ and phospholipid-dependent PKC isoforms (α, βI, βII, and γ); we also employed anti-PKC-α siRNA and shRNA strategies to downregulate the expression of PKC-α protein. Every one of these methods demonstrated that the silencing of PKC-α blocks gymnotic silencing of a targeted Bcl-2 mRNA. Reconstitution of the anti-PKC-α shRNA phenotype by transfecting a plasmid encoding a full length PKC-α mRNA restored ASO function. In addition, we demonstrated that activators of PKC (e.g., oleic acid and high glucose), stimulated gymnotic silencing of the targeted Bcl-2 mRNA. Our data suggest that if the PKC-α-dependent maturation of early endosomes to late endosomes (i.e., MVB) is blocked by the downregulation of PKC-α protein expression, gymnotic silencing can be suppressed. These data are critical for our understanding of the intracellular ASO trafficking after gymnosis, as it has been demonstrated that MVBs and GW bodies are in physical contact.30 Our results potentially provide the crucial link between intracellular trafficking and LNA gap-mer ASO induced gene silencing. They are important not only for our mechanistic understanding of the behavior of ASOs, but also because this technology has recently experienced a clinical renascence, as evidenced by the increased number of early stage clinical trials with PS ASOs and the continued clinical development of LNA ASOs.
Results
The maturation of early to late endosomes is essential for effective antisense function
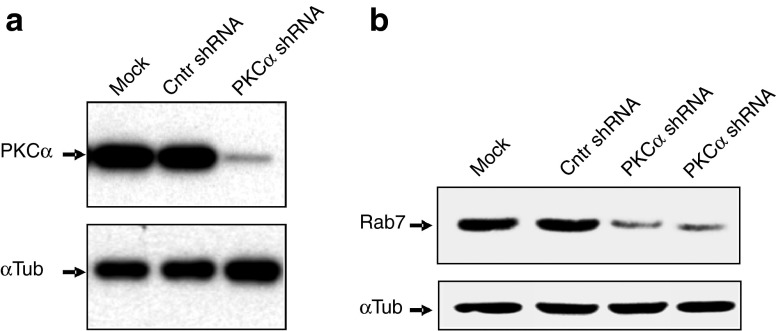
We have previously demonstrated that antisense LNA PS gapmer oligonucleotides (LNA-ASOs) bind to the PAZ domain of Ago-2 and, similar to siRNAs, colocalize to GW-bodies.21 The Ago-2 protein is important for LNA-ASO function, possibly by transporting the LNA-ASOs to the GW-bodies,21 which are physically in contact with late endosomes.30 We therefore speculated that blocking access to the late endosomal compartment should result in loss of LNA-ASO function. To evaluate this possibility, we generated lentiviral-transduced stable cell lines expressing an shRNA targeted to PKC-α (PKCα-shRNA) or a nontargeting shRNA control (Cntr-shRNA). The stable anti-PKC-α shRNA strongly suppressed its target, resulting in less than 10% residual PKC-α protein (Figure 1a). It has previously been demonstrated that silencing of PKC-α protein expression blocks early-to-late endosome maturation.31 Conversely, its overexpression induces early-to-late endosome maturation, as shown by the increase of the late endosomal marker rab9, in cells overexpressing PKC-α.29 As expected, silencing of PKC-α protein expression coincided with a block of early-to-late endosome maturation, as indicated by the strong reduction of Rab7 protein expression (Figure 1b). Rab7 is generally considered a good marker for late endosomes, although the function of this protein has not been completely settled.32 Of note, we have previously determined21 that once internalized after gymnosis, at least some of the LNA-ASO colocalizes with Rab7, placing it in late endsomes. However, LNA-ASOs can also be found in other compartments, including lysosomes. We then compared the activity of the anti-Bcl-2 LNA-ASO in the HT1080 wild-type cell line (Figure 2a), the control cell line (Figure 2b), and the PKC-α-depleted cell line (Figure 2c). In contrast to what was observed in the parental and control cell lines, where the anti-Bcl-2 LNA-ASO maintained its activity unaltered, we found that under the conditions of the experiment, the diminution in levels of PKC-α resulted in a dramatic decrease in the ability of the LNA-ASO to silence Bcl-2 protein expression (Figure 2a–c,e). The silencing of PKC-α protein expression in HT1080 cells—that express a specific shRNA directed to the PKC-α mRNA—results in the loss of Bcl-2 protein expression in a concentration-dependent manner when cells are treated with the anti-Bcl-2 LNA-ASO (Figure 3). However, we would like to carefully point out that at greatly increased concentrations of anti-Bcl-2 LNA-ASO, the block of Bcl-2 silencing after PKC-α silencing can be at least partially overcome (Supplementary Figure S1). At these higher concentrations (which are probably not physiologically relevant), other non-PKC-α-dependent mechanisms may regulate endosomal trafficking, LNA-ASO endosomal escape, and silencing ability
Figure 1.
Silencing of PKC-α reduces late endosome formation. (a) HT1080 cells were transduced with a nontargeting shRNA control (Cntr shRNA) or a specific shRNA directed to the PKC-α mRNA (PKCα shRNA). Western analysis demonstrates effective and specific silencing of PKC-α protein expression in the derived stable cell line. The mock control indicates the parental un-transduced cells. α-tubulin was the loading control. (b) Silencing of PKC-α protein expression reduces late endosome formation as indicated by the concomitant reduction of Rab7 protein (a marker for late endosomes) in cells expressing the PKC-α shRNA (PKC-α shRNA), but not the nontargeting shRNA control (Cntr shRNA). Two technical replicates are shown. α-tubulin was the loading control.
Figure 2.
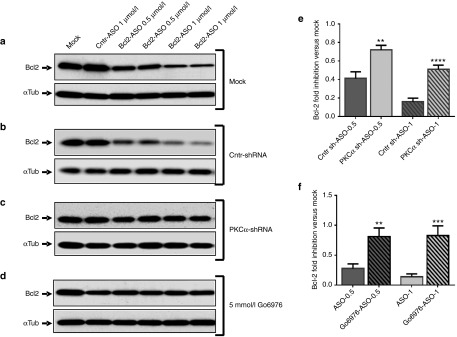
Silencing of PKC-α protein expression reduces anti-Bcl-2 LNA-ASO activity in cells. Intracellular levels of Bcl-2 protein were detected by western analysis: (a) HT1080 parental cells (mock) were treated with either a control ASO (Cntr-ASO) or varying concentrations of the anti-Bcl-2 LNA-ASO. In this representative Western, two experimental lanes for each concentration are shown. (b) The same as above, except that HT1080 parental cells were initially transduced with a nontargeting shRNA control. (c) Same as in a and b, except that the treated HT1080 cell line was initially transduced with a specific shRNA directed to the PKC-α mRNA (d) HT1080 cells were treated with Go6976, a chemical inhibitor of PKC-α activity. (e) LNA-ASO activity is impaired in a stable HT1080 cell line transduced with PKC-α shRNA (PKC-α sh-ASO) but not when transduced with a control shRNA (Cntr-sh-ASO). The LNA-ASO was delivered in both cell lines at 0.5 and 1 μmol/l. The graph represents a combined analysis of five different experiments including five technical replicates. Values were normalized to the α-tubulin loading control and to the mock (not shown), which was normalized to unity; P (Student's t-test) = 0.002 (0.5 μmol/l) and 1.7E−05 (1 μmol/l). (f) LNA-ASO activity is impaired in cells treated with Go6976, a chemical inhibitor of PKC-α. The LNA-ASO was delivered at 0.5 or 1 μmol/l in untreated (ASO-0.5 and ASO-1) or treated (Go6976-ASO-0.5 and Go6976-ASO-1) cells. The graph represents a combined analysis of three different experiments, including four technical replicates. Values were normalized to α-tubulin as loading control and to the mock control (not shown); P (Student's t-test) = 0.005 (0.5 μmol/l) and 0.001 (1 μmol/l).
Figure 3.
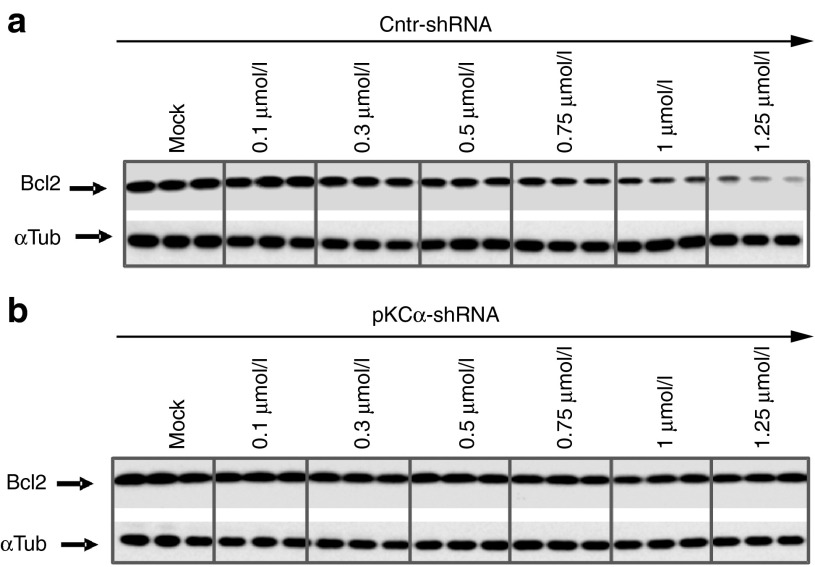
Cells with silenced PKC-α expression demonstrate a concentration-dependent block of anti-Bcl-2 LNA-ASO activity. (a) Western analysis of Bcl-2 protein expression in stable HT1080 cells expressing a nontargeting shRNA control (Cntr shRNA), and treated with increasing concentrations of the anti-Bcl-2 LNA-ASO. (b) Intracellular levels of Bcl-2 protein in HT1080 expressing a specific shRNA directed to the PKC-α mRNA (PKCα shRNA), and treated with increasing concentrations of the anti-Bcl-2 LNA-ASO. In this representative Western, three experimental lanes for each concentration are shown. The mock control indicates the parental un-transduced cells. α-tubulin was the loading control.
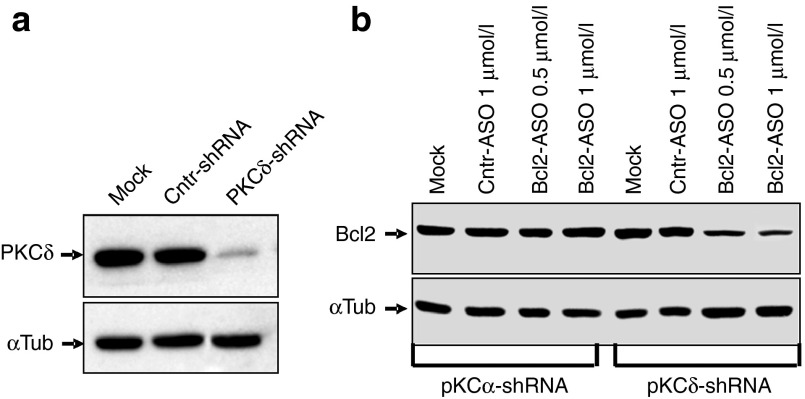
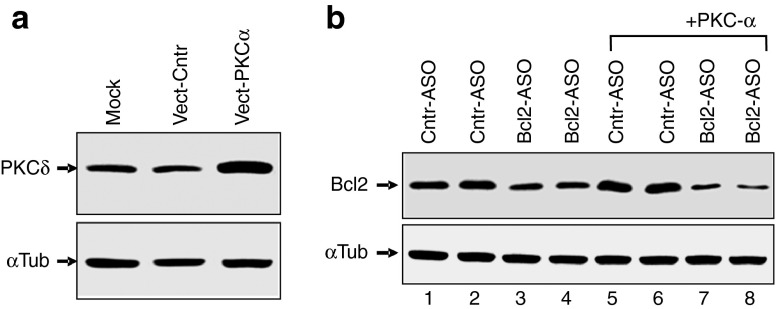
In addition to shRNA-directed silencing of PKC-α, we also treated the HT 1080 parental cell line with Go6976, a known small-molecule PKC-α inhibitor. Go6976 is an inhibitor of the classical isoforms of PKC (i.e., PKC-α, βI, βII and γ.33 However, PKC-γ is central nervous system-specific, and we could not detect any PKC-β in HT1080 cells by Western blotting (not shown)). In concordance with the results presented above, Go6976 treatment resulted in loss of LNA-ASO activity (Figure 2d,f). To evaluate if the block of ASO function was specific to the silencing of the PKC-α isoform, we performed similar experiments with shRNAs targeted to PKC-δ, a nonclassical isoform. Despite a profound reduction in protein expression (Figure 4a and Supplementary Figure S2a), ASO activity was not affected (Figure 4b and Supplementary Figure S2a).
Figure 4.
Silencing of PKC-δ does not affect anti-Bcl-2 LNA-ASO activity in cells. (a) HT1080 cells were transduced with a nontargeting shRNA control (Cntr-shRNA) or a specific shRNA directed to the PKC-δ mRNA (PKCδ-shRNA). Western analysis demonstrates effective and specific silencing of PKC-δ protein expression in the derived stable cell line. The mock control indicates the parental, un-transduced cells. α-tubulin was the loading control. (b) In this representative Western, HT1080 parental cells (Mock), were initially transduced with a specific shRNA directed to the PKC-α mRNA, or with a shRNA directed against the PKC-δ mRNA. They were then treated with either a control ASO (Cntr-ASO) or varying concentrations of the anti-Bcl-2 LNA-ASO. α-tubulin was the loading control. Supplementary Figure S2a presents an analysis of these data. α-tubulin was the loading and normalization control.
Increasing PKC-α expression improves ASO silencing function in mammalian cells
Our results indicated that in our system, impeding the maturation of late endosomes resulted in loss of LNA-ASO activity. Therefore, we hypothesized that driving the maturation of late endosomes by PKC-α overexpression29 should result in the opposite outcome, i.e., increasing the ability of the LNA-ASO to silence its target. To evaluate this possibility, we transfected HT1080 cells with a vector expressing either a full-length PKC-α mRNA (Vect-PKCα) or with the control vector backbone (Vect-Cntr). After confirming that PKC-α protein expression was significantly increased in the PKC-α-transfected cells (Figure 5a), we gymnotically delivered the LNA-ASO to the vector control (Figure 5b, lanes 1–4) or to the vector-PKCα (Figure 5b: +PKCα lanes 5–8) transfected cells and analyzed the ability of the ASO to silence Bcl-2. A scrambled LNA-ASO was used as a control (Figure 5b: Cntr-ASO). Western blot analysis demonstrated that increasing PKC-α expression (+PKCα) enhanced the ability of the LNA-ASO to silence Bcl-2 protein expression (Figure 5b: Compare Cntr-ASO versus Bcl-2-ASO in vector-transfected cells (lanes 1–4) with Cntr-ASO versus Bcl-2-ASO in PKC-α-transfected cells (lanes 5–8) and Supplementary Figure S2b).
Figure 5.
Overexpression of PKC-α protein enhances the intracellular function of the anti-Bcl-2 LNA-ASO. (a) Detection of PKC-α protein by Western analysis demonstrates its overexpression in cells transfected with the PKC-α expression vector (Vect-PKC-α), but not in cells transfected with the control vector (Vect-Cntr). The mock control indicates intracellular levels of PKC-α protein in un-transfected cells. α-tubulin (α-Tub) is the loading control. (b) Western analysis demonstrates intracellular levels of Bcl-2 protein in parental HT1080 cells transfected with a vector control (lanes 1–4) or with the same vector expressing PKC-α (+PKC-α; lanes 5–8). These cells were then treated with 1 μmol/l of the control, scrambled ASO (Cntr-ASO; lane 1–2 and 5–6) or with 1 μmol/l of the anti-Bcl-2 LNA-ASO (Bcl-2-ASO; lane 3–4 and 7–8). Silencing of Bcl-2 protein expression by the anti-Bcl-2 LNA-ASO increases when PKC-α is overexpressed (Bcl-2-ASO, +PKC; compare lanes 1–2 with 3–4, and lanes 5–6 with 7–8). Supplementary Figure S2b presents an analysis of these data. α-tubulin was the loading and normalization control.
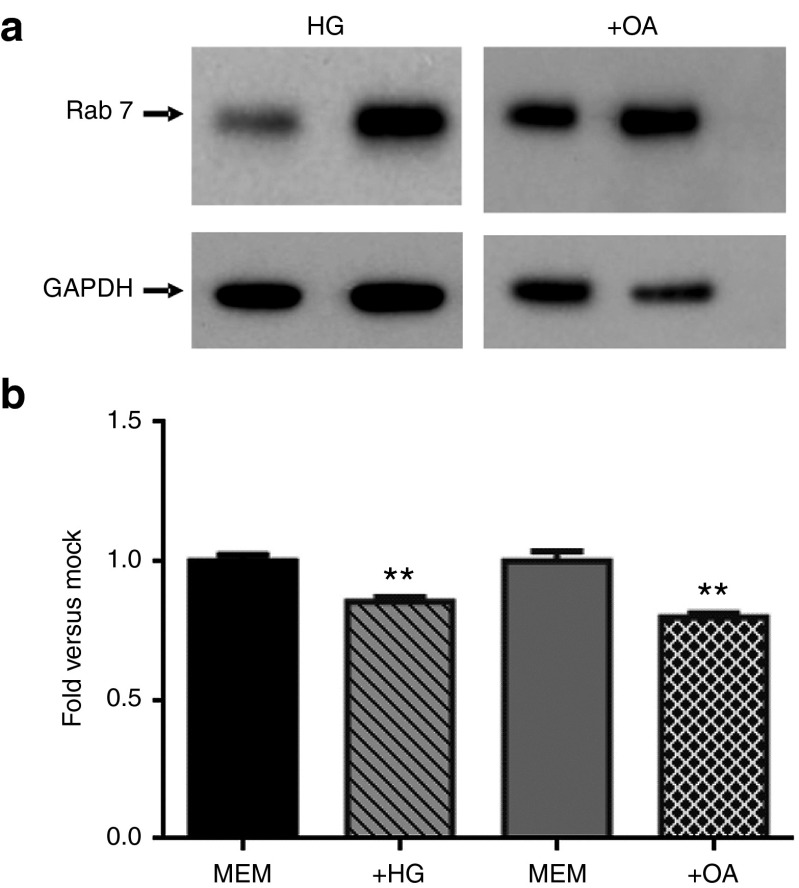
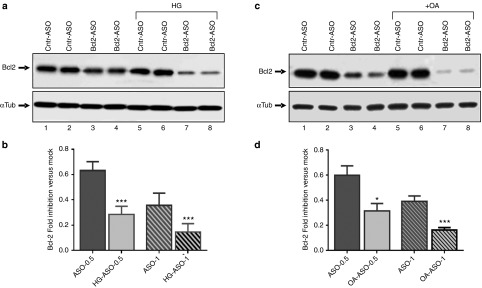
To confirm these results, we employed two other methods known to increase the activity of PKC-α (i) Growing the cells in high glucose (HG) media34; and (ii) treating the cells with oleic acid.35,36 Western blot detection confirmed that both experimental conditions (+HG and +OA) induced endosomal maturation, as shown by the increased expression of Rab7 (Figure 6a and Supplementary Figure S2c). Cells maintained in media supplemented with high glucose concentrations resulted in more potent anti-Bcl-2 LNA-ASO dependent silencing of Bcl-2 protein expression (Figure 7a,b: compare Cntr-ASO versus Bcl-2-ASO in low glucose maintained cells (lanes 1–4) with Cntr-ASO vs. Bcl-2-ASO in HG maintained cells (lanes 5–8)). Similarly, LNA-ASO-dependent Bcl-2 silencing was more potent in cells treated with OA (Figure 7c,d); compare Cntr-ASO versus Bcl-2-ASO in untreated cells (lanes 1–4) with Cntr-ASO vs Bcl-2-ASO in OA-treated cells (lanes 5–8). An experiment demonstrating the concentration-dependent, anti-Bcl-2 LNA-ASO silencing of Bcl-2 protein expression, when cells are maintained in HG or treated with OA, is shown in Supplementary Figure S3.
Figure 6.
High glucose and oleic acid treatment increases cellular Rab7 expression but do not alter LNA-ASO cellular uptake. (a) Western blot detection demonstrates that growing HT1080 cells in high glucose media (+HG) or treatment with oleic acid (+OA) induced endosomal maturation, as shown by the increased expression of the late endosomal marker Rab7. Supplementary Figure S2c presents an analysis of these data. α-tubulin was the loading and normalization control. These results are consistent with previously published observations35,36 (b) HT1080 parental cells were grown in 1 g/l glucose-containing media (MEM) or in 4 g/l high glucose-containing media (HG), or preincubated with 150 μmol/l OA in MEM media (+OA) prior to the addition of a Cy5-labeled anti-Bcl-2 LNA-ASO. Flow cytometry data analysis indicates that there is no greater ASO uptake in the treated cells. For +HG, P (Student's t-test) = 0.009 and for OA, P (Student's t-test) = 0.007
Figure 7.
High glucose and oleic acid increase the activity of PKC-α protein and, in turn, the intracellular function of the anti-Bcl-2 LNA-ASO. (a) HT1080 parental cells were grown in 1 g/l glucose-containing media or in 4 g/l high glucose-containing media (HG), which has been demonstrated to increase cellular activity of PKC-α34 (Figure 6a and Supplementary Figure S2c). 1 μmol/l of the Bcl-2 LNA-ASO (Bcl2-ASO) was delivered at low and high glucose (HG). A control, scrambled ASO (Cntr-ASO) demonstrates the absence of nonspecific activity either with or without 4 g/l of glucose in the media (Cntr-ASO and Cntr-ASO HG). Additional concentrations are shown in Supplementary Figure S3a (b) The Bcl-2 LNA-ASO at two concentrations, 0.5 and 1 μmol/l was delivered at low and high glucose (ASO 0.5 and HG-ASO 0.5) and (ASO 1 and HG-ASO 1). The graph represents the combined analysis of three different experiments, which include five technical replicates. Values representing the extent of Bcl-2 protein silencing by the LNA-ASO were normalized to α-tubulin loading control and to the mock control (not shown); P (Student's t-test) = 0.0003 (0.5 μmol/l ASO) and 0.0005 (1 μmol/l ASO). (c) Same as in a, with the exception that PKC-α activity was increased by treating the parental cells with oleic acid (+OA; refs. 35,36; Figure 6a and Supplementary Figure S2c). The anti-Bcl-2 LNA-ASO activity is enhanced in cells treated with OA. Cells were treated with 1 μmol/l LNA-ASO or pretreated for 2 hours with OA 150 μmol/l prior to LNA-ASO treatment. Additional concentrations are shown in Supplementary Figure S3b (d) Cells were treated with 0.5 and 1 μmol/l LNA-ASO (ASO 0.5 and ASO-1) or pretreated for 2 hours with OA 150 μmol/l prior to LNA-ASO treatment (OA-ASO-0.5 and OA-ASO-1). The graph represents the combined analysis of three different experiments, and includes five technical replicates. Values were normalized to α-tubulin as the loading control and to the mock control (not shown); P (Student's t-test) = 0.005 (0.5 μmol/l) and 0.001 (1 μmol/l).
To confirm that OA or HG had not simply altered the cellular uptake of ASO, we performed a flow cytometry analysis using the analogous 5'-Cy5-labeled ASO. The results demonstrated the lack of any significant increased uptake of the 5'-Cy5-labeled LNA-ASO in the HG or OA-treated cells versus the untreated cells (Figure 6b).
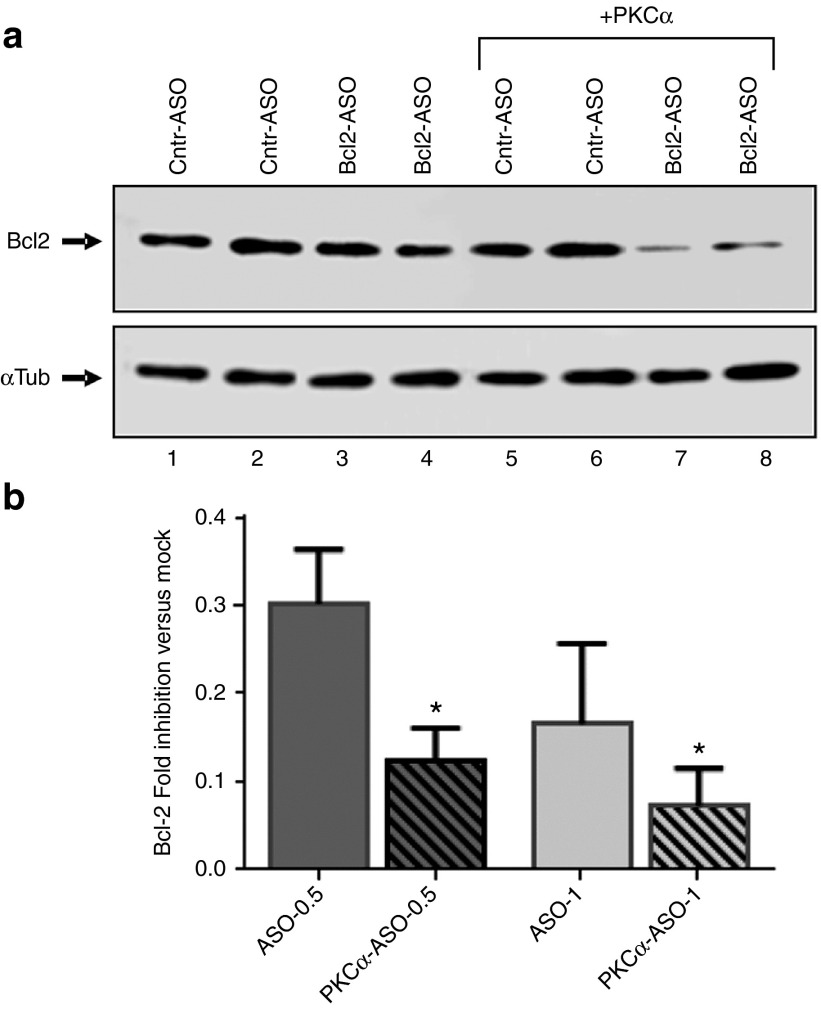
In all cases, increasing maturation of late endosomes via increased PKC-α expression (via transfection of Vect-PKCα) or activity (via HG or OA treatment) resulted in enhanced LNA-ASO silencing activity of the targeted Bcl-2 gene (Figures 5b and 7a–d and Supplementary >Figures S2b and S3). To further emphasize the role of PKC-α in facilitation LNA-ASO silencing, we performed a reconstitution experiment in which we reintroduced PKC-α into the stable cell line expressing the anti-PKC-α shRNA (Supplementary Figure S4, Figure 8a,b and Supplementary Figure S5). As previously demonstrated, gymnosis of the anti-Bcl-2 LNA-ASO in cells depleted of PKC-α resulted in loss of effective silencing of the target gene (Figure 8a: Compare Cntr-ASO vs. Bcl-2-ASO). However, the reinstatement of basal, endogenous levels of PKC-α protein by transfecting the PKC-α expression vector (+PKCα and Supplementary Figure S4) restored the ability of the LNA-ASO to silence Bcl-2 protein expression (Figure 8a,b and Supplementary Figure S5). Similar experiments were conducted in A431 cells, with essentially identical results (not shown).
Figure 8.
Overexpression of PKC-α protein reverts the loss-of-activity phenotype in the PKC-α knock-down cell line. (a) The anti-Bcl-2 LNA-ASO activity is restored in a reconstitution experiment in which a vector control or the identical vector expressing PKC-α (+PKC-α) were transfected into the stable transduced HT 1080 cell line expressing the PKC-α shRNA. Cells were treated with 1 μmol/l of the anti Bcl-2 LNA-ASO. An additional gel with 0.5 or 1 μmol/l is shown in Supplementary Figure S5. (b). Prior to anti-Bcl-2 LNA-ASO treatment at 0.5 or 1 μmol/l, stable cells with silenced PKC-α protein were transfected with either a vector control (ASO-0.5 and ASO-1) or with a PKC-α expression vector (PKC-α-ASO-0.5 and PKC-α-ASO-1). The graph represents the combined analysis of five different experiments including five technical replicates. Values were normalized to α-tubulin as loading control and to the mock control (not shown). P (Student's t-test) = 0.02 (0.5 μmol/l) and 0.01 (1 μmol/l).
We confirmed that the loss of LNA-ASO activity in cells in which PKC-α had been downregulated was not a phenomenon confined solely to the Bcl-2 gene. To accomplished this, we gymnotically delivered a control LNA-ASO (Supplementary Figure S6a, Cntr-ASO) or an anti-eGFP LNA-ASO (Supplementary Figure S6a, eGFP-ASO) to the control cell line (Supplementary Figure S6a, Cntr-shRNA), and the PKC-α-depleted cell line (Supplementary Figure S6a, PKCα-shRNA). Twenty-four hours following gymnotic delivery, we transfected an eGFP target mRNA. The results are consistent with our previous findings21 that demonstrated a reduction of the silencing ability of the anti-eGFP LNA-ASO in the PKC-α-depleted cells (Supplementary Figure S6a, compare Cntr-shRNA + eGFP-ASO with PKCα-shRNA + eGFP-ASO).
There are striking similarities between these observations and our previous21 and current studies with siRNAs. Therefore, in an identical experiment as above, we replaced the anti-eGFP LNA-ASO with an anti-eGFP siRNA. This was done to evaluate if PKC-α depletion could also affect siRNA function. The results demonstrate that reduction of PKC-α protein expression also affects the silencing ability of siRNAs (Supplementary Figure S6b, compare Cntr-shRNA + eGFP-siRNA with PKCα-shRNA + eGFP-siRNA).
Discussion
Protein kinase C is a family of serine/threonine protein kinases that regulate numerous cellular properties, including differentiation, proliferation, and apoptosis. The protein kinase C family is comprised of three classes: The classical isoforms (PKC-α, β, and γ) contain diacylglycerol and calcium binding domains, while the novel isoforms (PKC-δ, ɛ, η, and θ) retain the ability to bind diacylglycerol but not calcium. The atypical isoforms (ζ and ι/λ) are regulated independently of either diacylglycerol or calcium.37 Not all PKC isoforms are found in all cell lines. Of the classical isoforms, PKC-γ is essentially confined to neural tissue,38 while PKC-β is absent from porcine endothelial cells39 and from HeLa cells as well.40 Similarly, in the HT1080 cells employed in our experiments, PKC-β immunoreactivity was not detected, making PKC-α the dominant, if not the sole, classical PKC isoform present.
The PKC-α protein can reside on the plasma membrane, where it is found in caveolae26,41; from that site, PKC-α appears to regulate the internalization of caveolae.42 LNA-ASOs bind to numerous heparin-binding proteins on the cell surface, some of which may be located in caveolae. However, in our experiments, HT1080 cells in which PKC-α protein expression has been knocked down internalized LNA-ASOs at to the same extent as wild-type or control cells (not shown).
Nevertheless, PKC-α appears to play a significant role in the endocytosis process. For example, it has been shown27 that PKC-α is a critically important protein in the recycling of the PDGF-β receptor. In addition, activated PKC-α-GFP has been shown to localize, at least in part, to c-met-expressing endosomes, where it appears to control post-early endosomal trafficking of the receptor to a peri-nuclear compartment.40 Other cell surface molecules, such as the norepinephrine transporter43 and synaptotagmin IX44 also appear to be internalized and sequestered in a PKC-α dependent manner, and a PKC-α-dependent subset of recycling endosomes has also been characterized.44 PKC-α has been implicated in the maturation of phagosomes,31 and its overexpression has been shown to lead to an increase in the cellular production of Rab9,29 a marker of late endosomes.45 As it is “commonly accepted that antisense oligonucleotides usually enter cells via endocytosis”,3 the ability of PKC-α to regulate endosomal trafficking is thus concordant with our observations on its ability to regulate the endosomal trafficking of internalized LNA-ASOs. However, as mentioned above, the cell surface proteins/receptors to which LNA-ASO adsorb are as yet unknown, except in a few defined cases.
Protein kinase C isoforms regulate a multitude of cellular processes and can reversibly modulate protein function. Most of these isoforms, including PKC-α, are upstream of and contribute to the regulation of signaling networks. They also display a certain degree of redundancy by sharing several common substrates. However, each PKC enzyme also possesses its own unique role. We employed various strategies to determine if other PKC isoforms (δ, ɛ, γ, β I and β II) would reduce LNA-ASO silencing in cells comparable to after silencing of PKC-α protein expression. However, the effects on LNA-ASO silencing, if any, were negligible (Figure 4 and not shown). However, we cannot exclude the possibility that these or additional PKC isoforms could have a role in modulating LNA-ASO silencing in other systems, or under different experimental conditions.
We have used five different, complementary methods to prove that PKC-α activity is necessary for effective LNA-ASO antisense activity. Of note, LNA-ASO silencing is consistent with an antisense mechanism; this has been shown by the concomitant reduction of Bcl-2 mRNA and protein21. These complementary methods include: (i) Downregulating PKC-α expression by an siRNA strategy; (ii) inhibiting PKC-α activity by treatment with the PKC classical isoform inhibitor Go6976; (iii) increasing the expression of PKC-α by delivery of a PKC-α expressing plasmid; (iv) increasing the activity of PKC-α by treatment with HG or OA; and (v) performing a reconstitution experiment to restore ASO activity in PKC-α-depleted cell lines. Oleic acid is a well-known activator of PKC-α46,47 and causes a redistribution of the protein from the cytosol to the membrane. More recently, we have demonstrated14 that treatment of several cell lines by oleic acid and by other polyunsaturated fatty acids increases the gene silencing activity of LNA-ASOs. Increasing the ambient glucose concentration has also been shown to activate PKC-α in tissue culture experiments.34 It is of interest that the glucose concentrations employed in this work (low glucose = (5.5 mmol/l); high glucose = (22 mmol/l)) are almost identical to those employed by the authors of ref. 31; this speaks to the general nature of the observation.
Our data suggests that LNA-ASOs initially present in early endosomes will also be present as they mature to late endosomes and MVBs, which are the point, whether delivered by gymnosis or transfection, at which they pass through the endosomal membrane and into the cytoplasm. However, the manner by which a hydrophilic nucleic acid transits a hydrophobic lipid bilayer is still not understood. Nevertheless, as we have previously described,21 regardless of the precise mechanism, transiting through the late endosomal membrane seems to facilitate binding of a LNA-ASO to Ago-2, and subsequent formation of GW-bodies.21
Ago-2 has long been recognized as a membrane-associated protein.48 More recently, it has been demonstrated that Ago-2 and GW182 colocalize with MVBs.49,50 Blocking MVB formation has been shown to impair miRNA silencing and reduce siRISC loading.49,50 Our findings that PKC-α inhibition inhibits LNA-ASO and siRNA gene silencing provides further evidence that these ASOs, after internalization in early endosomes, become localized in late endosomes (MVBs) and that this step, as for miRNAs, is important for their function.
Exogenously delivered siRNAs also benefit from retention in late endosomes, as shown by increased target silencing in cells depleted of Niemann Pick type C1 protein, which regulates recycling of endosomes at the late endosome/lysosome step.51 Furthermore, it has been recently demonstrated that siRNAs delivered via lipofection or lipid nanoparticles are released from maturing endosomes.52 With the exception of enhanced nuclear uptake, we have to date not identified any clear differences between the molecular trafficking mechanisms of siRNAs delivered via lipofection or lipid nanoparticles, and gymnotically delivered LNA-ASOs (ref. 21 and not shown). Further, the data describing the release of siRNAs from maturing and late endosomes is consistent with our findings that LNA-ASO and siRNA function and release can be altered by PKC-α expression—an important component of the endosome maturation machinery. Our data is also in concordance with a recent study by Yang et al.53, in which a high-throughput screen identified compounds that increased ASO efficacy by promoting their release from late endosomes. An additional, recent, well-conducted study by Wagenaar et al.54 found that TSG101, a component of the ESRCT1 complex, decreases the efficacy of a miR-21 antagomir in vitro and in vivo. The authors proposed that depletion of TSG101 yields improved the cellular uptake of the antagomir because of the resulting block of MVB formation. This, in turn, avoided the shuttling of the antagomirs to the lysosomes for degradation.54 TSG101 depletion, however, also caused a dramatic change in shape and dynamics of the endosomal membrane. In fact, these structures no longer resembled early endosomes, but rather formed multi-cisternal endosomes.55 These altered endosomes, whose membranes may be more similar to those of MVBs rather than those of early endosomes, could permit more effective release/leakage of the antagomir into the cytoplasm. This possibility may therefore complement our results.
In contrast, it is not clear if any ASO “leaks” from early endosomes. The membranes of the early endosomes differ from those of the MVBs in that the latter's membrane is surrounded by a LAMP-1-rich glycocalyx. This membrane also contains bis(monoacylglycero)phosphate/lysobisphosphatidic acid, which is not found in the early endosomal membrane.56 However, how or even if these materials promote transit of an ASO through the membrane is also uncertain.
Endosomal sorting of proteins is based on the presence of Ub-tags (which are removed by deubiquitinating enzymes).56 Ub-tagged cargo molecules include a variety of activated growth factor and other signaling receptors, adhesion molecules such as integrins (e.g., α5β1), ion channels and other proteins. It is possible that LNA-ASOs, bound to either these or to similar cell surface proteins, are transferred with them into MVBs upon protein ubiquitination: In this respect, it is interesting to note that LNA-ASOs are known to bind to the αVβM integrin,17 the EGFR,57 and to the V-ATPase (CA Stein, unpublished data).
In summary, all the strands of our evidence, in addition to previous data accumulated by others, point to PKC-α as being an important regulator of endosomal maturation and of intercellular ASO trafficking. We do not mean to imply that PKC-α acts directly at the level of the endosome. More likely, it acts as an upstream kinase that regulates numerous effector molecules that have this direct function. In addition, given the narrow concentration-dependency window that we have described above, it seems clear that there are other mechanisms that are involved in intracellular trafficking that, unsurprisingly, are not PKC-α dependent. Regardless, the illumination of this mechanism helps to improve our understanding of the complexities of gene silencing by a highly clinically relevant technology.
Materials and Methods
Plasmid constructs and cells culture conditions. The lentiviral constructs expressing the PKC-α, PKC-δ shRNA, or the nontargeting shRNA control were purchased from Sigma (St. Louis, MO). The anti PKC-α shRNA sequence is CCGGGCTGTACTTCGTCATGGAATACTCGAGTATTCCATGACGAAGTACAGCTTTTTTG (TRCN0000196909 clone ID NM_002737.2-1280s1c1). The anti PKC-δ shRNA sequence is CCGGCAGAGCCTGTTGGGATATATCCTCGAGGATATATCCCAACAGGCTCTGTTTTTG (TRCN0000272637 clone ID NM_006254.3-1273s21c1 The PKC-α expression plasmid was purchased from Addgene, Cambridge, MA (plasmid # 21232). HT 1080 cells (ATCC, Rockville, MD) were used to titrate the virus, which was produced with standard calcium phosphate coprecipitation. A multiplicity of infection of 0.5 was used for all transductions and 1.5 μg/ml of puromycin was added to the media to select stable lines.
For most of the experiments, HT 1080 cells, were grown in Dulbecco's modified Eagle's medium (DMEM) (Media Tech/Cell gro, Manassas, VA) supplemented with 10% fetal calf serum (Gemini, West Sacramento, CA) and 1 mmol/l L-glutamine. For the oleic acid (OA, Sigma-Aldrich) or the Go6976-inhibitor (Millipore, Billerica, MA) studies, cells were preincubated with 150 μmol/l OA in DMEM media or 5 μmol/l Go6976 for 2–4 hours prior to the addition of the LNA-ASOs. Following the addition of the ASO, cells were kept in serum-free minimum essential medium (MEM) with fatty acid-free bovine serum albumin (BSA) (0.25 g/dl) or 5% charcoal dextran-treated fetal bovine serum (CDT-FBS). For the glucose studies, cells were maintained in either 1 g/l glucose MEM (Media Tech/Cellgro) or 4 g/l glucose DMEM (Media Tech/Cellgro). Cells were seeded at approximately 50% confluency 24 hours prior to treatment. After an overnight incubation, the media was replaced with DMEM containing 5% charcoal/dextran-treated fetal bovine serum (CDT-FBS, Gemini), varying concentrations of LNA-ASOs, as indicated in the text, were added to the culture media and the cells further incubated for 48 hours. At the end of this time, the cells were lysed with RIPA buffer and the lysates collected for analysis. All ASOs, except where otherwise specified, were delivered to cells via gymnosis.58 The sequence of the anti-Bcl-2 LNA-ASO (SPC2996) is 5′-mCsTsCsCsCsAsAsCsGsTsGsCsGsmCsmCsA-3′.58 This same Bcl-2 sequence was used for the Cy5-labeled oligo. 5′-The sequence of the scrambled oligo (SPC 3046) is 5′-mCsGsmCsAsGsAsTsTsAsTsAsAsAsmCsmCsT-3′.58 The anti PKC-α siRNAs were purchased from IDT, Coralville, IA (HSC.RNAI.N002737.12.1 and HSC.RNAI.N002737.12.2). The sequence of the eGFP LNA-ASO is 5′-GsAsAsCsTsTsCsAsGsGsGsTsCsAsGsC-3′.
The eGFP siRNA was purchased from Dharmacon, Boulder, CO (target sequence 5′-GCCACAACGUCUAUAUCAU-3′).
In this work, all ASOs are all-PS, with LNAs and at the 3' and 5' termini. Experiments performed to determine whether two or three LNAs at each terminus were the optimal number gave identical results.
Plasmids and siRNA transfection. HT 1080 cells were seeded at 50% confluency in DMEM containing 10% FBS, 24 hours prior to transfection. Five micrograms of the various plasmids per six-well dish were transfected using Lipofectamine 2000 (Life Technology, Camarillo, CA) or XFect (Clontech Mountain View, CA) as recommended by the manufacturer. XFect or Lipofectamine 3000 (Life Technology) were used exclusively when double treatments were performed. The siRNAs were delivered at a final concentration of 20–50 nmol/l using TransIT siQUEST (Mirus Bio LLC, Madison, WI) through a reverse transfection procedure, or using Lipofectamine 3000 (as recommended by the manufacturer). Gene silencing was achieved by transfecting the specific siRNA/shRNA or by employing the corresponding stably lentivirally-transduced line expressing a shRNA; both silencing procedures resulted in the identical outcome. For western analysis, all transfections were performed in six-well dishes. After approximately 32 hours of incubation, cells were lifted and reseeded in 12- or 24-well plates for gymnotic delivery of the LNA-ASOs. On the next day, cells were lysed in RIPA buffer and the lysates were collected for analysis.
The eGFP mRNA transfection was performed in a 96-well plate. HT1080 cells expressing a control shRNA or the anti-PKCα shRNA were seeded at approximately 60% confluency. Approximately 6 hours later, to allow reattachment of the cells, 0.5 μmol/l of a control LNA-ASO, or an anti-eGFP LNA-ASO were gymnotically delivered to both cell lines. In a parallel experiment, 10 nmol/l of a control siRNA or an anti-eGFP siRNA were transfected with Lipofectamine 3000. Following a 24-hour incubation the media was replaced with 90 microliters of fresh DMEM and 50 ng/well of eGFP mRNA (TriLink Bio Technologies, San Diego, CA) were delivered using Lipofectamine MessangerMax. All experiments were performed in triplicate. Fluorescence images were acquired the following day with the Invitrogen EVOS FL Auto microscope (ThermoFisher Scientific, Carlsbad, CA).
Immunoblotting. Proteins from cell lysates were resolved by 10% SDS-PAGE. The monoclonal mouse anti-human Bcl-2 antibody (Dako, Carpinteria, CA) was added at 1,000× dilution in Tris-buffered saline + Tween 20 (TBST) containing 5% fatty acid free BSA (Sigma). The monoclonal rabbit anti-human PKC-δ antibody (Cell Signaling, Danvers, MA) and the polyclonal rabbit anti-Rab7 antibody (Cell Signaling) were added at a 1,000× dilution in TBST containing 5% fat-free dry milk. The monoclonal mouse anti-human α-Tubulin antibody (Sigma) was added at 4,000× dilution in TBST containing 5% fat-free dry milk. The polyclonal rabbit anti-human PKC-α antibody (Cell Signaling) was added at 2,000× dilution in TBST containing 5% fatty acid free BSA (Sigma). The secondary enhanced chemiluminescence anti-Rabbit (GE Health care, Pasadena, CA) or Mouse IgG-horseradish peroxidase (GE Health care) whole antibody was added at a 1:7,000 dilution.
Flow cytometry analysis. Cells were seeded in 24-well plates at approximately 50% confluency 24 hours prior to LNA-ASO treatment and maintained in either: (i) 1 g/l glucose MEM (Media Tech/Cellgro); (ii) 4 g/l glucose DMEM (Media Tech/Cell gro); or (iii) 120 μmol/l OA (following 2–4 hours preincubation with 150 μmol/l OA) in MEM media. Following Cy5-labeled LNA-ASO treatment, cells were resuspended in phosphate-buffered saline containing 1% BSA and 0.2 μg/ml 4′,6-diamidino-2-phenylindole (Sigma). Twenty thousand to thirty thousand live cells were analyzed by flow cytometry; in most cases, viability was above 90%.
Statistical analysis. Each experiment was performed a minimum of three times (biological replicates) with at least four technical replicates per sample. The values corresponding to the P (Student's t-test) represent a combined analysis of these results and were obtained after normalization to the α-tubulin loading control and to the mock (see figure legends). Numbers were determined using ImageQuant software in a nonsaturation range and after subtracting the background signal.
SUPPLEMENTARY MATERIAL Figure S1. Greatly increased concentrations of anti-Bcl-2 LNA-ASO overcome the block to Bcl-2 silencing in cells with depleted PKC-a protein expression. Figure S2. Silencing of PKC-α does not affect anti-Bcl-2 LNA-ASO activity in cell. Figure S3. High glucose and oleic acid demonstrate a concentration-dependent increase in anti-Bcl-2 LNA-ASO intracellular function. Figure S4. Reintroduction of PKC-a into the stable cell line expressing the anti-PKC-a shRNA restores the basal, endogenous levels of PKC-a protein. Figure S5. Overexpression of PKC-a protein restores the cellular function of the anti-Bcl-2 LNA-ASO in the PKC-a knock-down cell line. Figure S6. Depletion of PKCa reduces LNA-ASO and siRNA function.
Acknowledgments
We thank Xiaowei Zhang for technical assistance and the NCI Cancer Center Core Grant P30CA33572 awarded to the City of Hope for support. The authors report no conflicts of interest. M.L. and D.C. performed most of the experiments; C.K. made the lentivirally-transduced stable cell lines; T.K., B.R.H., and H.O. provided the LNA-ASOs; D.C. and C.A.S. designed the project, analyzed the data, and wrote the manuscript.
Supplementary Material
References
- Goodchild, J (2011). Therapeutic oligonucleotides. Methods Mol Biol 764: 1–15. [DOI] [PubMed] [Google Scholar]
- Akhtar, S, Basu, S, Wickstrom, E and Juliano, RL (1991). Interactions of antisense DNA oligonucleotide analogs with phospholipid membranes (liposomes). Nucleic Acids Res 19: 5551–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano, RL, Ming, X and Nakagawa, O (2012). Cellular uptake and intracellular trafficking of antisense and siRNA oligonucleotides. Bioconjug Chem 23: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano, RL, Carver, K, Cao, C and Ming, X (2013). Receptors, endocytosis, and trafficking: the biological basis of targeted delivery of antisense and siRNA oligonucleotides. J Drug Target 21: 27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, XH, Shen, W, Sun, H, Prakash, TP and Crooke, ST (2014). TCP1 complex proteins interact with phosphorothioate oligonucleotides and can co-localize in oligonucleotide-induced nuclear bodies in mammalian cells. Nucleic Acids Res 42: 7819–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, P, Misteli, T, Baker, BF, Bennett, CF and Spector, DL (2000). Nucleocytoplasmic shuttling: a novel in vivo property of antisense phosphorothioate oligodeoxynucleotides. Nucleic Acids Res 28: 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz, P, Baker, BF, Bennett, CF and Spector, DL (1998). Phosphorothioate antisense oligonucleotides induce the formation of nuclear bodies. Mol Biol Cell 9: 1007–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias, N and Stein, CA (2002). Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther 1: 347–355. [PubMed] [Google Scholar]
- Grünweller, A and Hartmann, RK (2007). Locked nucleic acid oligonucleotides: the next generation of antisense agents? BioDrugs 21: 235–243. [DOI] [PubMed] [Google Scholar]
- Stein, CA, Hansen, JB, Lai, J, Wu, S, Voskresenskiy, A, Høg, A et al. (2010). Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res 38: e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y, Qu, Z, Kim, S, Shi, V, Liao, B, Kraft, P et al. (2011). Down-modulation of cancer targets using locked nucleic acid (LNA)-based antisense oligonucleotides without transfection. Gene Ther 18: 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y, Castaneda, S, Dumble, M, Wang, M, Mileski, M, Qu, Z et al. (2011). Reduced expression of the androgen receptor by third generation of antisense shows antitumor activity in models of prostate cancer. Mol Cancer Ther 10: 2309–2319. [DOI] [PubMed] [Google Scholar]
- Wu, Y, Zhang, Y, Wang, M, Li, Q, Qu, Z, Shi, V et al. (2013). Downregulation of HER3 by a novel antisense oligonucleotide, EZN-3920, improves the antitumor activity of EGFR and HER2 tyrosine kinase inhibitors in animal models. Mol Cancer Ther 12: 427–437. [DOI] [PubMed] [Google Scholar]
- Souleimanian, N, Deleavey, GF, Soifer, H, Wang, S, Tiemann, K, Damha, MJ et al. (2012). Antisense 2'-deoxy, 2'-fluroarabino nucleic acids (2'F-ANAs) oligonucleotides: in vitro gymnotic silencers of gene expression whose potency is enhanced by fatty acids. Mol Ther Nucleic Acids 1: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton, R, Sciabola, S, Salatto, C, Weng, Y, Moshinsky, D, Little, J et al. (2012). Chemical modification study of antisense gapmers. Nucleic Acid Ther 22: 344–359. [DOI] [PubMed] [Google Scholar]
- Beltinger, C, Saragovi, HU, Smith, RM, LeSauteur, L, Shah, N, DeDionisio, L et al. (1995). Binding, uptake, and intracellular trafficking of phosphorothioate-modified oligodeoxynucleotides. J Clin Invest 95: 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benimetskaya, L, Loike, JD, Khaled, Z, Loike, G, Silverstein, SC, Cao, L et al. (1997). Mac-1 (CD11b/CD18) is an oligodeoxynucleotide-binding protein. Nat Med 3: 414–420. [DOI] [PubMed] [Google Scholar]
- Butler, M, Crooke, RM, Graham, MJ, Lemonidis, KM, Lougheed, M, Murray, SF et al. (2000). Phosphorothioate oligodeoxynucleotides distribute similarly in class A scavenger receptor knockout and wild-type mice. J Pharmacol Exp Ther 292: 489–496. [PubMed] [Google Scholar]
- Hanss, B, Leal-Pinto, E, Bruggeman, LA, Copeland, TD and Klotman, PE (1998). Identification and characterization of a cell membrane nucleic acid channel. Proc Natl Acad Sci USA 95: 1921–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubov, LA, Deeva, EA, Zarytova, VF, Ivanova, EM, Ryte, AS, Yurchenko, LV et al. (1989). Mechanism of oligonucleotide uptake by cells: involvement of specific receptors? Proc Natl Acad Sci USA 86: 6454–6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanotto, D, Lin, M, Kowolik, C, Wang, L, Ren, XQ, Soifer, HS et al. (2015). A cytoplasmic pathway for gapmer antisense oligonucleotide-mediated gene silencing in mammalian cells. Nucleic Acids Res 43: 9350–9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg, SF (2008). Structural basis of protein kinase C isoform function. Physiol Rev 88: 1341–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen, D, Das, K and Grimes, KV (2012). Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov 11: 937–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idkowiak-Baldys, J, Becker, KP, Kitatani, K and Hannun, YA (2006). Dynamic sequestration of the recycling compartment by classical protein kinase C. J Biol Chem 281: 22321–22331. [DOI] [PubMed] [Google Scholar]
- Alvi, F, Idkowiak-Baldys, J, Baldys, A, Raymond, JR and Hannun, YA (2007). Regulation of membrane trafficking and endocytosis by protein kinase C: emerging role of the pericentrion, a novel protein kinase C-dependent subset of recycling endosomes. Cell Mol Life Sci 64: 263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson, TE, Zhang, J, Xia, SL, Kuchibhotla, S, Block, ER and Patel, JM (2012). Enhanced phosphorylation of caveolar PKC-α limits peptide internalization in lung endothelial cells. Mol Cell Biochem 360: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg, C, Schmees, C, Karlsson, S, Ahgren, A and Heldin, CH (2009). Activation of protein kinase C alpha is necessary for sorting the PDGF beta-receptor to Rab4a-dependent recycling. Mol Biol Cell 20: 2856–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, TA, Luan, H, Tom, E, Bielecki, TA, Mohapatra, B, Ahmad, G et al. (2014). A kinase inhibitor screen reveals protein kinase C-dependent endocytic recycling of ErbB2 in breast cancer cells. J Biol Chem 289: 30443–30458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamari, F, Chen, FW, Li, C, Chaudhari, J and Ioannou, YA (2013). PKC activation in Niemann pick C1 cells restores subcellular cholesterol transport. PLoS One 8: e74169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi, H and Siomi, MC (2009). RISC hitches onto endosome trafficking. Nat Cell Biol 11: 1049–1051. [DOI] [PubMed] [Google Scholar]
- Ng Yan Hing, JD, Desjardins, M and Descoteaux, A (2004). Proteomic analysis reveals a role for protein kinase C-alpha in phagosome maturation. Biochem Biophys Res Commun 319: 810–816. [DOI] [PubMed] [Google Scholar]
- Girard, E, Chmiest, D, Fournier, N, Johannes, L, Paul, JL, Vedie, B et al. (2014). Rab7 is functionally required for selective cargo sorting at the early endosome. Traffic 15: 309–326. [DOI] [PubMed] [Google Scholar]
- Gschwendt, M, Dieterich, S, Rennecke, J, Kittstein, W, Mueller, HJ and Johannes, FJ (1996). Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett 392: 77–80. [DOI] [PubMed] [Google Scholar]
- Hempel, A, Maasch, C, Heintze, U, Lindschau, C, Dietz, R, Luft, FC et al. (1997). High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res 81: 363–371. [DOI] [PubMed] [Google Scholar]
- Park, JY, Kim, YM, Song, HS, Park, KY, Kim, YM, Kim, MS et al. (2003). Oleic acid induces endothelin-1 expression through activation of protein kinase C and NF-kappa B. Biochem Biophys Res Commun 303: 891–895. [DOI] [PubMed] [Google Scholar]
- Hsu, KL, Fan, HJ, Chen, YC, Huang, YS, Chen, CH, Wu, JC et al. (2009). Protein kinase C-Fyn kinase cascade mediates the oleic acid-induced disassembly of neonatal rat cardiomyocyte adherens junctions. Int J Biochem Cell Biol 41: 1536–1546. [DOI] [PubMed] [Google Scholar]
- Konopatskaya, O and Poole, AW (2010). Protein kinase Calpha: disease regulator and therapeutic target. Trends Pharmacol Sci 31: 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito, N and Shirai, Y (2002). Protein kinase C gamma (PKC gamma): function of neuron specific isotype. J Biochem 132: 683–687. [DOI] [PubMed] [Google Scholar]
- Haller, H, Ziegler, W, Lindschau, C and Luft, FC (1996). Endothelial cell tyrosine kinase receptor and G protein-coupled receptor activation involves distinct protein kinase C isoforms. Arterioscler Thromb Vasc Biol 16: 678–686. [DOI] [PubMed] [Google Scholar]
- Kermorgant, S, Zicha, D and Parker, PJ (2004). PKC controls HGF-dependent c-Met traffic, signalling and cell migration. EMBO J 23: 3721–3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo, C, Ying, YS, Chapline, C, Jaken, S and Anderson, RG (1998). Targeting of protein kinase Calpha to caveolae. J Cell Biol 141: 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart, EJ, Ying, YS and Anderson, RG (1995). Hormonal regulation of caveolae internalization. J Cell Biol 131: 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi, LD, Samuvel, DJ and Ramamoorthy, S (2004). Regulated internalization and phosphorylation of the native norepinephrine transporter in response to phorbol esters. Evidence for localization in lipid rafts and lipid raft-mediated internalization. J Biol Chem 279: 19315–19326. [DOI] [PubMed] [Google Scholar]
- Haberman, Y, Ziv, I, Gorzalczany, Y, Fukuda, M and Sagi-Eisenberg, R (2005). Classical protein kinase C(s) regulates targeting of synaptotagmin IX to the endocytic recycling compartment. J Cell Sci 118(Pt 8): 1641–1649. [DOI] [PubMed] [Google Scholar]
- Chavrier, P, Parton, RG, Hauri, HP, Simons, K and Zerial, M (1990). Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62: 317–329. [DOI] [PubMed] [Google Scholar]
- Khan, WA, Blobe, GC and Hannun, YA (1992). Activation of protein kinase C by oleic acid. Determination and analysis of inhibition by detergent micelles and physiologic membranes: requirement for free oleate. J Biol Chem 267: 3605–3612. [PubMed] [Google Scholar]
- Khan, WA, Blobe, G, Halpern, A, Taylor, W, Wetsel, WC, Burns, D et al. (1993). Selective regulation of protein kinase C isoenzymes by oleic acid in human platelets. J Biol Chem 268: 5063–5068. [PubMed] [Google Scholar]
- Cikaluk, DE, Tahbaz, N, Hendricks, LC, DiMattia, GE, Hansen, D, Pilgrim, D et al. (1999). GERp95, a membrane-associated protein that belongs to a family of proteins involved in stem cell differentiation. Mol Biol Cell 10: 3357–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbings, DJ, Ciaudo, C, Erhardt, M and Voinnet, O (2009). Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol 11: 1143–1149. [DOI] [PubMed] [Google Scholar]
- Lee, YS, Pressman, S, Andress, AP, Kim, K, White, JL, Cassidy, JJ et al. (2009). Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol 11: 1150–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay, G, Querbes, W, Alabi, C, Eltoukhy, A, Sarkar, S, Zurenko, C et al. (2013). Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol 31: 653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittrup, A, Ai, A, Liu, X, Hamar, P, Trifonova, R, Charisse, K et al. (2015). Visualizing lipid-formulated siRNA release from endosomes and target gene knockdown. Nat Biotechnol 33: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, B, Ming, X, Cao, C, Laing, B, Yuan, A, Porter, MA et al. (2015). High-throughput screening identifies small molecules that enhance the pharmacological effects of oligonucleotides. Nucleic Acids Res 43: 1987–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar, TR, Tolstykh, T, Shi, C, Jiang, L, Zhang, J, Li, Z et al. (2015). Identification of the endosomal sorting complex required for transport-I (ESCRT-I) as an important modulator of anti-miR uptake by cancer cells. Nucleic Acids Res 43: 1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyotte, A, Russell, MR, Hopkins, CR and Woodman, PG (2005). Depletion of TSG101 forms a mammalian “Class E” compartment: a multicisternal early endosome with multiple sorting defects. J Cell Sci 118(Pt 14): 3003–3017. [DOI] [PubMed] [Google Scholar]
- Huotari, J and Helenius, A (2011). Endosome maturation. EMBO J 30: 3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwell, P, O'Connor, WJ, King, K, Goldstein, NI, Zhang, LM and Stein, CA (1997). Cell-surface perturbations of the epidermal growth factor and vascular endothelial growth factor receptors by phosphorothioate oligodeoxynucleotides. Proc Natl Acad Sci USA 94: 6523–6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soifer, HS, Koch, T, Lai, J, Hansen, B, Hoeg, A, Oerum, H et al. (2012). Silencing of gene expression by gymnotic delivery of antisense oligonucleotides. Methods Mol Biol 815: 333–346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.