Abstract
Background
Recent studies have shown an association between thyroid hormone levels and metabolic syndrome (MetS) among euthyroid individuals; however, there have been some inconsistencies between studies. Here, we evaluated the relationship between thyroid hormone levels and MetS in euthyroid middle-aged subjects in a large cohort.
Methods
A retrospective analysis of 13,496 euthyroid middle-aged subjects who participated in comprehensive health examinations was performed. Subjects were grouped according to thyroid stimulating hormone, total triiodothyronine (T3), total thyroxine (T4), and T3-to-T4 ratio quartile categories. We estimated the odds ratios (ORs) for MetS according to thyroid hormone quartiles using logistic regression models, adjusted for potential confounders.
Results
Of the study patients, 12% (n=1,664) had MetS. A higher T3 level and T3-to-T4 ratio were associated with unfavourable metabolic profiles, such as higher body mass index, systolic and diastolic blood pressure, triglycerides, fasting glucose and glycated hemoglobin, and lower high density lipoprotein cholesterol levels. The proportion of participants with MetS increased across the T3 quartile categories (P for trend <0.001) and the T3-to-T4 ratio quartile categories (P for trend <0.001). The multi-variate-adjusted OR (95% confidence interval) for MetS in the highest T3 quartile group was 1.249 (1.020 to 1.529) compared to the lowest T3 quartile group, and that in the highest T3-to-T4 ratio quartile group was 1.458 (1.141 to 1.863) compared to the lowest T3-to-T4 ratio quartile group, even after adjustment for potential confounders.
Conclusion
Serum T3 levels and T3-to-T4 ratio are independently associated with MetS in euthyroid middle-aged subjects. Longitudinal studies are needed to define this association and its potential health implications.
Keywords: Thyroid hormones, Triiodothyronine, Metabolic syndrome
INTRODUCTION
Thyroid dysfunction and metabolic syndrome (MetS) are both associated with an increased risk of cardiovascular morbidity and mortality [1,2,3,4]. Studies have reported the relationship between overt and subclinical thyroid dysfunction and components of MetS [5,6,7]. Recently, there has been growing interest in the potential impact of differences in thyroid function within the reference range on MetS and its components.
Many studies have reported an association between thyroid hormones within the reference range and components of MetS [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24]. Serum thyroid stimulating hormone (TSH) within the reference range has been positively related to the prevalence of obesity [18,19], high blood pressure (BP) [12], and unfavourable lipid profiles [13,17,24]. Low normal free thyroxine (fT4) levels have been correlated to components of MetS, such as unfavorable body composition [9,14,15,22,24], high BP [9,14], unfavourable lipid profiles [9,14,16,22], and insulin resistance [16,24]. High normal total triiodothyronine (T3) or free triiodothyronine (fT3) levels have been positively associated with unfavourable body composition [10,15,21,22,23,25], high BP [8,22], and insulin resistance [11,21,26].
However, few studies have evaluated the association between thyroid hormones within the reference range and MetS, and the association remains under debate. Some studies found an association between high normal TSH levels and MetS [18,20], while others did not [24]. One recent study reported an association between low normal fT4 levels and MetS [24], while another study identified no such association [14]. To date, no studies have investigated the association between T3 levels within the reference range and MetS.
The aim of the present study was to investigate the association of thyroid hormones with MetS and its components in euthyroid middle-aged subjects in a large cohort.
METHODS
Study population
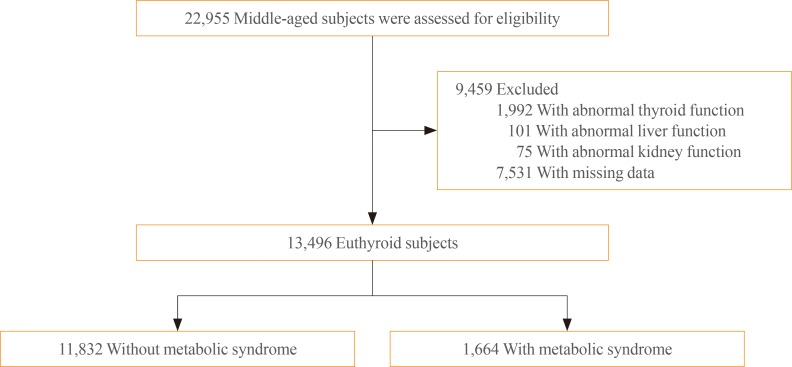
Middle-aged subjects between 35 and 65 years who participated in comprehensive health check-ups at the Health Promotion Center of Samsung Medical Center more than four times during a 6-year period (between January 2006 and December 2012; n=22,955) served as the source for the initial data (Fig. 1). Among these subjects, 9,459 were excluded for the following reasons, all of which were based on the 2006 records: abnormal thyroid function (TSH, T3, and/or T4 below or above the normal reference range; n=1,992), abnormal liver function (aspartate aminotransferase and/or alanine aminotransferase ≥ 2.5 above the upper limit of normal; n=101), abnormal kidney function (serum creatinine >1.3 mg/dL; n=75), and/or absence of data (questionnaire, anthropometric measurements, glycated hemoglobin [HbA1c], insulin, TSH, T3, and/or T4; n=7,531). Several subjects met more than two exclusion criteria. After exclusion of ineligible participants, 13,496 subjects were deemed eligible and therefore included in this study. All data containing anthropometric data, laboratory tests, results of radiologic images, and coded answers to self-reported questionnaires were stored in an electronic medical record. The requirement for informed consent for this study was waived by the Institutional Review Board of because researchers only accessed the database for analysis purposes and because personal identifying information was not accessed. The study protocol was approved by the Institutional Review Board of Samsung Medical Center.
Fig. 1. Flow chart of the study population.
Definitions of euthyroidism
Euthyroidism was defined as serum TSH (reference range, 0.4 to 5.0 mU/L), T3 (reference range, 1.10 to 2.90 nmol/L), and T4 (reference range, 64.0 to 155.0 nmol/L) levels within the normal reference ranges.
Definitions of metabolic syndrome
Using a Joint Interim Statement by the International Diabetes Federation and the American Heart Association/National Heart, Lung, and Blood Institute criteria [27] and World Health Organization body mass index (BMI) cut-off point for overweight [28], MetS was defined as three or more of the following: (1) fasting glucose ≥100 mg/dL or on antidiabetic medication; (2) BMI ≥25 kg/m2; (3) triglycerides (TG) ≥150 mg/dL; (4) high density lipoprotein cholesterol (HDL-C) <40 mg/dL in men and <50 mg/dL in women; and (5) BP ≥130/85 mm Hg or on antihypertensive medication.
Clinical and laboratory measurements
Smoking status was evaluated using a questionnaire completed during an interview and patients were defined as never smokers, former smokers, or current smokers.
Height and weight were measured while subjects were wearing light clothing without shoes. BMI was calculated as weight in kilograms divided by the height in meters squared (kg/m2). Percentage body fat and abdominal fat was estimated using a multi-frequency bioimpedance analyser (InBody 720, Biospace Co., Seoul, Korea), which has been validated with regards to reproducibility and accuracy for body composition [29], with eight-point tactile electrodes (two for each foot and hand). BP was measured using an automatic manometer with participants in a seated position after 5 minutes of quiet rest.
After overnight fasting, blood samples were drawn from the antecubital vein into vacuum tubes and subsequently analysed at a central, certified laboratory in Samsung Medical Center. Total cholesterol, low density lipoprotein cholesterol (LDL-C), HDL-C, and TG levels were measured with an enzymatic colorimetric method using a Modular D2400 (Roche Diagnostics, Basel, Switzerland). Blood glucose concentration was determined using a non-enzymatic method. HbA1c was measured using an immunoturbidimetric assay with Cobra Integra 800 automatic analyser (Roche Diagnostics) with a reference value range of 4.4% to 6.4%. HbA1c measurements were standardized to the reference method aligned with the Diabetes Control and Complications Trial and the National Glycohemoglobin Standardization Program standards. Serum insulin concentration was measured by an immunoradiometric assay (TFB Co. Ltd., Tokyo, Japan). As a marker of insulin resistance, the homeostatic model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR=[fasting insulin (µIU/mL)×fasting glucose (mmol/L)]/22.5 [30]. Serum TSH levels were measured using an immunoradiometric assay kit (Immunotech, Marseille Cedex, France). Serum T3 and T4 levels were measured using a radioimmunoassay kit (Immunotech).
Study design and statistical analysis
All 13,496 subjects were divided into two groups according to whether or not they had MetS based on the 2006 records. In addition, all subjects were categorized into quartiles based on the level of thyroid hormones within the reference range: (1) TSH (Q1, 0.4 to 1.4 mU/L; Q2, 1.5 to 2.1 mU/L; Q3, 2.2 to 3.0 mU/L; and Q4, 3.1 to 5.0 mU/L); (2) T3 (Q1, 1.10 to 1.51 nmol/L; Q2, 1.52 to 1.69 nmol/L; Q3, 1.70 to 1.89 nmol/L; and Q4, 1.90 to 2.90 nmol/L); (3) T4 (Q1, 64.0 to 96.0 nmol/L; Q2, 96.1 to 107.0 nmol/L; Q3, 107.1 to 119.5 nmol/L; and Q4, 119.6 to 155.0 nmol/L); and (4) T3-to-T4 ratio (Q1, 0.0060 to 0.0140; Q2, 0.0141 to 0.0159; Q3, 0.0160 to 0.0179; and Q4, 0.0180 to 0.0565). Continuous variables are expressed as the mean±SD, and categorical variables are expressed as the percentage (%). Clinical and laboratory data with respect to the presence of MetS were compared using an independent t test for continuous variables and chi-square test for categorical variables. Mean thyroid hormone levels of the study population with respect to the cut-off values of MetS components were compared using an independent t test or one-way analysis of variance as appropriate for the variable, and differences between groups were determined based on Tukey's multiple comparison test. After adjusting for confounding variables, logistic regression models were used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for MetS. All P values and 95% CI for OR were corrected by Bonferroni's method due to multiple testing. All statistical tests were two-sided, and analyses were executed using SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
RESULTS
Baseline clinical and biochemical characteristics of the 13,496 subjects are summarized in Table 1. The overall prevalence of MetS in this cohort was 12% (n=1,664). Subjects with MetS were more likely to be older, and current or former smokers. They also had higher BMI, body fat percentage, abdominal fat percentage, systolic and diastolic BP, total cholesterol, LDL-C, TG, fasting glucose, HOMA-IR, T3, and T3-to-T4 ratio, but lower HDL-C and TSH levels, than those without MetS. There was no significant difference in T4 levels between the two groups.
Table 1. Baseline Characteristics of Study Subjects.
| Variable | Metabolic syndrome | Overall (n=13,496) | ||
|---|---|---|---|---|
| No (n=11,832) | Yes (n=1,664) | P value | ||
| Male sex | 6,852 (58) | 1,316 (79) | <0.001 | 8,168 (61) |
| Age, yr | 50.5±6.6 | 52.3±6.5 | <0.001 | 50.7±6.6 |
| Smoking, current/former/never, % | 17/29/54 | 27/37/36 | <0.001 | 19/30/51 |
| BMI, kg/m2 | 23.4±2.6 | 26.5±2.4 | <0.001 | 23.8±2.7 |
| Overweight (25≤BMI<30) | 2,784 (24) | 1,258 (76) | <0.001 | 4,042 (30) |
| Obesity (BMI≥30) | 134 (1) | 109 (7) | <0.001 | 243 (2) |
| Body fat, % | 23.3±6.1 | 25.7±5.7 | 0.004 | 23.6±6.1 |
| Male | 20.2±4.4 | 23.8±4.1 | <0.001 | 20.8±4.5 |
| Female | 27.7±5.4 | 33.1±4.9 | <0.001 | 28.0±5.5 |
| Abdominal fat, % | 0.88±0.06 | 0.93±0.06 | <0.001 | 0.88±0.06 |
| Male | 0.89±0.06 | 0.93±0.05 | <0.001 | 0.90±0.06 |
| Female | 0.85±0.06 | 0.91±0.06 | <0.001 | 0.86±0.06 |
| Systolic BP, mm Hg | 109.9±14.2 | 121.0±15.3 | <0.001 | 111.3±14.8 |
| Diastolic BP, mm Hg | 67.7±9.8 | 74.4±10.0 | <0.001 | 68.5±10.0 |
| Total cholesterol, mg/dL | 188.0±30.7 | 191.9±33.2 | <0.001 | 188.5±31.0 |
| LDL-C, mg/dL | 122.8±27.9 | 125.4±29.9 | 0.001 | 123.1±28.2 |
| HDL-C, mg/dL | 59.4±13.5 | 47.3±10.9 | <0.001 | 57.9±13.8 |
| Male | 55.5±11.9 | 47.2±11.3 | <0.001 | 54.2±12.2 |
| Female | 64.8±13.8 | 47.7±9.2 | <0.001 | 63.6±14.2 |
| Triglycerides, mg/dL | 114.7±63.9 | 214.0±102.5 | <0.001 | 126.9±77.1 |
| Fasting glucose, mg/dL | 87.5±12.9 | 103.7±23.8 | <0.001 | 89.5±15.6 |
| HbA1c, % | 5.3±0.5 | 5.9±0.9 | <0.001 | 5.4±0.6 |
| HOMA-IR | 1.9±0.9 | 2.9±1.5 | <0.001 | 2.1±1.1 |
| TSH, mU/L | 2.4±1.3 | 2.3±1.3 | 0.017 | 2.3±1.3 |
| T3, nmol/L | 1.72±0.28 | 1.78±0.29 | <0.001 | 1.72±0.28 |
| T4, nmol/L | 108.6±17.9 | 108.3±18.4 | 0.521 | 108.6±17.9 |
| T3-to-T4 ratio | 0.016±0.003 | 0.017±0.003 | <0.001 | 0.016±0.003 |
Values are expressed as number (%) or mean±SD. Demographic and biochemical characteristics of the study population with respect to the development of metabolic syndrome were compared using an independent t test for continuous variables and chi-square test for categorical variables.
BMI, body mass index; BP, blood pressure; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; HbA1c, glycated hemoglobin; HOMA-IR, homeostasis model assessment of insulin resistance; TSH, thyroid stimulating hormone; T3, triiodothyronine; T4, thyroxine.
The mean thyroid hormone levels according to select components of MetS are shown in Table 2. The mean TSH levels were negatively related to BMI (P<0.001), HDL-C level (P=0.012), fasting glucose level (P<0.001), and HbA1c level (P=0.017). The mean T3 levels were positively related to BMI (P<0.001), systolic BP (P<0.001), diastolic BP (P<0.001), HDL-C level (P<0.001), TG level (P<0.001), and fasting glucose level (P<0.001). The mean T3 level in the HbA1c 5.7 to 6.4 group was significantly higher than that in the HbA1c <5.7 or HbA1c ≥6.5 groups (P<0.001). The mean T4 levels were highest in the obesity group, followed by the underweight and normal BMI groups, then the overweight group (P=0.019). Systolic BP (P<0.001), diastolic BP (P<0.001), and HbA1c (P=0.009) were positively related to the mean T4 levels. With regard to T3-to-T4 ratio, we observed similar trends as the ones shown between mean T3 levels and components of MetS.
Table 2. Means for Thyroid Hormone Levels by Select Metabolic Syndrome Components.
| Variable | No. (%) | Thyroid hormones | |||
|---|---|---|---|---|---|
| TSH, mU/L | T3, nmol/L | T4, nmol/L | T3-to-T4 ratio | ||
| BMI, kg/m2 | |||||
| Underweight (<18.5) | 219 (2) | 2.6±1.3a,* | 1.67±0.28a,* | 109.7±17.4a,‡ | 0.015±0.003a,* |
| Normal (18.5-24.9) | 8,992 (66) | 2.4±1.3a,* | 1.71±0.28b,* | 108.8±17.9a,‡ | 0.016±0.003b,* |
| Overweight (25-29.9) | 4,042 (30) | 2.2±1.2b,* | 1.75±0.28c,* | 108.0±17.9b,‡ | 0.017±0.003c,* |
| Obesity (≥30) | 243 (2) | 2.2±1.2b,* | 1.79±0.26d,* | 110.9±20.0c,‡ | 0.017±0.003c,* |
| Systolic BP, mm Hg | |||||
| <130 | 10,272 (76) | 2.3±1.3 | 1.71±0.28* | 108.3±17.8* | 0.016±0.003† |
| ≥130e | 3,224 (24) | 2.3±1.3 | 1.76±0.29* | 109.7±18.3* | 0.017±0.003† |
| Diastolic BP, mm Hg | |||||
| <85 | 10,756 (80) | 2.3±1.3 | 1.71±0.28* | 108.3±17.8* | 0.016±0.003‡ |
| ≥85e | 2,739 (20) | 2.3±1.3 | 1.76±0.29* | 109.9±18.5* | 0.017±0.003‡ |
| HDL-C, mg/dL | |||||
| Lowf | 1,555 (12) | 2.5±1.3* | 1.68±0.28* | 108.8±17.7 | 0.016±0.003* |
| Highg | 11,941 (88) | 2.3±1.3* | 1.73±0.28* | 108.6±17.9 | 0.017±0.003* |
| Triglycerides, mg/dL | |||||
| <150 | 9,966 (74) | 2.3±1.3 | 1.70±0.28* | 108.8±17.9 | 0.016±0.003* |
| ≥150 | 3,530 (26) | 2.4±1.3 | 1.80±0.28* | 108.1±18.1 | 0.017±0.003* |
| Fasting glucose, mg/dL | |||||
| <100 | 11,484 (85) | 2.4±1.3* | 1.72±0.28* | 108.6±17.8 | 0.016±0.003* |
| ≥100h | 2,012 (15) | 2.2±1.2* | 1.75±0.29* | 108.6±18.6 | 0.017±0.003* |
| HbA1c, % | |||||
| <5.7 | 10,636 (79) | 2.4±1.3a,‡ | 1.72±0.28a,* | 108.5±17.8a,† | 0.016±0.003a,* |
| 5.7-6.4 | 1,992 (15) | 2.3±1.3a,b,‡ | 1.75±0.29b,* | 108.6±17.8a,† | 0.017±0.003b,* |
| ≥6.5h | 868 (6) | 2.2±1.3b,‡ | 1.73±0.30a,* | 110.4±19.6b,† | 0.016±0.003a,* |
Values are expressed as mean±SD. Thyroid hormone levels of the study population with respect to the cut-off values of metabolic syndrome components were compared using an independent t test or one-way analysis of variance as appropriate for the variable.
TSH, thyroid stimulating hormone; T3, triiodothyronine; T4, thyroxine; BMI, body mass index; BP, blood pressure; HDL-C, high density lipoprotein cholesterol; HbA1c, glycated hemoglobin.
a,b,c,dThe same letters indicate non-significant difference between groups based on Tukey's multiple comparison test; eInclude patients on antihyper-tensive medication regardless of systolic or diastolic BP levels; fHDL-C <40 mg/dL in men or <50 mg/dL in women; gHDL-C ≥40 mg/dL in men or ≥50 mg/dL in women; hInclude patients on antidiabetic medication regardless of fasting glucose or HbA1c levels; *P<0.001; †0.001≤P<0.01; ‡0.01≤P<0.05.
The proportion of participants with MetS increased across the T3 quartile categories (P for trend <0.001) and T3-to-T4 ratio quartile categories (P for trend <0.001) (Supplementary Fig. S1). The risk of MetS according to thyroid hormone category is shown in Table 3. As the T3 quartile categories increased, the risk for MetS increased, with ORs of 1.254, 1.255, and 1.712 when compared to the lowest quartile. After adjustment for sex, age, body fat percentage, smoking, and HOMA-IR, the highest T3 quartile remained a significant risk factor for MetS (OR, 1.249; 95% CI, 1.020 to 1.529; P=0.031). The risk of MetS increased as the T3-to-T4 ratio quartile categories increased with ORs of 1.132, 1.350, and 1.692 when compared to the lowest quartile. The multivariate-adjusted OR (95% CI) for MetS in the highest T3-to-T4 ratio quartile group was 1.458 (1.141 to 1.863) compared to the lowest T3-to-T4 ratio quartile group (P<0.001), even after adjustment for sex, age, body fat percentage, smoking, and HOMA-IR. No significant differences were seen in the proportion of subjects with MetS (Supplementary Fig. S1) and in the risk of MetS (Table 3) across the TSH quartile categories and T4 quartile categories.
Table 3. Risk of Metabolic Syndrome in Euthyroid Subjects according to Thyroid Hormone Quartiles.
| Thyroid hormone category | Metabolic syndrome/total, n (%) | OR (95% CI) | ||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||
| TSH quartiles, mU/L | ||||
| 0.4-1.4 | 481/3,744 (13) | 1 | 1 | 1 |
| 1.5-2.1 | 395/3,414 (12) | 0.888 (0.747-1.055) | 0.919 (0.772-1.095) | 0.926 (0.727-1.178) |
| 2.2-3.0 | 423/3,085 (14) | 1.078 (0.909-1.279) | 1.170 (0.983-1.391) | 1.245 (0.982-1.577) |
| 3.1-5.0 | 365/3,253 (11) | 0.857 (0.719-1.023) | 1.016 (0.848-1.217) | 1.134 (0.887-1.452) |
| T3 quartiles, nmol/L | ||||
| 1.10-1.51 | 323/3,307 (10) | 1 | 1 | 1 |
| 1.52-1.69 | 403/3,373 (12) | 1.254 (1.074-1.464)† | 1.217 (1.040-1.424)‡ | 1.025 (0.829-1.268) |
| 1.70-1.89 | 415/3,470 (12) | 1.255 (1.076-1.464)† | 1.169 (1.001-1.366)‡ | 1.000 (0.809-1.235) |
| 1.90-2.90 | 523/3,346 (16) | 1.712 (1.476-1.984)* | 1.570 (1.351-1.824)* | 1.249 (1.020-1.529)† |
| T4 quartiles, nmol/L | ||||
| 64.0-96.0 | 481/3,631 (13) | 1 | 1 | 1 |
| 96.1-107.0 | 390/3,387 (12) | 0.852 (0.716-1.014) | 0.870 (0.730-1.038) | 0.867 (0.685-1.097) |
| 107.1-119.5 | 354/3,153 (11) | 0.828 (0.693-0.990)† | 0.814 (0.680-0.975)† | 0.746 (0.582-0.956)† |
| 119.6-155.0 | 439/3,325 (13) | 0.996 (0.841-1.180) | 0.942 (0.794-1.119) | 0.890 (0.706-1.121) |
| T3-to-T4 ratio quartiles | ||||
| 0.0060-0.0140 | 333/3,379 (10) | 1 | 1 | 1 |
| 0.0141-0.0159 | 375/3,406 (11) | 1.132 (0.936-1.368) | 1.132 (0.934-1.371) | 1.097 (0.843-1.428) |
| 0.0160-0.0179 | 428/3,329 (13) | 1.350 (1.122-1.624)* | 1.358 (1.126-1.638)* | 1.275 (0.988-1.646) |
| 0.0180-0.0565 | 528/3,382 (16) | 1.692 (1.416-2.022)* | 1.616 (1.349-1.936)* | 1.458 (1.141-1.863)* |
Model 1, unadjusted; Model 2, adjusted for sex and age; Model 3, adjusted for sex, age, body fat percentage, smoking and homeostasis model assessment of insulin resistance. OR and 95% CI for metabolic syndrome were estimated using logistic regression models. All P values and 95% CI for OR were corrected by Bonferroni's method due to multiple testing.
OR, odds ratio; CI, confidence interval; TSH, thyroid stimulating hormone; T3, triiodothyronine; T4, thyroxine.
*P<0.001; †0.001≤P<0.01; ‡0.01≤P<0.05.
DISCUSSION
Our study showed that a high T3 level and a high T3-to-T4 ratio were associated with unfavourable metabolic profiles in a healthy population. Moreover, a high normal T3 level and a higher T3-to-T4 ratio were independently associated with MetS in euthyroid middle-aged subjects. However, we found no association between serum TSH and T4 levels within the reference range and a risk of MetS, despite the significant correlation between those thyroid hormones and several metabolic parameters.
Recent cross-sectional studies have reported that high normal T3 or fT3 levels correlated with components of MetS [10,15,22]. Alevizaki et al. [15] demonstrated that T3 levels were positively associated with parameters of central obesity, including subcutaneous fat and preperitoneal fat, waist circumference, and waist-to-hip ratio. De Pergola et al. [10] and Kitahara et al. [23] observed significant associations of fT3 levels with both BMI and waist circumference. Moreover, Roef et al. [22] reported that T3 and fT3 levels and fT3-to-fT4 ratio were positively associated with BMI, waist circumference, and components of MetS, such as TG levels, BP, and fasting plasma glucose levels, and negatively with HDL-C levels. However, whether high normal T3 levels increased the risk of MetS remains unknown [10,15,22,23]. In the current study, a high normal T3 level and a high T3-to-T4 ratio were significantly associated with high BMI, BP, HDL-C, TG, and fasting glucose levels. Moreover, a high normal T3 level and a high T3-to-T4 ratio correlated with increased MetS, even after adjustment for sex, age, body fat percentage, smoking, and HOMA-IR. This increase in circulating levels of T3 may represent an adaptive protective response against the detrimental effects of obesity or insulin resistance. Tissue-specific regulation of deiodinases enzyme levels were suggested as an important mechanism in altered thyroid hormone homeostasis in obese subjects [31]. The moderate increases in fT3 and T3 levels in obese patients lead to an increase in energy expenditure [32]. Basal metabolic rate, total energy expenditure, and sleeping energy expenditure are positively correlated to serum T3 or fT3 concentrations [32]. Therefore, changes in thyroid hormone concentrations in obesity may be regarded as an adaptation process to increased body weight [32]. Furthermore, leptin may be implicated as a mediator of the effects of obesity, as it could influence the thyroid hormone axis. Previous studies demonstrated that the reduction in serum leptin levels during weight loss could lead to decreased energy expenditure [33]. Apart from the central effects of leptin on the hypothalamic-pituitary axis, decreased serum thyroid hormone levels during caloric deprivation may also be related to reduced liver type 1 deiodinase activity [34]. Conversely, increased circulating leptin levels in obese subjects may elevate serum T3 concentrations, which could serve as a mechanism to increase metabolic rate and confer a relative protection against the development of obesity [31].
In contrast, inverse associations between fT4 levels within reference range and metabolic parameters have been reported [9,14,15,22,24]. Two cross-sectional studies investigated the association of fT4 levels with MetS, and discordant results were found between studies [14,24]. Kim et al. [14] showed that patients in the high normal fT4 quintile had a significantly lower prevalence of MetS than those in the lowest fT4 quintile, but such differences disappeared after adjustment for age and sex. More recently, Mehran et al. [24] found that lower normal fT4 levels were significantly related to a higher risk of insulin resistance and MetS. In addition, only one study by Roef et al. [21] described positive associations between T4 levels within the reference range and body fat mass and insulin resistance, but the relationship between T4 levels and MetS was not investigated. In current study, data on fT4 levels were not available in most subjects, so we focused on T4 rather than fT4. High normal T4 levels correlated with unfavourable metabolic parameters, such as high BP and HbA1c levels. However, we found no association between serum T4 levels within the reference range and a risk of MetS.
Although TSH was regarded as the first factor to be used for the detection of thyroid dysfunction, it was not associated with MetS in our study. A few studies showed a positive relation between TSH levels within the reference range and prevalent MetS [18,20], but the association remains under debate. One study in euthyroid subjects in Germany reported that high normal TSH levels were associated with obesity, higher TG levels, and MetS [18]. Another study of euthyroid postmenopausal women in Korea found that high normal TSH levels were associated with an increased prevalence of MetS [20]. Conversely, Mehran et al. [24] found no association between TSH within the reference range and a risk of presenting with MetS, despite a significant correlation between TSH levels and insulin resistance, which is consistent with our results. Although we found BMI, HDL-C, fasting glucose, and HbA1c levels to be associated with TSH, no significant differences were seen in the proportion of subjects with MetS and in the risk of MetS according to TSH. The discrepancy in results between reports may be due to differences in study populations, in the categorization of thyroid function, and in the factors included for adjustments in the analyses.
The main limitation of our current study is its retrospective nature. We did not measure anti-thyroid peroxidase antibodies to exclude potential underlying chronic autoimmune thyroid disease. We also lacked information on other exposures, including alcohol intake and physical activity, which influence metabolic profiles. In addition, the measurements of waist circumference in the assessment of obesity were not available. However, we hope that the inclusion of other anthropometric data including BMI and percentage of body fat and abdominal fat overcomes this limitation. The strength of our study is the large sample size, suitable exclusion criteria, and assessment of TSH, T3, and T4 levels.
In conclusion, serum T3 levels and T3-to-T4 ratio are independently associated with MetS in euthyroid middle-aged subjects. Further studies are warranted to confirm these findings. In addition, longitudinal studies are needed to define this association and its potential health implications.
ACKNOWLEDGMENTS
This study was supported by the Research Grant Number CB-2011-03-02 of the Korean Foundation for Cancer Research and Soonchunhyang University Research Fund.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
Supplementary Material
The proportion of subjects with metabolic syndrome by thyroid hormone quartile categories within the euthyroid range. TSH, thyroid stimulating hormone.
References
- 1.Isomaa B, Almgren P, Tuomi T, Forsen B, Lahti K, Nissen M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care. 2001;24:683–689. doi: 10.2337/diacare.24.4.683. [DOI] [PubMed] [Google Scholar]
- 2.Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- 3.Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA. 2002;288:2709–2716. doi: 10.1001/jama.288.21.2709. [DOI] [PubMed] [Google Scholar]
- 4.Iglesias P, Ridruejo E, Munoz A, Prado F, Macias MC, Guerrero MT, et al. Thyroid function tests and mortality in aged hospitalized patients: a 7-year prospective observational study. J Clin Endocrinol Metab. 2013;98:4683–4690. doi: 10.1210/jc.2012-3849. [DOI] [PubMed] [Google Scholar]
- 5.Fommei E, Iervasi G. The role of thyroid hormone in blood pressure homeostasis: evidence from short-term hypothyroidism in humans. J Clin Endocrinol Metab. 2002;87:1996–2000. doi: 10.1210/jcem.87.5.8464. [DOI] [PubMed] [Google Scholar]
- 6.Uzunlulu M, Yorulmaz E, Oguz A. Prevalence of subclinical hypothyroidism in patients with metabolic syndrome. Endocr J. 2007;54:71–76. doi: 10.1507/endocrj.k06-124. [DOI] [PubMed] [Google Scholar]
- 7.Waring AC, Rodondi N, Harrison S, et al. Thyroid function and prevalent and incident metabolic syndrome in older adults: the Health, Ageing and Body Composition Study. Clin Endocrinol (Oxf) 2012;76:911–918. doi: 10.1111/j.1365-2265.2011.04328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakker SJ, ter Maaten JC, Popp-Snijders C, Heine RJ, Gans RO. Triiodothyronine: a link between the insulin resistance syndrome and blood pressure? J Hypertens. 1999;17:1725–1730. doi: 10.1097/00004872-199917120-00009. [DOI] [PubMed] [Google Scholar]
- 9.Lin SY, Wang YY, Liu PH, Lai WA, Sheu WH. Lower serum free thyroxine levels are associated with metabolic syndrome in a Chinese population. Metabolism. 2005;54:1524–1528. doi: 10.1016/j.metabol.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 10.De Pergola G, Ciampolillo A, Paolotti S, Trerotoli P, Giorgino R. Free triiodothyronine and thyroid stimulating hormone are directly associated with waist circumference, independently of insulin resistance, metabolic parameters and blood pressure in overweight and obese women. Clin Endocrinol (Oxf) 2007;67:265–269. doi: 10.1111/j.1365-2265.2007.02874.x. [DOI] [PubMed] [Google Scholar]
- 11.Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92:491–496. doi: 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- 12.Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. Association between blood pressure and serum thyroid-stimulating hormone concentration within the reference range: a population-based study. J Clin Endocrinol Metab. 2007;92:841–845. doi: 10.1210/jc.2006-2208. [DOI] [PubMed] [Google Scholar]
- 13.Asvold BO, Vatten LJ, Nilsen TI, Bjoro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007;156:181–186. doi: 10.1530/eje.1.02333. [DOI] [PubMed] [Google Scholar]
- 14.Kim BJ, Kim TY, Koh JM, Kim HK, Park JY, Lee KU, et al. Relationship between serum free T4 (FT4) levels and metabolic syndrome (MS) and its components in healthy euthyroid subjects. Clin Endocrinol (Oxf) 2009;70:152–160. doi: 10.1111/j.1365-2265.2008.03304.x. [DOI] [PubMed] [Google Scholar]
- 15.Alevizaki M, Saltiki K, Voidonikola P, Mantzou E, Papamichael C, Stamatelopoulos K. Free thyroxine is an independent predictor of subcutaneous fat in euthyroid individuals. Eur J Endocrinol. 2009;161:459–465. doi: 10.1530/EJE-09-0441. [DOI] [PubMed] [Google Scholar]
- 16.Garduno-Garcia Jde J, Alvirde-Garcia U, Lopez-Carrasco G, Padilla Mendoza ME, Mehta R, Arellano-Campos O, et al. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 2010;163:273–278. doi: 10.1530/EJE-10-0312. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Tan Y, Wang C, Zhang X, Zhao Y, Song X, et al. Thyroid-stimulating hormone levels within the reference range are associated with serum lipid profiles independent of thyroid hormones. J Clin Endocrinol Metab. 2012;97:2724–2731. doi: 10.1210/jc.2012-1133. [DOI] [PubMed] [Google Scholar]
- 18.Ruhla S, Weickert MO, Arafat AM, Osterhoff M, Isken F, Spranger J, et al. A high normal TSH is associated with the metabolic syndrome. Clin Endocrinol (Oxf) 2010;72:696–701. doi: 10.1111/j.1365-2265.2009.03698.x. [DOI] [PubMed] [Google Scholar]
- 19.Lai Y, Wang J, Jiang F, Wang B, Chen Y, Li M, et al. The relationship between serum thyrotropin and components of metabolic syndrome. Endocr J. 2011;58:23–30. doi: 10.1507/endocrj.k10e-272. [DOI] [PubMed] [Google Scholar]
- 20.Lee YK, Kim JE, Oh HJ, Park KS, Kim SK, Park SW, et al. Serum TSH level in healthy Koreans and the association of TSH with serum lipid concentration and metabolic syndrome. Korean J Intern Med. 2011;26:432–439. doi: 10.3904/kjim.2011.26.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roef G, Lapauw B, Goemaere S, Zmierczak HG, Toye K, Kaufman JM, et al. Body composition and metabolic parameters are associated with variation in thyroid hormone levels among euthyroid young men. Eur J Endocrinol. 2012;167:719–726. doi: 10.1530/EJE-12-0447. [DOI] [PubMed] [Google Scholar]
- 22.Roef GL, Rietzschel ER, Van Daele CM, Taes YE, De Buyzere ML, Gillebert TC, et al. Triiodothyronine and free thyroxine levels are differentially associated with metabolic profile and adiposity-related cardiovascular risk markers in euthyroid middle-aged subjects. Thyroid. 2014;24:223–231. doi: 10.1089/thy.2013.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitahara CM, Platz EA, Ladenson PW, Mondul AM, Menke A, Berrington de Gonzalez A. Body fatness and markers of thyroid function among U.S. men and women. PLoS One. 2012;7:e34979. doi: 10.1371/journal.pone.0034979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehran L, Amouzegar A, Tohidi M, Moayedi M, Azizi F. Serum free thyroxine concentration is associated with metabolic syndrome in euthyroid subjects. Thyroid. 2014;24:1566–1574. doi: 10.1089/thy.2014.0103. [DOI] [PubMed] [Google Scholar]
- 25.Taneichi H, Sasai T, Ohara M, Honma H, Nagasawa K, Takahashi T, et al. Higher serum free triiodothyronine levels within the normal range are associated with metabolic syndrome components in type 2 diabetic subjects with euthyroidism. Tohoku J Exp Med. 2011;224:173–178. doi: 10.1620/tjem.224.173. [DOI] [PubMed] [Google Scholar]
- 26.Lambadiari V, Mitrou P, Maratou E, Raptis AE, Tountas N, Raptis SA, et al. Thyroid hormones are positively associated with insulin resistance early in the development of type 2 diabetes. Endocrine. 2011;39:28–32. doi: 10.1007/s12020-010-9408-3. [DOI] [PubMed] [Google Scholar]
- 27.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 28.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 29.Malavolti M, Mussi C, Poli M, Fantuzzi AL, Salvioli G, Battistini N, et al. Cross-calibration of eight-polar bioelectrical impedance analysis versus dual-energy X-ray absorptiometry for the assessment of total and appendicular body composition in healthy subjects aged 21-82 years. Ann Hum Biol. 2003;30:380–391. doi: 10.1080/0301446031000095211. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Araujo RL, Carvalho DP. Bioenergetic impact of tissuespecific regulation of iodothyronine deiodinases during nutritional imbalance. J Bioenerg Biomembr. 2011;43:59–65. doi: 10.1007/s10863-011-9327-x. [DOI] [PubMed] [Google Scholar]
- 32.Reinehr T. Obesity and thyroid function. Mol Cell Endocrinol. 2010;316:165–171. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Ahima RS. Leptin and the neuroendocrinology of fasting. Front Horm Res. 2000;26:42–56. doi: 10.1159/000061014. [DOI] [PubMed] [Google Scholar]
- 34.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The proportion of subjects with metabolic syndrome by thyroid hormone quartile categories within the euthyroid range. TSH, thyroid stimulating hormone.