Abstract
Background
Pulmonary hydatid is caused by larval stage of parasite Echinococcus granulosus. Although surgery still remains the definitive therapy, various workers have tried albendazole and sterilization of cysts with varying result.
Methods
32 patients(21 males, 11 females) of pulmonary hydatid disease with average age 32.5 years(21-51 years) treated by us between Jan 97 to Apr 2001 were analysed. Diagnosis was established clinically, radiologically and by serological testing. 16 patients who had simple cyst were treated with 20 ml percutaneous hypertonic(20%) saline irrigation of the cyst along with albendazole (400 mg twice a day, 6 cycles of 4 weeks with 2 weeks drug free period between the cycles). 13 patients of complicated cysts were treated with 6 cycles of albendazole. All cases were followed up for one year. 16 patients including three fresh cases were subjected to surgical resection.
Results
Pleural involvement was noted in 10 patients. On chest radiography 19 patients had homogenous oval or circular cysts, 6 patients had crescent sign and 10 had water lily sign. After percutaneous hypertonic saline irrigation all patients showed initial regression in size and developed complicated cysts with water lily sign but subsequently there was no regression. Of 13 patients treated with albendazole, 3 patients showed complete resolution and 2 patients showed regression of cyst. All these 5 patients had shown regression during first cycle of albendazole. 16 patients were subjected to surgery (6 after saline irrigation, 7 after albendazole course and 3 fresh cases). No difference was noted in these groups on histopathological examination.
Conclusion
From this study it was evident that those patients who demonstrate regression in size during first cycle of albendazole are likely to benefit and improve with further cycles of it. Those who do not respond should be subjected to surgery. Result of percutaneous hypertonicsaline irrigation as scolicidal was not encouraging.
Key Words: Albendazole, Complicated cyst, Hypertonic saline irrigation, Pulmonary hydatid
Introduction
Hydatid disease is caused by the larval stage of Echinococcus granulosus where man is the accidental intermediate host [1]. This is a zoonotic disease with worldwide distribution. In India cases occur in all regions. At our tertiary referral center, 6 to 9 cases per 2000 admissions have been reported every year. Lungs happen to be the second most common site of infection. The incidence of pulmonary involvement is known to be 10-30% in different reported series[2]. In two different Delhi hospitals incidence has been reported to be 2.3 to 3.3 per 10,000 patients admitted for various illnesses [3]. At our tertiary referral center 6 to 9 cases per 2000 admissions have been reported every year. It is one of the rare helminthic infections, which has not benefited from progress in chemotherapy. Definitive treatment still remains surgery although there had always been a need for medical treatment when the cyst ruptured with chances of dissemination. Albendazole has been used with promising results [4, 5, 6]. We have thus carried out this study to analyse various clinical and radiological presentations of pulmonary hydatid disease and to assess response to percutaneous hypertonic(20%) saline irrigation of the cyst and albendazole therapy.
Material and Methods
All patients of pulmonary hydatid disease treated by us between January 1997 to April 2001 were included in this study. A detailed history and meticulous clinical evaluation was carried out. Detailed investigation included chest X-ray, total and differential blood count, liver function tests, urinalysis and biochemical parameters and ELISA for Echinococcus. Ultrasonography (USG) chest, CT scan thorax, fiberoptic bronchoscopy and pleural aspiration were done whenever there was requirement. Patients with simple spherical cysts were treated with 20 ml percutaneous hypertonic (20%) saline instillation under USG guidance, along with course of albendazole (400 mg twice a day, 6 cycles of 4 weeks with 2 weeks drug free interval between the cycles). Patients with complicated cysts were treated with 6 cycles of albendazole.
Follow-up was done 3 monthly with clinical, radiological, hematological and biochemical profiles for one year. 16 patients were subjected to resectional surgery, which included 6 patients after inadequate response to hypertonic saline irrigation with albendazole course, 7 patients after inadequate response toalbendazole and 3 patients who were freshly diagnosed. Histopathological examination was carried out in detail in all patients.
Results
During the study period a total of 32 patients were diagnosed as pulmonary hydatid disease. There were 21 males and 11 females, their ages ranging from 21 years to 51 years. Average age of this cohort was 32.5 years. 19 patients had presented with well-circumscribed homogeneous oval or spherical cyst. 13 patients had presented with complicated cysts (rupture, bronchial communication or pleural effusion). 21 patients had solitary cyst, 10 patients had two cysts (Fig-1) of which it was bilateral in 4 and one patient had multiple pulmonary cysts. 3 patients had associated cysts in liver, one in spleen and one in retroperitoneum. Pleural involvement was noted in 10 patients (31%). Commonest site of cyst was found in both lower lobes (17 cysts in right lower lobe and 13 cysts in left lower lobe). Water lily sign was seen in 10 patients and crescent sign was noted in 6 patients. Chest-pain, cough, haemoptysis, dyspnea and fever were the commonest symptoms (Table 1). 9 patients were asymptomatic in which hydatid cyst was detected on chest radiography. On investigation ELISA for Echinococcus was found positive in 20 out of 24 patients (83%). Eosinophilia was found only in 5 patients out of whom 4 patients had complicated cyst. Radiological signs have been mentioned in Table 2. One patient showed all the radiological signs during hospitalization (spherical homogenous cyst, Fig-2), crescent sign, double crescent sign (Fig-3), water lily sign, pneumonia, pleural effusion, and solid lesions due to crumpled and collapsed membranes. 3 cases had significant pleural effusions. Pleural aspirate was exudative with predominance of lymphocytes. 2 patients presented with hydropneumothorax. Rest all 5 patients had minimal pleural effusion, which could not be tapped. Two patients had history of severe reaction and collapse at the time of rupture before presentation.
Fig. 1.
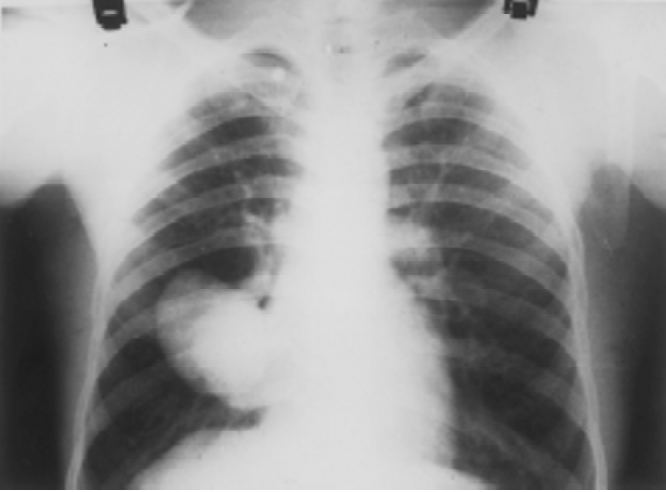
Chest radiograph showing two well-circumscribed homogenous cysts over right lower lobe
Table 1.
Pulmonary hydatid symptoms
| Symptoms | No of patients (32) |
|---|---|
| Asymptomatic | 9 |
| Cough | 13 |
| Chest pain | 15 |
| Haemoptysis | 9 |
| Dyspnoea | 8 |
| Fever | 6 |
| Expectoration of cyst, fluid | 3 |
| Allergic manifestations | 2 |
Table 2.
Pulmonary hydatid : radiological features
| X-ray sign | No of patients (32) |
|---|---|
| Spherical cyst | 19 |
| Crescent sign | 6 |
| Water lily sign | 10 |
| Double arch | 1 |
| Pneumonia | 3 |
| Hydropneumothorax | 2 |
| Blunting of CP angle | 5 |
Fig. 2.
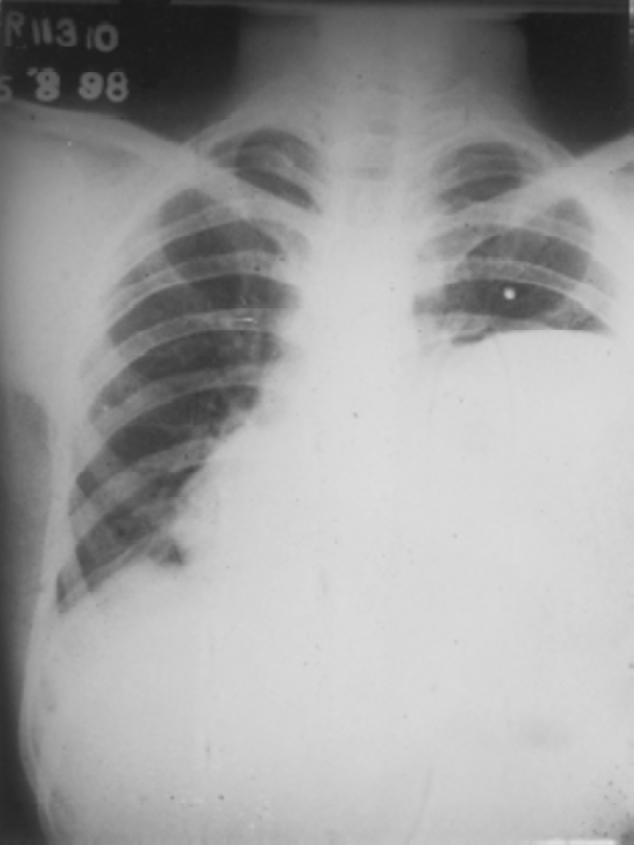
Chest radiograph showing large hydatid cyst left lower lobe causing mediastinal shift to opposite side
Fig. 3.
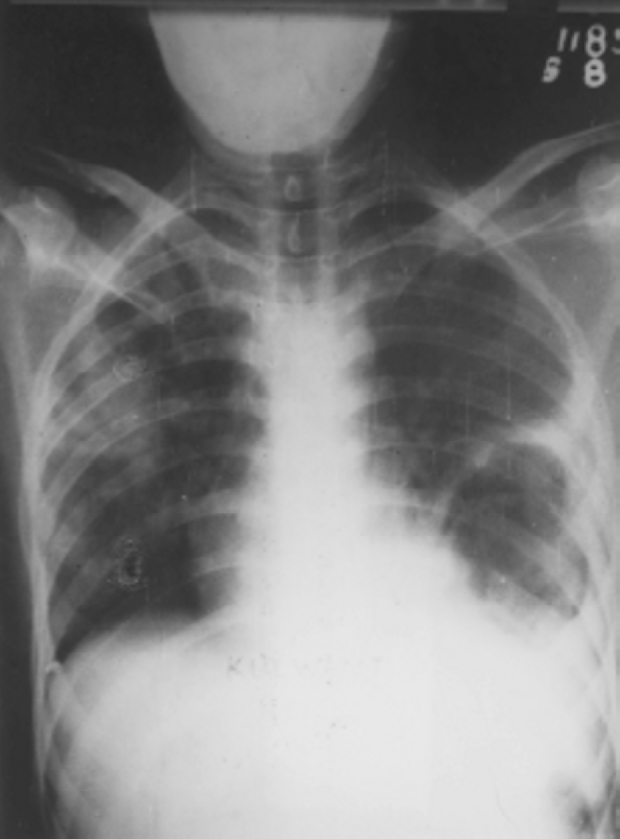
Chest radiograph showing double arch sign left lower lobe with pneumonia right upper lobe
After hypertonic saline irrigation, all patients showed initial regression in size and developed complicated cysts with water-lily sign but subsequently there was no regression. One patient developed lung abscess and one patient had allergic reaction. Of 13 patients who were treated with albendazole, 3 patients showed complete resolution and 2 patients showed regression of cyst. All these 5 patients had shown regression during the first cycle of albendazole. Histology of resected specimen did not show any difference in subgroups that were treated with albendazole, hypertonic saline irrigation or those who had not received any medical therapy.
Discussion
Indian experience of hydatosis shows that rural folk are more affected than urban dwellers and male to female ratio is 4-5:1[1]. Male to female ratio in our study was 2:1. The effect of the hepatic and pulmonary capillary sieves in containing the larvae is largely responsible for the localization of the diseases. 65-70% in liver, 10-30% in lungs[2]. Clinical picture depends on site and size of cyst and whether it is simple or complicated. Small simple cyst located peripherally usually remains asymptomatic. In present study 9 patients were asymptomatic and all of them had simple cysts, which were detected on X-ray for other reasons. We found chest pain, cough, haemoptysis, dyspnea and fever as the main symptoms, predominantly in complicated cysts. Coughing out of salty fluid is an important pointer in history to be looked for and is reported to occur in 20% patients[3]. We found this history in 3 patients (9%). Severe allergic manifestation and collapse due to intra-pleural rupture at presentation was found in 2 cases. Incidents of multiple cysts have been reported in 20-30% cases [2]. In present study 11 patients (30%) had two or more cysts. The majority of cysts were located in lower lobes, 17 in right (40%) and 13 in left (31%) which is consistent with other studies. In contrast to liver cysts in which calcification occurs in 20 to 30%, calcification of pulmonary hydatid cysts is rare (.07%)[2, 7], it was not seen in any patient in this study. Hepatic and extrapulmonary involvement has been reported 5-10% from various thoracic centers [7, 8, 9]. We found associated extrapulmonary cysts in 4 patients (12.5%), two in liver, one in liver and spleen and one in retroperitoneum. Presentation as pleural effusion can confuse many and it may prompt the clinician to a diagnostic tap. A careful palpation of liver and USG of chest and abdomen help us to get a diagnostic pointer. Approximately 5% of patients with pulmonary hydatid cyst have a pleural effusion [2, 7, 10]. Pleural involvement occurs because of (a) rupture of cyst into pleural space (b) rarely pleura may be primarily involved by the enlarging cyst or (c) a pulmonary hydatid cyst may be accompanied by a pleural effusion. The characteristic of the pleural fluid in this situation has not been characterized earlier. Out of 3 pleural aspirations, we found exudative fluid with predominance of lymphocyte in 2 patients. Incidence of pleural involvement was high in our study. 3 cases had pleural effusion and 3 cases had hydropneumothorax. 4 patients had only blunting of cardiophrenic angle and pleural tap was dry. Probably it was due to reactionary effusion as we see in synpneumonic effusions.
Diagnosis is suspected by noticing single or multiple rounded homogenous cysts (Fig-1) on chest skiagram [2, 4, 7, 11, 12] that can change its shape on valsalva manoeuver. Bronchial fistulisation is an important event in the evolution of the cyst. When communication develops between the cyst and the bronchial tree, air may enter the space between the pericyst and exocyst and produce the “meniscus” or “crescent” sign. In present study thin crescent of hydatid did not pose and problem in differentiating from other causes of crescent sign. It was seen in 6 patients(19.4%). After the cyst has ruptured into the bronchial tree, its membranes may float on the fluid within the cyst and give rise to “water-lily sign” of “sign of the camalote”. It was present in 10 patients (31%). In other radiological signs “double arch sign” and eosinophilic pneumonia was seen in one patient (Fig-3). USG and CT scan can indicate fluid density of contents and can confirm its regression after medication. Blood eosinophilia occurs in 25-50% patients[7, 10] but in this study mild eosinophilia was noted only in 5 patients (16%) of complicated cysts. ELISA for Echinococcus was done in 24 patients and it was found positive in 20 patients (83%).
Till now surgery is usually the only successful method of treatment for uncomplicated cyst [2, 4, 8]. In general, chemotherapy is used as a complement to surgery to prevent recurrence. There has been much debate about sterilizing the cyst. Various workers have tried aspiration of some fluid and its replacement with dilute iodine, formaldehyde, silver nitrate or hypertonic saline. Others argue that it increases risk of rupture and dissemination. We have tried USG guided hypertonic (20%) saline instillation in 16 patients. We had only one intraprocedural allergic reaction and infection but overall the results were not encouraging. New benzimidazole carbonate (albendazole) has been used with promising results by various workers [4, 5, 6]. In our study, out of 13 patients, 3 patients (23%) showed complete resolution and 2 patients (15%) showed regression in size. All these 5 patients who had shown improvement, improved during first cycle of albendazole. As per some workers cysts of smaller size (less than 2-3 cm) can regress completely with albendazole whereas larger cysts (more than 4-6 cm) do not.
The present study concludes that all patients of pulmonary hydatid disease should be treated with 3 to 4 weeks of albendazole. If patients respond, 4 to 6 cycles of albendazole should be given. If there is no response, surgical resection should be done and post operatively 3-4 cycles of albendazole should be given to avoid recurrence. Cyst sterilisation with hypertonic saline has got no role in treatment, however diagnostic aspiration of cyst can be done in selected patients, as intraprocedural allergic reaction and dissemination are not common.
References
- 1.Fossier . Larval cestodes. In: Collier L, Balows A, Sussman M, editors. 9th ed. Volume 5. 1998. pp. 539–960. (Topley and Wilson's Microbiology and microbial infections). parasitology. Arnold. [Google Scholar]
- 2.Fraser RS, Muller NL, Coleman N, Pare PD. Fraser an dPare's Diagnosis of diseases of the chest. 4th ed. WB Saunders; Philadelphia: 1999. Protozoa, Helminths, Arthopods and Leeches; pp. 1033–1066. [Google Scholar]
- 3.Maniar DR, Iyer RK, Joshi JM. Pulmonary Hydatidosis Medical and Surgical Management. JAPI. 1996;44:63–64. [PubMed] [Google Scholar]
- 4.Ramos G, Orduna A, Garcia-Yuste M. Hydatid cyst of the lung: diagnosis and treatment. World J Surg. 2001;25:46–57. doi: 10.1007/s002680020007. [DOI] [PubMed] [Google Scholar]
- 5.Anadol D, Ozcelik U, Kiper N, Gocmen A. Treatment of hydatid disease. Paediatr Drugs. 2001;3:123–135. doi: 10.2165/00128072-200103020-00005. [DOI] [PubMed] [Google Scholar]
- 6.Keshmiri M, Baharvahdat H, Fattahi SH, Davachi B, Dabiri RH, Baradaran H. Albendazole versus placebo in treatment of echinococcosis. Trans R Soc Trop Med Hyg. 2001;95:190–194. doi: 10.1016/s0035-9203(01)90162-2. [DOI] [PubMed] [Google Scholar]
- 7.Jerry M, Benzarti M, Garrouche A, Klales N, Hayouni A. Hydatid disease of lungs. Study of 386 cases. Am Rev Respir Dis. 1992;146:185–189. doi: 10.1164/ajrccm/146.1.185. [DOI] [PubMed] [Google Scholar]
- 8.Burgos R, Varela A, Castedo E, Roda J, Montero CG, Serrano S, Tellez G. Pulmonary hydatidosis: surgical treatment and follow-up of240 cases. Eur J Cardiothorac Surg. 1999;16:628–634. doi: 10.1016/s1010-7940(99)00304-8. [DOI] [PubMed] [Google Scholar]
- 9.Thameur H, Chenik S, Abdelmoulah S, Bey M, Hachicha S, Chemingui M, Mestiri T. Thoracic hydatidosis. A review of 1619 cases. Rev Pneumol Clin. 2000;56:7–15. [PubMed] [Google Scholar]
- 10.Kulapati DDS. Pulmonary Hydatid Disease. In: Ahuja MMS, editor. Progress in Clinical Medicine in India. 3rd series. Arnold-Heinmann; Delhi: 1979. pp. 439–458. [Google Scholar]
- 11.Shanker PS. Chest Medicine. 4th ed. Oxford and 1BH publishing Company; 1994. Parasitic pulmonary disease; pp. 139–142. [Google Scholar]
- 12.Tor M, Ozvaran K, Ersoy Y, Senol T, Altuntas N, Kilicoglu G, Celik L. Pitfalls in the diagnosis of complicated pulmonary hydatid disease. Respir Med. 2001;95:237–239. doi: 10.1053/rmed.2000.1024. [DOI] [PubMed] [Google Scholar]


