Abstract
Pseudomonas aeruginosa strain NB1 uses chloromethane (CM) as its sole source of carbon and energy under nitrate-reducing and aerobic conditions. The observed yield of NB1 was 0.20 (±0.06) (mean ± standard deviation) and 0.28 (±0.01) mg of total suspended solids (TSS) mg of CM−1 under anoxic and aerobic conditions, respectively. The stoichiometry of nitrate consumption was 0.75 (±0.10) electron equivalents (eeq) of NO3− per eeq of CM, which is consistent with the yield when it is expressed on an eeq basis. Nitrate was stoichiometrically converted to dinitrogen (0.51 ± 0.05 mol of N2 per mol of NO3−). The stoichiometry of oxygen use with CM (0.85 ± 0.21 eeq of O2 per eeq of CM) was also consistent with the aerobic yield. Stoichiometric release of chloride and minimal accumulation of soluble metabolic products (measured as chemical oxygen demand) following CM consumption, under anoxic and aerobic conditions, indicated complete biodegradation of CM. Acetylene did not inhibit CM use under aerobic conditions, implying that a monooxygenase was not involved in initiating aerobic CM metabolism. Under anoxic conditions, the maximum specific CM utilization rate (k) for NB1 was 5.01 (±0.06) μmol of CM mg of TSS−1 day−1, the maximum specific growth rate (μmax) was 0.0506 day−1, and the Monod half-saturation coefficient (Ks) was 0.067 (±0.004) μM. Under aerobic conditions, the values for k, μmax, and Ks were 10.7 (±0.11) μmol of CM mg of TSS−1 day−1, 0.145 day−1, and 0.93 (±0.042) μM, respectively, indicating that NB1 used CM faster under aerobic conditions. Strain NB1 also grew on methanol, ethanol, and acetate under denitrifying and aerobic conditions, but not on methane, formate, or dichloromethane.
Chloromethane (CM, or methyl chloride) enters the environment primarily via biogenic sources, including marine algae, giant kelp, wood-rotting fungi, various types of cedars, and phytoplankton. Lesser amounts are released via industrial processes, such as the manufacture of silicones, synthetic rubber, and methylcellulose (13). CM constitutes one of the largest reservoirs of gaseous chlorine in the atmosphere, accounting for approximately 55% of total chlorine emissions (6, 17) and causing as much as 15% of the stratospheric ozone destruction (17, 32). CM is also a groundwater contaminant of concern. It is present at approximately 8% of the sites currently on the National Priority List (http://www.epa.gov/superfund/sites/query/advquery.htm), often as a daughter product from reductive dechlorination of polychlorinated methanes. The U.S. Environmental Protection Agency has classified CM as a possible human carcinogen and a priority pollutant (16, 32), and they have issued a health advisory limit of 3 μg of CM liter−1 (http://sd.water.usgs.gov/nawqa/vocns/2020.qw.std.fy01.list).
Biodegradation plays a significant role in the global cycling of CM. Microbial use of CM as a sole source of carbon and energy under aerobic conditions has been known for nearly two decades (15). Doronina et al. (5) isolated eight strains of Hyphomicrobium and Methylobacterium that are able to grow on CM but not methane. One of the isolates, Methylobacterium sp. strain CM4, was used to evaluate the aerobic catabolic pathway (34). The involvement of a monooxygenase was ruled out, as was hydrolytic dechlorination to methanol (29, 33). Instead, the pathway proceeds via a corrinoid-dependent methyltransferase-dehydrogenase system. Following an initial dechlorination reaction, the tetrahydrofolate-bound C1 moiety is oxidized to carbon dioxide via formate or funneled into the serine pathway for biosynthesis. The four genes involved have been identified in several CM-grown strains (4, 22).
Use of CM as a growth substrate has also been demonstrated under fermentative conditions. Acetobacterium dehalogenans utilizes CM via a homoacetogenic pathway, with 4 mol of CM plus 2 mol of CO2 being fermented to 3 mol of acetate plus 4 mol of Cl− (31). A methyl chloride dehalogenase catalyzes the transfer of the methyl group in CM to tetrahydrofolate, which plays a central role in homoacetogenesis. The methyl transfer system involved in A. dehalogenans shows sequence similarity to genes of related function used in the pathway by strain CM4 under aerobic conditions (22, 24, 29).
Given the oxygen-independent pathway for CM catabolism under aerobic and fermentative conditions, it would seem reasonable to expect that CM may serve as a growth substrate under other terminal-electron-accepting conditions. We first demonstrated this possibility by developing an enrichment culture that consumed CM as the sole source of carbon and energy and nitrate as the terminal electron acceptor (8). The objective of this study was to obtain an isolate from the enrichment culture and characterize its physiology during anoxic and aerobic growth on CM, including the yield, stoichiometry of nitrate and oxygen consumption, chloride and nitrogen gas release, and kinetics.
(Some preliminary results of this study were presented at the 100th annual meeting of the American Society for Microbiology, Los Angeles, Calif.)
MATERIALS AND METHODS
Chemicals and medium.
CM gas (99.9%) was obtained from Matheson Gas Products, helium (99.998%) and nitrogen (99.998%) were from Holox, and compressed oxygen (99.6%) was obtained from National Welders Supply Co. Gases were filter sterilized (0.2-μm-pore-size sterile polytetrafluoroethylene) to maintain aseptic conditions. All other chemicals were of reagent grade.
The minimal salts medium (MSM) used consisted of (in grams per liter): NaH2PO4 · H2O, 4.0; K2HPO4, 6.0; (NH4)2SO4, 1.0; MgSO4 · 7H2O, 0.5; CaSO4, 0.02; KNO3, 1.5; and a trace metal solution (1 ml per liter of MSM) described previously (7). Nitrate was added (as KNO3) based on the amount of CM added, to match the stoichiometric requirement for complete oxidation (1.2 mol of NO3− per mol of CM, ignoring cell synthesis). The MSM was filter sterilized (0.2-μm polyethersulfone filter; Gelman).
Culture isolation and identification.
An enrichment that grew anoxically on CM (8) was used as the source of culture to obtain an isolate (designated strain NB1). The enrichment was filtered (5-μm pore size) to remove clumps of cells, followed by serial dilution to extinction. Dilutions were prepared in 160-ml serum bottles with 99 ml of MSM. CM was provided as the sole source of carbon and energy, with nitrate as the terminal electron acceptor. The purity of the isolate obtained in the 10−8 dilution was verified through Gram staining and observation of consistent cell morphology based on microscopy and when streaked on Trypticase soy agar plates.
Strain NB1 was identified by sequencing its 16S rRNA gene, as previously described (36). The BBL CRYSTAL identification system was also used to characterize NB1 by subculturing it on Luria-Bertani agar enrichment plates, followed by an overnight incubation at 37°C under aerobic conditions. Single colonies (less than 12 h old) between 2 and 3 mm were added aseptically to BBL inoculum fluid and vortexed (10 to 30 s; maximum speed). The inoculum fluid (2 ml) was added to the base plate and incubated at 40 to 60% humidity for 18 h. Reactions were read using the BBL CRYSTAL panel viewer and compared to the BBL identification chart.
Growth conditions.
Growth of NB1 on CM under aerobic and anoxic conditions was quantified in 160-ml serum bottles that were modified by fusing a test tube (1-cm inside diameter) at a right angle to the side of the bottles near the base (final bottle volume = 170 ml). These modified bottles (custom manufactured by Glass Warehouse, Millville, N.J.) resemble culture flasks with a side arm (e.g., Bellco Biotechnology or Ace Glass), making it possible to monitor growth by optical density (620 nm). Triplicate bottles were sealed with slotted gray butyl rubber septa and aluminum crimp caps and incubated at room temperature (22 to 24°C) on a gyratory shaker table. MSM (109 ml) was inoculated with a stock culture of NB1 (1 ml) obtained from the serial dilution isolation process. The bottles were purged with helium before they were fed. CM (240 μmol per bottle) was repeatedly added (along with stoichiometric amounts of nitrate or oxygen) and consumed. The pH was adjusted (8 M NaOH) periodically to 7.0 ± 0.5. The final amounts of N2 and oxygen in the headspace and aqueous nitrate and total suspended solids (TSS) were measured in order to calculate the yield and stoichiometry of CM biodegradation. Oxygen concentrations in aerobic bottles did not exceed 21%. A parallel experiment was run with a headspace of pure oxygen (100%) to test for possible inhibitory effects of oxygen on CM biodegradation. Killed controls were prepared by autoclaving (121°C, 15 min); live controls did not receive CM.
The ability of NB1 to grow on acetate, ethanol, methane, methanol, formate, dichloromethane (DCM), and glucose was tested under anoxic conditions in 160-ml serum bottles incubated at room temperature (22 to 24°C) on a gyratory shaker table. Serum bottles were also used for volatile substrates (DCM, methanol, and methane) under aerobic conditions, with enough oxygen added to the headspace to meet the stoichiometric demand. For nonvolatile substrates under aerobic conditions, 250-ml Erlenmeyer flasks with foam stoppers were used on a shaker table. A stock culture of NB1 (1 ml) was added to 109 ml of MSM containing 20 mM acetate, ethanol, methanol, formate, or glucose. The initial amounts of DCM and methane added were 24 and 120 μmol per bottle, respectively. Consumption of DCM and methane was monitored by gas chromatograph (GC) analysis of headspace samples, using a similar method as for CM. Growth on all of the substrates was based on an increase in optical density.
Analytical methods.
CM was monitored by analysis of headspace samples on a Hewlett-Packard 5890 series II GC equipped with a flame ionization detector and a 2.44- by 3.175-mm column packed with 1% SP-1000 on 60/80 Carbopak B (Supelco). The GC was run isothermally (40°C) with nitrogen as the carrier gas (30 ml min−1). The GC response to a headspace sample was calibrated to give the total mass of CM in that bottle. Assuming that the headspace and aqueous phases were in equilibrium, the total mass present was converted to an aqueous-phase concentration with equation 1:
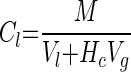 |
(1) |
where Cl = CM concentration in the aqueous phase (micromolar), M = total mass present (micromoles per bottle), Vl = volume of the liquid in the bottle (liters), Vg = volume of the headspace in the bottle (liters), and Hc = Henry's constant (dimensionless) at 23°C (0.314; calculated from Gossett [9]). The aqueous-phase detection limit for CM was 7.0 nM. The validity of assuming equilibrium between headspace and aqueous phases was verified during kinetic tests (see below).
Nitrogen and oxygen were monitored by analysis of headspace samples on a Hewlett-Packard 5890 series II GC equipped with a thermal conductivity detector. Helium served as the carrier gas (30 ml min−1). Samples were separated isothermally (45°C) on a Porapak Q (3.66 by 3.175 mm; Alltech) and Molecular Sieve 5A columns (1.83 by 3.175 mm; Alltech), arranged in a series bypass mode by using a six-port switching valve.
Chloride was measured with an ion-selective electrode (Orion) attached to a pH-millivolt meter (Corning). Samples were prepared by filtration (0.2-μm polytetrafluoroethylene; Nalgene), followed by addition of ionic strength adjustor (5 M NaNO3). Nitrate and nitrite were measured by ion chromatography (Dionex DX 100) by using an ion exchange column (4 by 250 mm; IonPac AS4A; Dionex). The eluant (1.7 mM NaHCO3 and 1.8 mM Na2CO3) was delivered at 2 ml min−1. Samples were prepared by filtration (0.2-μm polytetrafluoroethylene).
Standard methods (12) were used to measure TSS. Soluble chemical oxygen demand (COD) was measured with a Hach kit or a BioScience kit (range, 0 to 150 mg liter−1). Samples for soluble COD were prepared by filtration (0.45-μm polytetrafluoroethylene).
Kinetic experiments.
Monod kinetic constants were determined using an approach similar to ones reported for other volatile organics, including vinyl chloride, ethene, and ethane (35-37). In order to provide a consistent source of culture for the kinetic experiments, NB1 was maintained in an anoxic reactor operated in a semicontinuous, draw-and-fill mode, at a hydraulic retention time of 20 days. The reactor consisted of a 2.5-liter glass bottle (Wheaton) containing 1.5 liters of culture. The reactor was capped with a gray butyl rubber septum held in place with a screw cap. Two additional septa were installed in the side of the bottle (held in place with hose clamps) for the purpose of feeding and wasting. After CM was consumed (100 ml in approximately 3 days), 225 ml of culture was removed using a peristaltic pump (Masterflex L/S pump head; 0.12-in. inside diameter; LS 16 Tygon tubing), and the same volume of MSM was added by reversing the direction of the pump. Anoxic conditions in the reactor were maintained during withdrawal and feeding operations by purging the headspace with helium. Aseptic conditions were maintained during all manipulations. After several months of operation in this mode, the biomass concentration in the reactor stabilized between 250 and 280 mg of TSS liter−1. Maintenance of the culture in this manner provided a reproducible source of NB1 for the kinetic experiments (30).
A second reactor was operated under aerobic conditions. It was constructed and operated in the same manner as the anoxic reactor, except that stoichiometric amounts of pure oxygen were added instead of nitrate. The biomass concentration in this reactor stabilized between 340 and 360 mg of TSS liter−1 prior to use in the kinetic experiments (30).
Kinetic experiments were started by diluting samples from the reactors 10-fold with MSM, in order to slow the rate of CM consumption and permit a more precise measurement of the kinetic parameters. Experiments were performed in 70-ml serum bottles containing 25 ml of diluted culture, purged with helium, and fed CM. Initial biomass concentrations were 27.3 mg of TSS liter−1 for anoxic conditions and 34.0 mg of TSS liter−1 for aerobic conditions. Nitrate or oxygen was added stoichiometrically. Bottles were agitated on a gyratory shaker table (150 rpm).
Kinetic parameters were determined by fitting batch CM depletion data to the Monod expression:
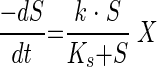 |
(2) |
where k = maximum specific substrate utilization rate (micromole of CM per milligram of TSS per day), Ks = half-saturation coefficient (micromolar CM), S = aqueous CM concentration (micromolar), and X = biomass concentration (milligrams of TSS per liter). In order to determine kinetic parameters, the data were fit to equation 2 while simultaneously calculating an increase in biomass concentration during the course of the experiment, using equation 3:
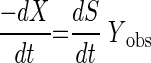 |
(3) |
where Yobs = observed yield coefficient. Nonlinear fitting was done numerically using Aquasim (26), as previously described (36). Linear, absolute relative sensitivity functions for k and Ks were also calculated with Aquasim.
The effect of mass transfer on the evaluation of the kinetic parameters was determined by simultaneously solving equations 2 and 3 with equation 4:
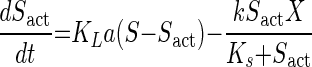 |
(4) |
where Sact is the actual liquid-phase concentration of CM experienced by the culture and KLa is the mass transfer coefficient for CM, 43.434 ± 1.8540 h−1 (30). KLa was measured under conditions identical to those of the kinetic experiments, as previously described (28).
The maximum specific growth rate, μmax (per day) was calculated as shown in equation 5:
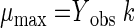 |
(5) |
Chloride stoichiometry.
The stoichiometry of chloride release from CM was measured using effluent from the anoxic and aerobic reactors. The effluent was centrifuged and resuspended in MSM (in order to lower the large background concentration of Cl−), placed in triplicate serum bottles, and provided with CM. After the bottles went through three cycles of CM consumption, enough Cl− was released to allow for precise measurement.
Nucleotide sequence accession number.
The complete sequence (1,477 bases) of the 16S rRNA gene of NB1 was deposited in the GenBank database under accession no. AF193514.
RESULTS
Strain identification.
The isolate used in this study originated from a mixed culture developed with activated sludge as the inoculum and enriched on CM as the sole source of carbon and energy, with nitrate as the terminal electron acceptor (8). Following numerous transfers of the enrichment into MSM, an isolate was obtained by serial dilution to extinction. Strain NB1 is gram negative, rod shaped, and motile. Based on the sequence of strain NB1's 16S rRNA gene, the closest match using BLAST (GenBank) is to Pseudomonas aeruginosa. Strain NB1 shares 99.19% identity with 10 strains of P. aeruginosa, including strain AL 98 (accession no. AJ249451), a potent degrader of natural rubber and synthetic cis-1,4-polyisoprene (19). Evaluation of NB1 using the BBL CRYSTAL test also resulted in a match closest to P. aeruginosa (99.1% confidence).
Growth of NB1.
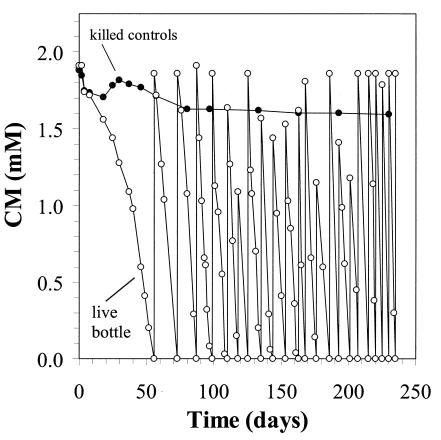
The ability of NB1 to use CM as a sole source of carbon and energy when nitrate serves as the terminal electron acceptor was demonstrated by the repeated consumption of CM (Fig. 1). Minimal loss of CM occurred in the killed controls (15% average over a 237-day period), confirming the lack of significant abiotic degradation in the live treatments. NB1 was able to switch from nitrate to oxygen as the terminal electron acceptor, following a lag period of approximately 3 weeks. Results similar to those shown in Fig. 1 were obtained with oxygen, as long as the headspace oxygen was kept below approximately 21%. When an atmosphere of pure oxygen was used, CM biodegradation stopped after several days.
FIG. 1.
Use of CM as a sole source of carbon and energy by strain NB1 under anoxic conditions. The live treatment represents a single serum bottle; replicates behaved similarly. Results for the killed control treatment represent the average for triplicate bottles; the standard deviation was less than 1.5% for all points.
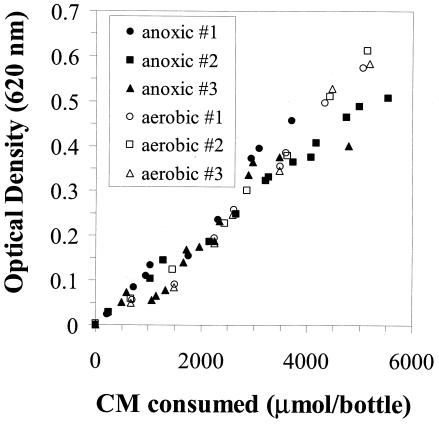
Consumption of CM correlated with an increase in optical density under both anoxic and aerobic conditions (Fig. 2). There was no increase in optical density in the killed controls or live controls that were not fed CM (data not shown). Observed yields (Yobs) (Table 1) were calculated based on the final amount of TSS measured and cumulative CM utilized. Yobs was higher when oxygen was the terminal electron acceptor versus that with nitrate.
FIG. 2.
Correlation between the amount of CM consumed and an increase in optical density in live bottles under anoxic and aerobic conditions. There was no increase in optical density in killed controls or live bottles that were not fed CM.
TABLE 1.
Kinetic parameters for growth of strain NB1 using CM as a substrate
| Parameter | Value under:
|
|
|---|---|---|
| Anoxic conditions | Aerobic conditions | |
| Yobs (mg of TSS mg of CM−1) | 0.20 ± 0.06 | 0.27 ± 0.01 |
| k (μmol of CM mg of TSS−1 day−1) | 5.01 ± 0.06 | 10.7 ± 0.11 |
| μmax (day−1) | 0.0506 | 0.145 |
| Ks (μM) | 0.067 ± 0.004 | 0.093 ± 0.042 |
In addition to CM, strain NB1 grew on methanol, ethanol, acetate, and glucose as sole sources of carbon and energy under aerobic and anoxic conditions. NB1 was unable to use methane, formic acid, or DCM as a substrate. Although the mixed culture from which strain NB1 was isolated utilized DCM as a growth substrate (8), strain NB1 did not. Apparently an organism other than NB1 in the enrichment culture was responsible for DCM biodegradation.
Stoichiometry of CM biodegradation.
Under anoxic conditions, an average of 0.90 mol (±0.12) (mean ± standard deviation) of NO3− was consumed per mol of CM. When expressed on an electron equivalent (eeq) basis, this ratio is 0.75 (±0.10) eeq of NO3− per eeq of CM. The balance of electron flow presumably went to biomass synthesis (27), which would result in a predicted yield of 0.25 eeq of biomass per eeq of CM. This value is within 1 standard deviation of the observed yield; when converted to an eeq basis, Yobs = 0.21 (±0.06) eeq of biomass per eeq of CM.
The observed ratio of dinitrogen produced to nitrate consumed (0.51 ± 0.05 mol of N2 per mol of NO3−) was very close to the expected ratio of 0.5 mol of N2 per mol of NO3−, assuming complete denitrification. Nitrate was not used as a nitrogen source for cell synthesis because ammonia was available in excess in the MSM. Correspondingly, the ratio of dinitrogen produced to CM consumed (0.46 ± 0.05 mol of N2 per mol of CM) was very close to one-half of the ratio of nitrate consumed to CM consumed (0.90 mol of NO3− per mol of CM). Complete denitrification was also confirmed by the lack of any nitrite accumulation.
Under aerobic conditions, the ratio of oxygen consumed to CM consumed averaged 1.07 mol (±0.29) of O2 per mol of CM. When expressed on an eeq basis, this ratio was 0.85 (±0.21) eeq of O2 per eeq of CM. The balance of electron flow presumably went to biomass synthesis (27), which would result in a predicted yield of 0.15 eeq of biomass per eeq of CM. The observed aerobic yield of 0.28 (±0.01) eeq of biomass per eeq of CM was higher than the predicted yield based on oxygen stoichiometry, although the difference was not statistically significant, due to the large standard error associated with the oxygen measurement.
Extent of CM biodegradation.
The expected stoichiometry for complete dechlorination of CM is 1 mol of Cl− per mol of CM. Under nitrate-reducing conditions, the observed chloride release was 1.09 ± 0.13 mol of Cl− per mol of CM. Under aerobic conditions, the observed chloride release was 0.98 ± 0.02 mol of Cl− per mol of CM consumed.
The amount of CM converted to carbon dioxide and biomass was estimated based on a COD balance, which was determined when the reactors were operating at steady state (based on no major change in TSS or the rate of CM consumption between feeding cycles). The total COD consumed was estimated based on the amount of CM consumed, since at the end of a feeding cycle, GC analysis of the reactor's headspace indicated that no other volatile compounds were detected. In the anoxic reactor, the average effluent soluble COD concentration was 38.43 (±10.74) mg liter−1. Based on removal of 0.225 liter during each feeding cycle, the amount of soluble COD removed was 8.65 mg (0.225 liters × 38.43 mg of COD liter−1). This represents 4.5% of the COD fed as CM (100 ml; equivalent to 192 mg of COD), indicating that 95.5% of the CM was mineralized or utilized for biosynthesis under anoxic conditions.
For the aerobic reactor, the average effluent-soluble COD was 9.17 mg liter−1. Using the same approach as above, 98.9% of the CM consumed was mineralized or converted to biomass. Thus, the chloride stoichiometry and soluble COD results indicated essentially complete biodegradation of CM when it was used as a growth substrate by NB1 under anoxic or aerobic conditions.
Kinetic experiments.
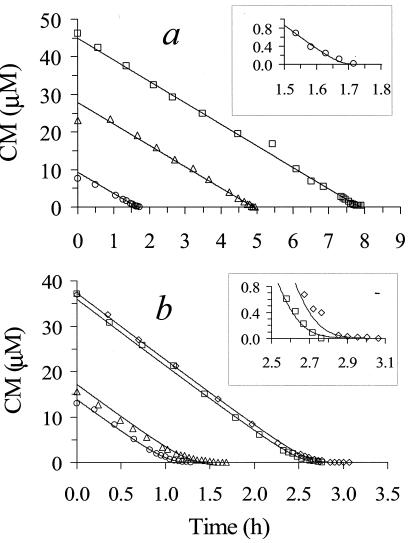
The kinetics of CM utilization for strain NB1 were measured using batch depletion data (Fig. 3). Initial CM concentrations were high enough to demonstrate a maximum rate of utilization (i.e., zero order). Simultaneously fitting all of the depletion data to equations 2 and 3 resulted in the k and Ks values shown in Table 1, along with the calculated value for μmax using equation 5. As indicated by correlation matrix elements of 0.658 for the anoxic data and 0.763 for the aerobic data (both less than 0.9), the estimates of k and Ks were accurate and unique (2). The insets in Fig. 3 demonstrate how well the Monod model fit the data in the low-concentration range.
FIG. 3.
CM batch depletion data used to determine k and Ks under anoxic (a) and aerobic (b) conditions. Symbols represent individual bottles fed various amounts of CM. Lines represent a simultaneous nonlinear fit of equations 2 and 3 to the entire data set.
The aqueous-phase data used to determine k and Ks were calculated (equation 1) based on the total mass of CM in the 70-ml serum bottles, as determined by GC analysis of headspace samples. This approach assumes that the gas and aqueous phases are continuously in equilibrium, which would not be valid if the rate of mass transfer of the volatile substrate between phases was much lower than the rate of biodegradation. To evaluate this possibility, the solution to equation 4 (which includes biodegradation and mass transfer) was compared to those for equations 2 and 3 (which assume equilibrium). The nonlinear curve fit for the mass-transfer coefficient of CM showed that equilibrium between the gas and the aqueous phase was achieved at approximately 0.05 h (under anoxic and aerobic conditions), which was before the first CM data point was measured. This result demonstrated that the equilibrium assumption used to calculate aqueous-phase concentrations was appropriate and that mass transfer did not affect the estimation of kinetic parameters.
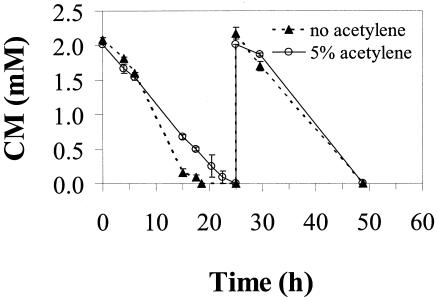
Effect of acetylene.
Acetylene is a known inhibitor of monooxygenase activity (3, 25). To evaluate the possible involvement of a monooxygenase in catabolism of CM under aerobic conditions, duplicate serum bottles were set up with and without acetylene added to the headspace (5%). This level of acetylene is sufficient to shut down substrate utilization when monooxygenase activity is required (36). As shown in Fig. 4, there was a relatively small difference in the rate of CM consumption with acetylene present, suggesting that a monooxygenase is not involved in initiating aerobic CM metabolism in strain NB1.
FIG. 4.
Comparison of CM utilization in the presence and absence of acetylene (5% headspace concentration) under aerobic conditions. Results are the average of duplicate bottles. Error bars represent the data range.
DISCUSSION
Previous studies have demonstrated use of CM as a sole carbon and energy source by several isolates under aerobic (5, 15, 21, 23, 34) and fermentative (24) conditions. The results of this research extend the range of possible terminal electron acceptors for CM to nitrate. It is not yet known what pathway NB1 uses for CM biodegradation. The lack of inhibition by acetylene indicates that a monooxygenase is probably not involved in aerobic catabolism of CM, suggesting that the same pathway may be in use by NB1 when oxygen or nitrate serves as the terminal electron acceptor. Like Methylobacterium sp. strain CM4, NB1 is unable to grow on methane. Strain CM4 uses a corrinoid-dependent methyltransferase-dehydrogenase system when growing aerobically on CM; oxygen is not involved as a reactant (33). It will be interesting to learn if the same system is operative when nitrate serves as the terminal electron acceptor.
The observed yield for aerobic growth of NB1 (0.27 mg of TSS mg of CM−1) is on the high end of the yields reported (0.14 to 0.24 mg [dry weight] mg of CM−1) for six Hyphomicrobium isolates, Aminobacter sp. CMC, Nocardioides sp. SAC-4 (21), and other strains (4, 5). The yields for nonchlorinated C1 compounds such as methanol are similar to the yield for CM when compared on the basis of eeq (10). This suggests that the free energy available from dechlorination is not conserved during CM catabolism. Microbes that use DCM as their sole source of carbon and energy under aerobic and nitrate-reducing conditions also do not appear to conserve the energy from dechlorination (7). With these compounds, dechlorination appears to simply produce intermediates that can enter central metabolic pathways.
The maximum specific growth rate for NB1 (0.006 h−1) is 1 to 2 orders of magnitude lower than that for other isolates that grow aerobically on CM (0.08 to 0.16 h−1) (21, 34). Half-saturation coefficients have not been reported for other aerobic isolates. The Ks values for NB1 under aerobic and anoxic conditions were considerably lower than the 100 μM value for strain MC when it grows by fermentation of CM. Low Ks values mean that NB1 continues to consume CM at its maximum rate until very low concentrations are reached. This may be of greatest relevance in natural environments, where the concentration of CM likely never approaches the high levels used in most laboratory studies.
It should be noted that the kinetic parameters reported in Table 1 were measured under extant conditions (11), i.e., the ratio of CM to biomass (on an eeq basis) was comparatively low. The physiological state of cells used in extant tests has an impact on the rate of substrate depletion during batch tests. It is likely that some of the differences in k and Ks between strain NB1 and other cultures grown aerobically with CM are attributable to differences in how the cultures were grown prior to measurement of the parameters. Even this consideration, however, is unlikely to alter the conclusion that NB1 grows comparatively slowly with CM.
The lag phase required for NB1 to switch from nitrate to oxygen as the terminal electron acceptor when growing on CM was unexpectedly long. For example, a lag phase of several hours has been reported when Pseudomonas denitrificans is switched from oxygen to nitrate (18, 20). Initially, we attributed the longer lag phase for NB1 to use of too high an oxygen concentration. However, even after using a headspace concentration below 21%, several weeks were still required. Cultivation of NB1 with nitrate as the sole electron acceptor for more than 1 year before attempting the switch to oxygen may have been a factor. Other studies have employed much more frequent cycling between oxygen and nitrate (1, 18, 20).
The ubiquity of bacteria capable of growing aerobically on CM (21) is consistent with the large amounts of this compound produced biogenically (14). Some of the natural environments from which isolates have been obtained, such as estuaries, are often subject to periods of little or no oxygen supply, when nitrate frequently becomes a significant alternate electron acceptor. The results of this study indicate that additional attention should be given to the role of CM biodegradation under anoxic conditions when modeling the global cycling of this important C1 compound. The potential for anoxic biodegradation of other methyl halides such as methyl bromide should also be examined.
Acknowledgments
The assistance of Ricky Ulrich in sequencing the 16S rRNA gene of NB1 is gratefully acknowledged.
REFERENCES
- 1.Baumann, B., M. Snozzi, J. R. van der Meer, and A. J. B. Zehnder. 1997. Development of stable denitrifying cultures during repeated aerobic-anaerobic transient periods. Water Res. 31:1947-1954. [Google Scholar]
- 2.Beck, J. V., and K. J. Arnold. 1977. Parameter estimation in engineering and science. Wiley and Sons, New York, N.Y.
- 3.Bedard, C., and R. Knowles. 1989. Physiology, biochemistry, and specific inhibitors of CH4, NH4+, and CO oxidation by methanotrophs and nitrifiers. Microbiol. Rev. 53:68-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coulter, C., J. T. G. Hamilton, W. C. McRoberts, L. Kulakov, M. J. Larkin, and D. B. Harper. 1999. Halomethane:bisulphide/halide ion methyltransferase, an unusual corrinoid enzyme of environmental significance isolated from an aerobic methylotroph using chloromethane as the sole carbon source. Appl. Environ. Microbiol. 65:4301-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doronina, N. V., A. P. Sokolov, and Y. A. Trotsenko. 1996. Isolation and initial characterization of aerobic chloromethane-utilizing bacteria. FEMS Microbiol. Lett. 142:179-183. [Google Scholar]
- 6.Edwards, P. R., I. Campbell, and G. S. Milne. 1982. The impact of chloromethanes on the environment, part 2: methyl chloride and methylene chloride. Chem. Ind. 4:619-622. [Google Scholar]
- 7.Freedman, D. L., C. R. Smith, and D. R. Noguera. 1997. Dichloromethane biodegradation under nitrate-reducing conditions. Water Environ. Res. 69:115-122. [Google Scholar]
- 8.Freedman, D. L., J. Woertz, M. F. Verce, I. M. Reis, and R. A. Barbosa. 1997. Biodegradation of chloromethane under anoxic conditions, p. 1-6. In B. C. Alleman and A. Leeson (ed.), In situ and on-site bioremediation, vol. 5. Battelle Press, Columbus, Ohio. [Google Scholar]
- 9.Gossett, J. M. 1987. Measurement of Henry's Law constants for C1 and C2 chlorinated hydrocarbons. Environ. Sci. Technol. 21:202-208. [Google Scholar]
- 10.Grady, C. P. L., Jr., G. T. Daigger, and H. C. Lim. 1999. Biological wastewater treatment, 2nd ed. Marcel Dekker, Inc., New York, N.Y.
- 11.Grady, C. P. L., Jr., B. F. Smets, and D. S. Barbeau. 1996. Variability in kinetic parameter estimates: possible causes and a proposed terminology. Water Res. 30:742-748. [Google Scholar]
- 12.Greenberg, A. E., L. S. Clesceri, and A. D. Eaton (ed.). 1992. Standard methods for the examination of water and wastewater, 18th ed. American Public Health Association, American Water Works Association, and Water Environment Federation, Washington, D.C.
- 13.Gribble, G. W. 1994. The natural production of chlorinated compounds. Environ. Sci. Technol. 28:310A-319A. [DOI] [PubMed] [Google Scholar]
- 14.Harper, D. B. 2000. The global chloromethane cycle: biosynthesis, biodegradation and metabolic role. R. Soc. Chem. Nat. Prod. Rep. 17:337-348. [DOI] [PubMed] [Google Scholar]
- 15.Hartmans, S., A. Schmuckle, A. M. Cook, and T. Leisinger. 1986. Methyl chloride: naturally occurring toxicant and C-1 growth substrate. J. Gen. Microbiol. 132:1139-1142. [Google Scholar]
- 16.Keith, L. H., and W. A. Telliard. 1979. Priority pollutants. I. A perspective view. Environ. Sci. Technol. 13:416-423. [Google Scholar]
- 17.Khalil, M. A. K., and R. A. Rasmussen. 1999. Atmospheric methyl chloride. Atmosph. Environ. 33:1305-1321. [Google Scholar]
- 18.Kornaros, M., and G. Lyberatos. 1998. Kinetic modelling of Pseudomonas denitrificans growth and denitrification under aerobic, anoxic and transient operating conditions. Water Res. 32:1912-1922. [DOI] [PubMed] [Google Scholar]
- 19.Linos, A., R. Reichelt, U. Keller, and A. Steinbuchel. 2000. A gram-negative bacterium, identified as Pseudomonas aeruginosa AL98, is a potent degrader of natural rubber and synthetic cis-1,4-polyisoprene. FEMS Microbiol. Lett. 182:155-161. [DOI] [PubMed] [Google Scholar]
- 20.Liu, P.-H., S. A. Svoronos, and B. Koopman. 1998. Experimental and modeling study of diauxic lag of Pseudomonas denitrificans switching from oxic to anoxic conditions. Biotechnol. Bioeng. 60:649-655. [PubMed] [Google Scholar]
- 21.McAnulla, C., I. R. McDonald, and J. C. Murrell. 2001. Methyl chloride utilising bacteria are ubiquitous in the natural environment. FEMS Microbiol. Lett. 201:151-155. [DOI] [PubMed] [Google Scholar]
- 22.McAnulla, C., C. A. Woodall, I. R. McDonald, A. Studer, S. Vuilleumier, T. Leisinger, and J. C. Murrell. 2001. Chloromethane utilization gene cluster from Hyphomicrobium chloromethanicum strain CM2T and development of functional gene probes to detect halomethane-degrading bacteria. Appl. Environ. Microbiol. 67:307-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald, I. R., N. V. Doronina, Y. A. Trotsenko, C. McAnulla, and J. C. Murrell. 2001. Hyphomicrobium chloromethanicum sp. nov. and Methylobacterium chloromethanicum sp. nov., chloromethane-utilising bacteria isolated from a polluted environment. J. Syst. Evol. Microbiol. 51:119-122. [DOI] [PubMed] [Google Scholar]
- 24.Meβmer, M., S. Reinhardt, G. Wohlfarth, and G. Diekert. 1996. Studies on methyl chloride dehalogenase and o-demethylase in cell extracts of the homoacetogen strain MC based on a newly developed coupled enzyme assay. Arch. Microbiol. 165:18-25. [Google Scholar]
- 25.Prior, S. D., and H. Dalton. 1985. Acetylene as a suicide substrate and active site probe for methane monooxygenase from Methylococcus capsulatus (Bath). FEMS Microbiol. Lett. 29:105-109. [Google Scholar]
- 26.Reichert, P. 1994. Concepts underlying a computer program for the identification and simulation of aquatic systems. Schriftenr. EAWAG report CH-8600. EAWAG Swiss Federal Institute for Environmental Science and Technology, Duebendorf, Switzerland.
- 27.Rittmann, B. E., and P. L. McCarty. 2001. Environmental biotechnology: principles and applications. McGraw Hill, New York, N.Y.
- 28.Smatlak, C. R., J. M. Gossett, and S. H. Zinder. 1996. Comparative kinetics of hydrogen utilization for reductive dechlorination of tetrachloroethene and methanogenesis in an anaerobic enrichment culture. Environ. Sci. Technol. 30:2850-2858. [Google Scholar]
- 29.Studer, A., S. Vuilleumier, and T. Leisinger. 1999. Properties of the methylcobalamin:H4folate methyltransferase involved in chloromethane utilization by Methylobacterium sp. strain CM4. Eur. J. Biochem. 264:242-249. [DOI] [PubMed] [Google Scholar]
- 30.Swamy, M. H. 2001. M.S. thesis. Clemson University, Clemson, S.C.
- 31.Traunecker, J., A. Preuβ, and G. Diekert. 1991. Isolation and characterization of a methyl chloride utilizing, strictly anaerobic bacterium. Arch. Microbiol. 156:416-421. [Google Scholar]
- 32.U.S. Environmental Protection Agency. 1994. Health effects notebook for hazardous air pollutants. Draft. no. EPA-452/D-95-00, PB95-503579. U.S. Environmental Protection Agency, Washington, D.C.
- 33.Vannelli, T., M. Meβmer, A. Studer, S. Vuilleumier, and T. Leisinger. 1999. A corrinoid-dependent catabolic pathway for growth of a Methylobacterium strain with chloromethane. Proc. Natl. Acad. Sci. USA 96:4615-4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vannelli, T., A. Studer, M. Kertesz, and T. Leisinger. 1998. Chloromethane metabolism by Methylobacterium sp. strain CM4. Appl. Environ. Microbiol. 64:1933-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verce, M. F., and D. L. Freedman. 2001. Modeling the kinetics of vinyl chloride cometabolism by an ethane-grown Pseudomonas sp. Biotechnol. Bioeng. 71:274-285. [DOI] [PubMed] [Google Scholar]
- 36.Verce, M. F., R. L. Ulrich, and D. L. Freedman. 2000. Characterization of an isolate that uses vinyl chloride as a growth substrate under aerobic conditions. Appl. Environ. Microbiol. 66:3535-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verce, M. F., R. L. Ulrich, and D. L. Freedman. 2001. Transition from cometabolic to growth-linked biodegradation of vinyl chloride by a Pseudomonas sp. isolated on ethene. Environ. Sci. Technol. 35:4242-4251. [DOI] [PubMed] [Google Scholar]