Abstract
Well-established biodegradation tests use biogenously evolved carbon dioxide (CO2) as an analytical parameter to determine the ultimate biodegradability of substances. A newly developed analytical technique based on the continuous online measurement of conductivity showed its suitability over other techniques. It could be demonstrated that the method met all criteria of established biodegradation tests, gave continuous biodegradation curves, and was more reliable than other tests. In parallel experiments, only small variations in the biodegradation pattern occurred. When comparing the new online CO2 method with existing CO2 evolution tests, growth rates and lag periods were similar and only the final degree of biodegradation of aniline was slightly lower. A further test development was the unification and parallel measurement of all three important summary parameters for biodegradation—i.e., CO2 evolution, determination of the biochemical oxygen demand (BOD), and removal of dissolved organic carbon (DOC)—in a multicomponent biodegradation test system (MCBTS). The practicability of this test method was demonstrated with aniline. This test system had advantages for poorly water-soluble and highly volatile compounds and allowed the determination of the carbon fraction integrated into biomass (heterotrophic yield). The integrated online measurements of CO2 and BOD systems produced continuous degradation curves, which better met the stringent criteria of ready biodegradability (60% biodegradation in a 10-day window). Furthermore the data could be used to calculate maximal growth rates for the modeling of biodegradation processes.
Biodegradability has always been considered an important attribute for chemicals, but until recently it was rarely quantitatively incorporated into safety assessments. Concern about environmental quality and advances in models for predicting environmental concentrations have significantly increased a demand for reliable biodegradation data (9, 11, 31-36). Therefore, various laboratory test methods for investigating and monitoring biodegradation processes have been developed and standardized (2). Most efforts have been concentrated on biodegradation tests in the aerobic aquatic environment. Methods for measuring biodegradability can be divided into two principal groups: direct measurement of parent compound concentrations and indirect measurement of parent compound bioconversion, such as carbon dioxide production, decrease in dissolved organic carbon (DOC), cumulative oxygen consumption (biochemical oxygen demand [BOD]), and decrease in chemical oxygen demand (COD). Direct measurement can quantify the disappearance of the parent compound even at low concentrations and is frequently used for the determination of primary biodegradation by using substance-specific analytical methods. Otherwise, it can necessitate complicated analytical procedures depending on the molecule structure (e.g., polymers) and physicochemical properties (e.g., highly volatile or poorly water-soluble compounds). Furthermore, it is not possible to determine the mineralization of the parent compound if not all intermediate metabolites can be quantified. The indirect measurement of biodegradation by using summary parameters, such as BOD, COD, or DOC, is often easy and can be automated. In the case of DOC or COD measurements, it may be necessary to determine physical/chemical elimination processes, such as adsorption to biomass or stripping processes, to differentiate biodegradation from abiotic elimination. The assessment of CO2 and BOD allows an accurate determination of biodegradation processes, and continuous methods can be used for analysis. Especially the determination of the end product carbon dioxide is an important parameter in the estimation of the mineralization of a test compound. However, the use of only one indirect biodegradation parameter for biodegradation may be misleading (13). For example, oxygen can be consumed during biochemical oxidation of ethanol to acetic acid without any decrease in DOC (40).
For practical and legislative purposes, a number of biodegradation test procedures have been standardized. An overview is given by ISO 15462 (15-22) and Pagga (38). The most important and most frequently used tests are those for ready biodegradability. These are the most stringent tests, offering only limited opportunities for biodegradation and acclimatization of the inoculum. On the other hand, they have a fairly high predictive value for the real environment. The ready biodegradability tests are based solely on the indirect summary parameters, such as the removal of DOC or COD, the production of the catabolic end product carbon dioxide, and the determination of the BOD. In recent years, there has been increasing interest in the determination of carbon dioxide. Especially for testing polymers in composting and soil tests or for testing the biodegradability of detergents, the determination of CO2 production has proven to be an important parameter (7, 10, 39).
The existing biodegradation methods based on CO2 measurement and DOC often use discontinuous analytical techniques. However, continuous measurement of the BOD is possible with a respirometer (37). The major advantage of continuous measurement is not only the involvement of less manual work during the test but also the possibility of obtaining much better biodegradation curves, which is very advantageous for the determination of ready biodegradability. According to the Organisation for Economic Co-operation and Development (OECD) guidelines (31-36), a test substance is regarded as readily biodegradable if the degree of biodegradation based on DOC removal is >70%. In the case of CO2 production or BOD determination, 60% of the theoretical values have to be reached. These limit values have to be achieved within a period of 10 days (10-day window) during a test of 28 days. Methods that allow continuous measurement of the analytical parameter reduce the risk of missing the 10-day window.
After the development of an elegant new pressure-based respirometer, the Oxitop method (41), we used the continuous measurement of conductivity for an online CO2 evolution test. This method used the direct linear relationship between CO2 production and change of conductivity in a well-specified, calibrated system. Together with the ions of an aquatic potassium hydroxide (KOH) solution, the biogenously evolved CO2 produced potassium carbonate (K2CO3). The carbonate was less dissociated and therefore showed less conductivity. The linear correlation between the amount of CO2 liberated and the change in conductivity could be used to determine the formed CO2 very accurately. The main purpose of this study was the establishment of a conductivity-based online CO2 evolution test. This test fulfilled the requirements of standardized biodegradation tests and may serve as a basis for further development of biodegradation tests in different areas.
As a consequence of the development of the new Oxitop respirometer and the conductivity-based online CO2 evolution test, we also developed a test with parallel measurement of all three important summary parameters for ultimate biodegradation (CO2, BOD, and DOC), the multicomponent biodegradation test system (MCBTS). Such a test is not required for the usual standard investigations but may have advantages for compounds that are poorly water soluble or volatile and show no clear results in any standard biodegradation test. In such a case, information obtained from three independent analytical parameters may lead to a better and more reliable prediction of biodegradability.
MATERIALS AND METHODS
Chemicals.
Aniline, sodium benzoate, trace elements, and mineral salts were analytical-grade chemicals purchased from Merck, Darmstadt, Germany.
Activated sludge.
Activated sludge was collected from a laboratory wastewater treatment plant fed with municipal sewage. The inoculum was preconditioned to reduce the endogenous CO2 production rate. This was done by sieving the sludge with sieves with an 0.8-mm pore size to remove coarse particles, washing it once with tap water, bringing it to a concentration of 5 g of dry matter liter−1, and finally aerating it for 2 days. The usual concentration of activated sludge in the tests was 30 mg of dry substance liter−1.
Conventional CO2 evolution test.
The principle of the widely used CO2 evolution test (OECD 301 B [32] and ISO 9439 [16]), also known as the Sturm test, was the determination of the ultimate biodegradability of organic compounds by aerobic microorganisms, using a static aqueous test system and the evolution of CO2 as the analytical parameter. A 1.50-liter test mixture was prepared in 2-liter vessels containing an inorganic medium and the organic compound as the sole source of carbon at a concentration of 10 to 40 mg of organic carbon liter−1. Usually activated sludge, obtained from a wastewater treatment plant or from another source in the environment, was used as a mixed inoculum. The vessels were aerated with 1 to 2 bubbles of CO2-free air per s (50 ml min−1) and incubated at 20 ± 2°C for usually 28 days. Agitation was increased by stirring with a magnetic bar (length, 40 mm) at about 800 rpm. The biogenous CO2 formed during the microbial degradation was trapped in two external adjacent vessels (volume, 200 ml) containing an aqueous sodium hydroxide (NaOH) solution (0.05 M, 100 ml). Samples were taken at regular intervals to determine the amount of dissolved inorganic carbon (DIC) and to calculate the amount of CO2 produced. This evolved CO2 was compared with the calculated theoretical amount (ThCO2), and the degree of biodegradation was expressed as a percentage.
Online CO2 evolution test.
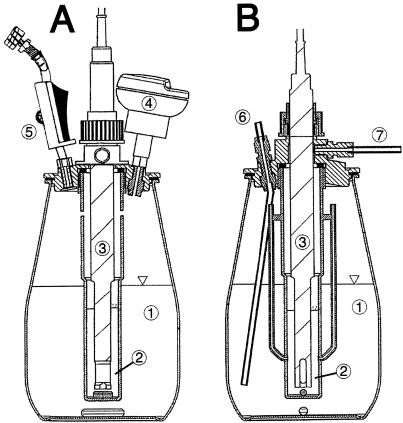
The degradation of chemical compounds in the online CO2 evolution were based on the same principle as the conventional CO2 evolution test. Test vessels were cylindrical bottles with a 2-liter volume containing the same mixture of inorganic medium, organic test substance, and mixed inoculum in a liquid volume of 1.5 liter. Incubation, aeration with CO2-free air, and agitation of the test mixture, blank, and control vessels were also comparable. The exhaust gas from the vessels was passed through a glass chamber (volume, 150 ml) that was immersed in the test mixture and filled with 50 ml of a 0.25 M aqueous NaOH or KOH solution. Here, CO2 was trapped and continuously measured by changes in conductivity (Fig. 1B), using an electrode. The absorption solution was continuously stirred with a magnetic bar (length, 10 mm) at 400 to 500 rpm. After conversion of the measured conductivity (millisiemens per centimeter) to CO2 (milligrams per liter) and subtracting the blank values, biodegradation was calculated and expressed as a percentage of the ThCO2. There was a linear correlation between the amount of carbon dioxide liberated and the change in conductivity. This correlation was determined for each volume and concentration of absorption solution used.
FIG. 1.
Schematic views of the test apparatus used for the MCBTS system (A) and the online CO2 evolution test (B). The total volume of the two vessels was 2.5 liters, and the culture volume was 1.5 liter. The parts are labeled as follows: 1, test vessel; 2, CO2 trap with NaOH or KOH; 3, conductivity electrode; 4, pressure sensor for BOD measurement; 5, sampling port; 6, aeration unit; and 7, de-aeration unit.
Respirometric biodegradation tests.
The respirometric tests were carried out in the Sapromat and Oxitop systems. A major difference between the two systems was the supply of oxygen. The Sapromat was able to replace the oxygen that had been used for biodegradation from the electrochemical oxygen production unit. In the Oxitop, such a replacement of oxygen was not necessary, but care had to be taken that the headspace over the liquid phase in the test vessels was large enough so that it contained sufficient oxygen and allowed complete oxidative biodegradation of the test compound (8, 13, 41). The concentration of inoculum was 30 mg of dry matter ml of activated sludge−1, and the concentration of the test compound was 100 mg of substance liter−1, or 50 to 100 mg liter−1 of theoretical oxygen demand (ThOD). The test duration was 28 days, and the tests were performed at an incubation temperature of 20°C. The measured BOD was compared with the calculated ThOD to obtain the biodegradation degree of the test substance.
MCBTS.
The system described for the online CO2 evolution test was additionally equipped with an Oxitop pressure indicator (Fig. 1A). Vessels were fitted with a glass chamber (volume, 150 ml) immersed in the test mixture, filled with 50 ml of 0.25 M aqueous NaOH or KOH solution and containing a conductivity electrode. In the MCBTS, the electronic pressure indicator for BOD determinations was not equipped with an absorber unit as usual and integrated into the stopper. The bottles were filled with test mixtures and closed gastight. The biogenically evolved CO2 passed through the absorber solution and changed the conductivity of the absorber. Thus, CO2 was permanently removed from the atmosphere, resulting in a pressure decrease in the closed vessel. To the side of the test vessel, a neck with a diaphragm was located, which allowed liquid samples for DOC determination to be taken from the test mixture by a syringe (Fig. 1A). Using MCBTS, CO2 and BOD could be recorded continuously and automatically and the removal of DOC of sufficiently water-soluble test substances could be measured, depending on the frequency of samples taken.
Determination of μmax.
The maximal growth rate (μmax) was determined according to Blok and Struys (5) in the increasing exponential phase. Based on CO2 production, BOD consumption, and DOC removal, the μmax was calculated according to the equation
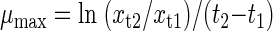 |
where xt1 and xt2 were the appropriate parameters (CO2 produced, oxygen consumed, and DOC removed) at times t1 and t2 in the increasing exponential phase.
RESULTS
Absorption of CO2.
Aqueous NaOH and KOH solutions were tested as absorbing materials in a concentration range of 0.25 to 1.0 M. KOH solutions proved to absorb CO2 faster than NaOH solutions. Furthermore, an increasing absorbance pattern was observed with an increasing volume of the absorber. The initial pH of the absorber solution was 13.3, which decreased proportionally to the amount of CO2 absorbed. To check the reliability of the system (absorber, 40 ml of 0.25 M KOH solution), sodium carbonate (Na2CO3) was added with a syringe as a concentrated stock solution (c = 21.2 g liter−1) to simulate different CO2 concentrations. At an initial total inorganic carbon (TIC) concentration of 20 mg liter−1, the pH decreased by 0.3 to 0.4. At an initial TIC concentration of 40 mg liter−1, it decreased by 2.4. For an initial TIC concentration of 40 mg liter−1, the absorber volume was increased to 50 ml and the pH decreased by 0.9. During the test period of 28 days, we did not observe a significant diminution of the absorber volume due to evaporation. At an initial volume of 30 ml, the reduction was low (mean, 0.18 ml; coefficient of variation, 43%; 95% confidence interval of the mean, 0.10 to 0.26 ml). The total carbon dioxide binding capacity of the absorbing solution was calculated according to the equation
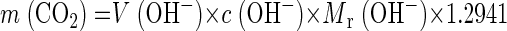 |
where m (CO2) was the mass of carbon dioxide (grams), m (OH−) was the mass of hydroxide ions (grams), V (OH−) was the volume of the absorbing hydroxide ion solution (liters), c (OH−) was the concentration of the absorbing hydroxide ion solution (moles per liter), and Mr (OH−) was the relative molar mass of hydroxide ions (grams per mole). Therefore the total carbon dioxide binding capacities were 165, 220, and 275 mg of CO2 for 30, 40, and 50 ml of a 0.25 M absorption solution (NaOH or KOH), respectively. The influence of the pH in the test medium on the liberation of CO2 is shown in Table 1. As expected, the lower the pH the faster was the liberation of CO2.
TABLE 1.
Influence of the pH of the test medium on the liberation of CO2a
| pH | Time (days) for liberation of
|
||
|---|---|---|---|
| 20% CO2 | 50% CO2 | 90% CO2 | |
| 4.0 | 0.2 | 0.5 | 1.2 |
| 7.0 | 0.6 | 1.8 | 4.2 |
| 8.0 | 1.3 | 3.0 | 5.2 |
Carbon dioxide was added as Na2CO3 to get a final concentration of 2 mM.
The long-term stability of six conductivity electrodes (WTW, Weilheim, Germany) was tested over a period of 19 months by monitoring an electrode-specific cell constant. During this period, three electrodes remained in a 0.25 M NaOH solution and three electrodes remained in a 1.00 M NaOH solution. During this period, the coefficient of variation of the cell constant varied between 1.26 and 1.31% in the 0.25 M solution and between 0.05 and 1.02% in the 1.00 M solution. According to technical information of the supplier, the cell constant should be 0.475 cm−1 and variations should not exceed ±1.5%. Therefore, the conductivity sensors tested were very suitable even for long-term studies.
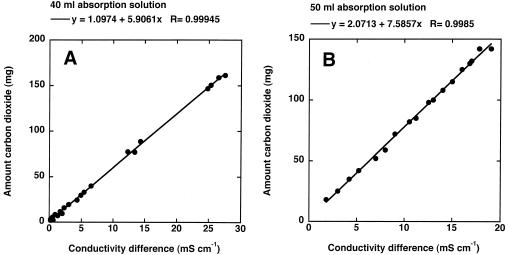
The calibration of the conductivity electrodes occurred by direct addition of different amounts of gaseous CO2 (1 to 160 mg) via a rubber septum into a closed stirred system with a total volume of 100 ml containing 40 or 50 ml of 0.25 M KOH as the absorption solution. After an equilibration period of 2 h, the difference in conductivity was recorded and a sample was withdrawn to determine the concentration of inorganic carbon (IC). The calibration functions for two different volumes of absorption solution are shown in Fig. 2.
FIG. 2.
Calibration curves with 0.25 M KOH as an absorber. (A) Forty milliliters of absorption solution. (B) Fifty milliliters of absorption solution. The capacity of the absorber was 220 mg of CO2, with 40 ml of absorption solution and 275 mg of CO2 with 50 ml of absorption solution. The number of replicate tests was six. The coefficients of variation were 6.4% for 40 ml of absorption solution and 7.2% for 50 ml of absorption solution.
Blank values.
CO2 production or cumulative BOD in the vessels for the blank values was due to endogenous oxidation activity of the microorganisms of the inoculum and took place without any addition of a biodegradable carbon source. In biodegradation tests, it was essential to determine this endogenous activity and to correct the CO2 and BOD measured from the test assays with test substance by subtracting the blank values. In the online CO2 test system, the CO2 production in the blanks proved to be a continuous and linear process over the whole incubation period of 28 days. In 13 independent tests, the final concentration of CO2 in blank vessels with an inoculum concentration of 30 mg of dry matter liter of suspended solids−1 ranged from 22 to 36 mg liter−1 with a mean value of 30 mg liter−1 (coefficient of variation, 20.7%; 95% confidence interval of the mean, 24.8 to 35.2 mg liter−1).
Variation of parallel measurements.
The degrees of biodegradation measured in the plateau phase in parallel vessels were used to calculate the mean degree of biodegradation of the test substance. Therefore it was important to find out whether parallel measurements gave comparable plateaus. Eight different parallel tests with aniline as a test compound were performed, and the differences between the plateaus were determined. The mean of the differences was 3.5%, with a coefficient of variation of 0.732%. The 95% confidence interval of the mean of the differences ranged from 1.36 to 5.64%. The mean of the final degree of biodegradation was 79.8% (coefficient of variation, 13.1%; 95% confidence interval of the mean, 74.3 to 85.4%).
Biodegradation patterns of aniline as a reference compound.
Aniline is a reference compound for many OECD tests and therefore well characterized. It was used to compare the conventional CO2 evolution test (OECD 302 B) with the new online CO2 evolution test. The data obtained in the two test systems are summarized in Table 2. Maximal growth rates and lag periods were in a comparable range, whereas the degree of biodegradation was slightly lower in the online CO2 evolution test.
TABLE 2.
Biodegradation of aniline in the modified CO2 evolution test and the online CO2 evolution testa
| CO2 evolution test | Mean degree of biodegradation (%)b | Mean μmax (day−1)b | Lag period (days)b |
|---|---|---|---|
| Modified | 86 (12.0) | 2.4 (15.0) | 3.8 (16.0) |
| Online | 75 (14.1) | 2.6 (18.0) | 3.4 (18.0) |
The test period was 28 days. The numbers of tests performed were 51 for the modified CO2 evolution test and 13 for the online CO2 evolution test.
Coefficients of variation (percent) are indicated in parentheses.
Influence of aeration in the online CO2 evolution test.
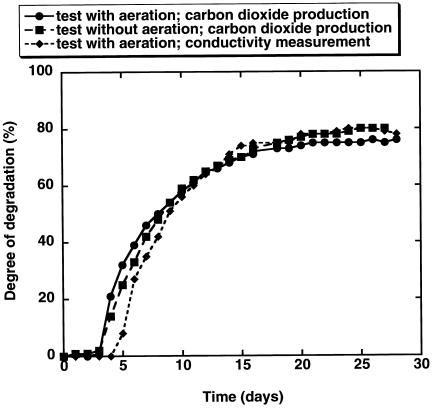
The influence of air supply in the online CO2 evolution test was tested by using aerated open systems (12 replicates) with about 50 ml of CO2-free air min−1 and comparing the results with those from nonaerated open systems (10 replicates). The total volume of the test vessels was 2.5 liters, and the volume of the test mixture was 1.5 liter. In the aerated and nonaerated systems, we found comparable degrees of degradation (75.9 and 78.1%, respectively). The lag periods (3.2 and 3.8 days, respectively) and the μmax (2.4 day−1 in both systems) were also in the same range (Fig. 3). The coefficients of variation for all indicated parameters ranged between 10.6 and 22.9%. A prerequisite for these results was that sufficient oxygen was available in the nonaerated system. The total oxygen content in the system was about 270 mg. This exceeded the total oxygen demand in a test vessel containing a completely biodegradable organic test substance at a usual test concentration of 20 mg of carbon liter−1 (ThOD, about 130 to 150 mg liter−1 including the blank). Therefore, we concluded that the closed system as used in the MCBTS contained sufficient oxygen for complete biodegradation of organic substances under standard conditions.
FIG. 3.
Degradation of aniline in aerated and nonaerated CO2 evolution test based on CO2 production and in a CO2 evolution test system with online measurement. The numbers of replicate tests were 12 for the aerated system, 10 for the nonaerated system, and 13 for the online system. The coefficients of variation of the mean final biodegradation were 10.6% for the aerated system, 6.3% for the nonaerated system, and 14.1% for the online system.
Biodegradation of aniline in the MCBTS.
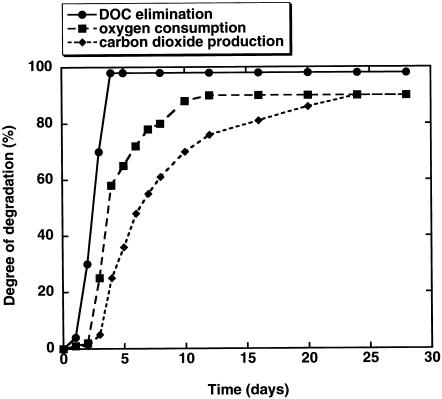
The biodegradation results for aniline in the MCBTS are summarized in Table 3. It is obvious that the elimination of DOC is the fastest process, followed by the BOD and the production of CO2. The lag periods for DOC elimination were the shortest and the degree of biodegradation was highest, followed by the values for BOD and CO2 evolution (Fig. 4).
TABLE 3.
Biodegradation parameters for aniline in the multicomponent biodegradation test systema
| Parameterb | DOC elimination
|
Oxygen consumption
|
CO2 production
|
|||
|---|---|---|---|---|---|---|
| Biodegradation (%) | μmax (day−1) | Biodegradation (%) | μmax (day−1) | Biodegradation (%) | μmax (day−1) | |
| Mean value | 96.0 | 6.0 | 82.5 | 3.0 | 83.0 | 1.7 |
| CV (%) | 5.4 | 13.2 | 18.0 | 32.4 | 10.4 | 22.1 |
| 95% CIM | 83.0-100.0 | 4.1-8.0 | 70.2-95.3 | 2.2-3.9 | 77.5-90.6 | 1.4-2.0 |
The test period was 28 days, and the number of experiments performed was 6. Lag periods were as follows: 2.0 days for DOC elimination, 2.8 days for oxygen consumption, and 3.9 days for CO2 production.
CV, coefficient of variation; CIM, confidence interval of the mean.
FIG. 4.
Degradation of aniline in an MCBTS. The number of replicate tests was six. A statistical analysis of the data is summarized in Table 3.
Biodegradation of sodium benzoate in different test systems.
Sodium benzoate was a readily biodegradable organic compound and was also frequently used as a reference substance in standardized biodegradation tests. The disadvantage compared to aniline was that it was degraded more easily and therefore gave no characteristic sigmoid-shaped biodegradation curves. The degradation pattern of sodium benzoate was nevertheless intensively studied in a coulombometric respirometer (Sapromat), a respirometer with direct pressure metering (Oxitop), the online CO2 evolution test, and the multicomponent biodegradation test system. The data obtained (Table 4) show that the lag periods and the degrees of biodegradation were in a similar range for all test systems, whereas the μmax was highest in the Oxitop and MCBTS (oxygen consumption) and lowest in the Sapromat and the CO2 evolution test.
TABLE 4.
Degradation pattern of sodium benzoate in different biodegradation test systemsa
| Test system/parameterb | Sapromat
|
Oxitop
|
CO2 evolution test
|
MCBTS
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Oxygen consumption
|
CO2 production
|
|||||||||
| Biodegradation (%) | μmax (day−1) | Biodegradation (%) | μmax (day−1) | Biodegradation (%) | μmax (day−1) | Biodegradation (%) | μmax (day−1) | Biodegradation (%) | μmax (day−1) | |
| Mean | 85.0 | 1.5 | 89.5 | 2.8 | 93.0 | 1.1 | 78.5 | 2.9 | 78.4 | 2.1 |
| CV (%) | 3.6 | 7.2 | 2.9 | 19.2 | 10.2 | 9.6 | 4.2 | 13.9 | 9.4 | 48.2 |
| 95% CIM | 81.9-89.7 | 1.3-1.6 | 86.8-92.2 | 2.2-3.3 | 77.8-100 | 1.0-1.3 | 73.2-83.3 | 2.2-3.5 | 72.2-84.6 | 1.2-2.9 |
The test period was 28 days. The number of tests performed was 4 to 6. The lag period varied between 0.8 and 1.5 days.
CV, coefficient of variation; CIM, confidence interval of the mean.
DISCUSSION
In recent years, several authors have reported difficulties with CO2 detection in the CO2 evolution test (OECD 301B [32] ISO 9439 [16]) due to the indirect method of CO2 determination (44, 51). As a consequence, the 10-day window was not passed in some cases, whereas it was passed without any difficulties in the DOC removal test (OECD 301A [31] and ISO 7827 [15]). Therefore, several authors have described alternative methods for the detection of carbon dioxide. These include sampling of headspace gas and subsequent infrared analysis (7, 11) and the trapping of carbon dioxide in a 0.1 M KOH and measuring changes in electrical conductivity of the base solution (23). A combination of an electrolytic respirometer and carbon dioxide detection was also proposed (42). In our study, the continuous online determination of the biodegradation product CO2 using a conductivity electrode was optimized for biodegradation tests based on the OECD 301B guideline (32) and the ISO 9439 standard (16). On account of the accuracy and easy performance of the method, the well-established CO2 evolution test can be used with a new and better analytical technique. The CO2 online test system is fully based on the existing OECD guideline and ISO standard and can be realized without any major technical effort.
An important practical application of an online CO2 test system will be in the field of biodegradation of polymers, where direct analytical methods are restricted or complicated. Using the online method, reliable biodegradation studies may also be performed in complex matrices, such as soil, waste, or compost. The suitability of carbon dioxide trapping methods for biodegradation of starch-based materials (23), cellulose, polyhydroxyalkonates, poly(3-hydroxybutyrate), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (30) has been described.
Aqueous KOH and NaOH solutions were tested as absorbing solutions. The KOH solution proved to absorb CO2 faster than the NaOH solution. Fifty milliliters of a 0.25 M KOH solution with a theoretical CO2 binding capacity of 275 mg was very suitable for the CO2 online test. The conductivity electrodes showed their suitability even for long-term studies.
The experiments performed in our laboratories showed that the variation of the parallel measurements was rather low and the relevant biodegradation patterns were in the same range compared with the existing method based on DIC measurements. Only the degree of biodegradation of aniline was slightly lower in the online system, but clearly above the usual limit values for ready biodegradability (60 and 70%, respectively). The major advantage of a continuous CO2 measurement based on conductivity is the possibility of obtaining much better biodegradation curves. CO2 measurement does not depend on the frequency of sampling for DIC measurement any longer and the risk of missing the important 10-day window is therefore reduced.
According to ISO standards and OECD guidelines, blank values for endogenous CO2 formation should not exceed 70 mg liter−1. Otherwise, the experimental technique has to be reviewed and the test has to be repeated with another inoculum (31-36). The data reported in this study for the CO2 online method were about 30 mg liter−1 over the usual test period of 28 days and were therefore in the allowed range. The endogenous production of CO2 was due to aerobic biodegradation of residual carbon sources of the inoculum and self-digestion processes of the microorganisms. A CO2 concentration of 30 to 70 mg liter−1 was equivalent to about 8.2 to 19 mg of carbon liter−1. The endogenous CO2 production represented a fraction of 0.21 to 0.95 of the ThCO2 of an added test substrate at the recommended test concentrations of 20 to 40 mg of carbon liter−1 and was therefore significant. The most convenient way of reducing endogenous CO2 production is to starve the inoculum. The drawback of this method is the possible reduction in the number of viable bacterial cells and subsequently the loss of biodegradation potential. The influence of a starvation process on the viability of degrading bacteria has been discussed controversially (50). Improved survivability has been reported (48), while another study found no significant effects on survivability (49). In our study, a short starvation period of about 18 to 24 h was sufficient to reduce endogenous CO2 production effectively. Extended starvation periods up to 10 days cause a significant decrease in respiration and dehydrogenase activity (50) and may also result in a decrease in biodegradation potential.
The further development of the online CO2 test and the Oxitop system for BOD determinations together with a port for taking samples for DOC analysis resulted in an MCBTS that offered the opportunity of monitoring two or even three relevant biodegradation parameters in parallel. This new system is very suitable for testing biodegradability of compounds, which are poorly water soluble, highly volatile, or have a high adsorption capacity to biomass. These properties may cause experimental difficulties in a system based on a single parameter only and may show no clear results in usual standard tests. In such a case, information obtained from three independent analytical parameters may lead to a more reliable prediction of biodegradability.
The MCBTS has the advantage that better differentiation can be made between the oxidized part of a test compound and the part incorporated into new biomass, which can be expressed by the heterotrophic yield coefficient (24, 46). Heterotrophic yield coefficients vary between 0.40 and 0.80 and depend on the quality of the organic substrate used, the source of the microbial inoculum, the substrate/inoculum ratio, and the test conditions applied. Higher degrees of biodegradation for the same test substance in tests based on carbon removal in comparison to tests based on oxygen consumption or CO2 formation can be explained by the heterotrophic yield. The usual limit values indicating sufficient biodegradation in BOD- and CO2-based tests (>60% BOD of ThOD or CO2 of ThCO2) consider this fact, but they are still discussed controversially (31-36, 41). Test results based on DOC removal seem to be much more reliable, as values of >90% indicate good biodegradation. This discrepancy can be solved by monitoring BOD and CO2 formation together with DOC removal. Both the MCBTS and the Oxitop rely on the oxygen being available in closed test vessels. For standard test conditions, the oxygen content is sufficient. An additional oxygen supply, as in the coulombometric Sapromat, may only be necessary when working under test conditions with a higher oxygen demand (higher substrate and inoculum concentrations), extended test periods, or in the case of highly volatile test substances, when the volume of the test medium is increased so that the headspace is limited to supply enough oxygen.
For biodegradation studies, aniline is a frequently used reference compound, since it has a characteristic biodegradation curve with a lag period of 3 to 5 days. In standardized test systems, it is used to show the biodegradation capability of the inoculum used. The aerobic microbial degradation of aniline has been described by Boon et al. (6). The first steps of the aerobic degradation pathway involve oxidative deamination resulting in the formation of catechol. The catechol is further degraded by ortho-cleavage (1, 28) or meta-cleavage (27).
The biodegradation patterns or curves of standardized batch tests are described by the lag phase, the final degree of degradation in the plateau phase, and the time needed to reach the plateau phase. A modern approach for describing biodegradation kinetics is the use of mathematical models. A variety of such methods exist that can be applied (2, 41). In this study, we focused on the determination of the μmax. According to Blok (3, 4), the classic theory of Monod (29) to describe bacterial growth offers suitable quantitative parameters that can be used to describe the biodegradability of substances. Blok and Struys (5) reported calculated μmax values for different classes of chemicals (aliphatic and aromatic substances and amino- and nitro-substituted aromatic substances) based on respirometric biodegradation experiments. The μmax values reported varied between 0.4 day−1 (diethyleneglycol) and 9.8 day−1 (p-nitrophenol). The μmax value for aniline of 2.5 day−1 is in accordance with data found in this study (2.81 day−1 in the MCBTS, 2.77 day−1 in the Oxitop, and 1.46 day−1 in the Sapromat). Tabak et al. (47) measured μmax from the oxygen uptake curves with an electrolytic respirometer. The μmax values for 15 substances varied between 1.6 day−1 and 11.5 day−1. Kincannon et al. (25, 26) and Stover and Kincannon (45) gave μmax values between 1 and 4.7 day−1 for 25 substances, and the values of Goldsmith et al. (12) were between 3.1 and 6.2 day−1 for aromatic substances. The discrepancies between μmax values were discussed in detail by Blok and Struys (5) and are mainly due to adaptation processes and the source of the inoculum used. Based on their studies, the authors proposed a critical μmax value of 1.5 day−1 to differentiate between readily and inherently biodegradable compounds.
Other methods frequently used for modeling biodegradation processes are the zero and first-order plot model, the modified logistic model, and the logarithmic model. Critical discussions of these models have been published by Simkins and Alexander (43), Battersby (2), Hales et al. (14), and Reuschenbach et al. (41). For future standards, it would be advantageous to integrate these models into standardized methods. For example, the 10-day window could be replaced with the μmax model and the first-order kinetic model and the modified logistic model could be appropriate for estimating the lag phase as well as the final degree of degradation. There are already ISO standards available for determining biodegradation kinetics (ISO 14592 parts 1 [20] and 2 [21]), indicating the positive future perspectives of biodegradation kinetics.
REFERENCES
- 1.Aoki, K., K. Ohtsuka, R. Shinke, and H. Nishina. 1984. Rapid biodegradation of aniline by Frateuria species ANA-18 and its aniline metabolism. Agric. Biol. Chem. 48:865-872. [Google Scholar]
- 2.Battersby, N. S. 1990. A review of biodegradation kinetics in the aquatic environment. Chemosphere 21:1243-1284. [Google Scholar]
- 3.Blok, J. 1994. Classification of biodegradability by growth kinetic parameters. Ecotoxicol. Environ. Saf. 27:294-305. [DOI] [PubMed] [Google Scholar]
- 4.Blok, J. 1994. Extrapolation of biodegradability test data to percentage removal and biological half life by use of growth kinetic parameters. Ecotoxicol. Environ. Saf. 27:306-315. [DOI] [PubMed] [Google Scholar]
- 5.Blok, J., and J. Struys. 1996. Measurement and validation of kinetic parameter values for prediction of biodegradation rates in sewage treatment. Ecotoxicol. Environ. Saf. 33:217-227. [DOI] [PubMed] [Google Scholar]
- 6.Boon, N., J. Goris, P. de Vos, W. Verstraete, and E. M. Top. 2001. Genetic diversity among 3-chloroaniline- and aniline-degrading strains of the Comamonadaceae. Appl. Environ. Microbiol. 67:1107-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calmon, A., L. Dusserre-Bresson, V. Bellon-Maurel, P. Feuiloley, and F. Silvestre. 2000. An automated test for measuring polymer biodegradation. Chemosphere 41:645-651. [DOI] [PubMed] [Google Scholar]
- 8.Conzelmann, F., and R. Beitlich. 1996. Einfache Bestimmung der biologischen Abbaubarkeit nach OECD 301 F mit dem Oxitop. Melliand 10:700. [Google Scholar]
- 9.Cowan, C. E., D. J. Versteeg, R. J. Larson, and P. J. Kloepper-Sams. 1995. Integrated approach for environmental assessment of new and existing substances. Regul. Toxicol. Pharmacol. 21:3-31. [DOI] [PubMed] [Google Scholar]
- 10.European Detergent Regulation. 2002. Regulation of the European Parliament and the Council of detergents. COM (2002) 485 final. Brussels. Belgium.
- 11.Federle, T. W., S. D. Gasior, and B. A. Nuck. 1997. Extrapolating mineralization rates from the CO2 screening test to activated sludge, river water and soil. Environ. Toxicol. Chem. 16:127-134. [Google Scholar]
- 12.Goldsmith, C. D., Jr., K. Russell, and K. Balderson. 1988. Biodegradation and growth kinetics of enriched isolates on benzene, toluene and xylene. Water Sci. Technol. 20:505-507. [Google Scholar]
- 13.Govind, R., C. Gao, L. Lai, and H. H. Tabak. 1997. Continuous, automated and simultaneous measurement of oxygen uptake and carbon dioxide evolution in biological systems. Water Environ. Res. 69:73. [Google Scholar]
- 14.Hales, S. G., T. Feijtel, H. King, K. Fox, and W. Verstraete. 1997. Biodegradation kinetics. SETAC Europe Publication, Brussels, Belgium.
- 15.International Organization for Standardization. 1994. ISO 7827. Water quality. Evaluation in an aqueous medium of the “ultimate” aerobic biodegradability of organic compounds. Method by analysis of dissolved organic carbon. International Organization for Standardization, Geneva, Switzerland.
- 16.International Organization for Standardization. 1999. ISO 9439. Water quality. Evaluation of ultimate aerobic biodegradability of organic compounds in aqueous medium. Carbon dioxide evolution test. International Organization for Standardization, Geneva, Switzerland.
- 17.International Organization for Standardization. 1999. ISO 9408. Water quality. Evaluation of ultimate aerobic biodegradability of organic compounds in aqueous medium by determination of oxygen demand in a closed respirometer. International Organization for Standardization, Geneva, Switzerland.
- 18.International Organization for Standardization 1994. ISO 10707. Water quality. Evaluation of ultimate aerobic biodegradability of organic compounds in aqueous medium. Method by analysis of biochemical oxygen demand (closed bottle test). International Organization for Standardization, Geneva, Switzerland.
- 19.International Organization for Standardization. 1997. ISO 10708. Water quality. Evaluation of ultimate aerobic biodegradability of organic compounds in aqueous medium. Method by determining the biochemical oxygen demand in a two-phase closed bottle test. International Organization for Standardization, Geneva, Switzerland.
- 20.International Organization for Standardization. ISO 14592. Water quality. Evaluation of the aerobic biodegradability of organic compounds at low concentrations. Part 1. Shake flask batch test with surface water or surface water sediment suspensions, in press. International Organization for Standardization, Geneva, Switzerland.
- 21.International Organization for Standardization. ISO 14592. Water quality. Evaluation of the aerobic biodegradability of organic compounds at low concentrations. Part 2. Continuous river flow model with attached biomass, in press. International Organization for Standardization, Geneva, Switzerland.
- 22.International Organization for Standardization. 1997. ISO 15462. Water quality. Selection of tests for biodegradability (technical report). International Organization for Standardization, Geneva, Switzerland.
- 23.Itävaara, M., and M. Vikman. 1996. An overview of methods for biodegradability testing of biopolymers and packaging materials. J. Env. Polym. Degrad. 4:29-36. [Google Scholar]
- 24.Kappeler, J., and W. Gujer. 1992. Estimation of kinetic parameters of heterotrophic biomass under aerobic conditions and characterization of wastewater for activated sludge modelling. Water Sci. Technol. 25:125-139. [Google Scholar]
- 25.Kincannon, D. F., E. L. Stover, V. Nichols, and D. Medley. 1983. Removal mechanisms for toxic priority pollutants. J. Water Pollut. Control Fed. 55:157-163. [Google Scholar]
- 26.Kincannon, D. F., A. Weinert, R. Padorr, and E. L. Stover. 1983. Predicting treatibility of multiple organic priority pollutant wastewaters from single pollutant treatability studies. In J. M. Bull (ed.), Proceedings of the 37th Industrial Waste Conference, p. 641-650. Ann Arbor Science Publishers, Ann Arbor, Mich.
- 27.Konopka, A., D. Knight, and R. F. Turco. 1989. Characterization of a Pseudomonas sp. capable of aniline degradation in the presence of secondary carbon sources. Appl. Environ. Microbiol. 48:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loidl, M., C. Hinteregger, G. Ditzelmueller, A. Ferschl, and F. Streichsbier. 1990. Degradation of aniline and monochlorinated anilines by soil-borne Pseudomonas acidovorans strains. Arch. Microbiol. 155:56-61. [Google Scholar]
- 29.Monod, J. 1949. The growth of bacterial cultures. Annu. Rev. Microbiol. 3:371-394. [Google Scholar]
- 30.Müller, R. J., J. Augusta, T. Walter, and H. Widdecke. 1994. The development and modification of some special test methods and the progress in standardization of test methods in Germany, p. 237-249. In Y. Doi and K. Fukuda (ed.), Biodegradable plastics and polymers, Elsevier Science B.V., Amsterdam, The Netherlands.
- 31.Organisation for Economic Co-operation and Development. 1993. OECD guidelines for testing of chemicals. OECD 301 A. DOC die-away test. Organisation for Economic Co-operation and Development, Paris, France.
- 32.Organisation for Economic Co-operation and Development. 1993. OECD guidelines for testing of chemicals. OECD 301 B. CO2 evolution test. Organisation for Economic Co-operation and Development, Paris, France.
- 33.Organisation for Economic Co-operation and Development. 1993. OECD guidelines for testing of chemicals. OECD 301 C. Modified MITI test (I). Organisation for Economic Co-operation and Development, Paris, France.
- 34.Organisation for Economic Co-operation and Development. 1993. OECD guidelines for testing of chemicals. OECD 301 D. Closed bottle test. Organisation for Economic Co-operation and Development, Paris, France.
- 35.Organisation for Economic Co-operation and Development. 1993. OECD guidelines for testing of chemicals. OECD 301 E. Modified OECD screening test. Organisation for Economic Co-operation and Development, Paris, France.
- 36.Organisation for Economic Co-operation and Development. 1993. OECD guidelines for testing of chemicals. OECD 301 F. Manometric respirometry test. Organisation for Economic Co-operation and Development, Paris, France.
- 37.Pagga, U. 1980. Respirometric degradation and toxicity test with activated sludge for substances and waste water. Vom Wasser 55:313-326. [Google Scholar]
- 38.Pagga, U. 1997. Testing biodegradability with standardized methods. Chemosphere 35:2953-2972. [DOI] [PubMed] [Google Scholar]
- 39.Pagga, U. 1999. Compostable packaging materials—test methods and limit values for biodegradation. Appl. Microbiol. Biotechnol. 51:125-133. [DOI] [PubMed] [Google Scholar]
- 40.Pitter, P., and J. Chudoba. 1990. Biodegradability of organic substances in the aquatic environment. CRC Press. Inc., Cleveland. Ohio.
- 41.Reuschenbach, P., U. Pagga, and U. Strotmann. 2003. A critical comparison of respirometric biodegradation tests based on OECD 301 and related test methods. Water Res. 37:1571-1582. [DOI] [PubMed] [Google Scholar]
- 42.Schäfer, A., W. R. Müller, and C. Engel. 1997. Kontinuierliche CO2-Messung im Respirometer als zusätzliches Abbaukriterium. Wasser Abwasser 138:570-576. [Google Scholar]
- 43.Simkins, S., and M. Alexander. 1984. Models for mineralization kinetics with variables of substrate concentration and population density. Appl. Environ. Microbiol. 47:1299-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan, P. T., and T. Viraraghavan. 2000. An analysis of the “modified Sturm test” data. Chemosphere 40:99-102. [DOI] [PubMed] [Google Scholar]
- 45.Stover, E. L., and D. F. Kincannon. 1983. Biological treatability of specific organic compounds found in chemical industry wastewaters. J. Water Pollut. Control Fed. 55:97-109. [Google Scholar]
- 46.Strotmann, U. J., A. Geldern, A. Kuhn, C. Gendig, and S. Klein. 1999. Evaluation of a respirometric test method to determine the heterotrophic yield coefficient of activated sludge bacteria. Chemosphere 38:3555-3570. [Google Scholar]
- 47.Tabak, H. H., S. Desai, and R. Govind. 1990. Determination of biodegradability kinetics of RCRA compounds using respirometry for structure-activity relationships. In J. M. Bell (ed.), Industrial Waste Conference, Purdue University, Proceedings, 44th. Lewis Publishers, Inc., Boca Raton, Fla.
- 48.Van Elsas, J. D., A. C. Wolters, C. D. Clegg, H. M. Lappin-Scott, and J. M. Anderson. 1994. Fitness of genetically modified Pseudomonas fluorescens in competition for soil and root colonization. FEMS Microbiol. Ecol. 13:259-272. [Google Scholar]
- 49.Van Overbeck, L. S., L. Eberl, M. Givskov, S. Molin, and J. D. van Elsas. 1995. Survival of, and induced stress resistance in, carbon-starved Pseudomonas fluorescens cells residing in soil. Appl. Environ. Microbiol. 61:4202-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe, K., M. Miyashita, and S. Harayama. 2000. Starvation improves survival of bacteria introduced into activated sludge. Appl. Environ. Microbiol. 66:3905-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weytjens, D., I. van Ginneken, and H. A. Painter. 1994. The recovery of carbon dioxide in the Sturm test for ready biodegradability. Chemosphere 28:801-812. [Google Scholar]