Abstract
Background
Resveratrol (RSV) is a polyphenolic phytoalexin that has many effects on metabolic diseases such as diabetes and obesity. Given the importance of brown adipose tissue (BAT) for energy expenditure, we investigated the effects of RSV on brown adipocytes.
Methods
For the in vitro study, interscapular BAT was isolated from 7-week-old male Sprague Dawley rats. For the in vivo study, 7-week-old male Otsuka Long Evans Tokushima Fatty (OLETF) rats were divided into four groups and treated for 27 weeks with: standard diet (SD); SD+RSV (10 mg/kg body weight, daily); high fat diet (HFD); HFD+RSV. RSV was provided via oral gavage once daily during the in vivo experiments.
Results
RSV treatment of primary cultured brown preadipocytes promoted mitochondrial activity, along with over-expression of estrogen receptor α (ER-α). In OLETF rats, both HFD and RSV treatment increased the weight of BAT and the differentiation of BAT. However, only RSV increased the mitochondrial activity and ER-α expression of BAT in the HFD-fed group. Finally, RSV improved the insulin sensitivity of OLETF rats by increasing the mitochondrial activity of BAT, despite having no effects on white adipocytes and muscles in either diet group.
Conclusion
RSV could improve insulin resistance, which might be associated with mitochondrial activity of brown adipocyte. Further studies evaluating the activity of RSV for both the differentiation and mitochondrial activity of BAT could be helpful in investigating the effects of RSV on metabolic parameters.
Keywords: Adipocytes, brown; Estrogen receptor alpha; Diet, high-fat; Mitochondria; Resveratrol
INTRODUCTION
Brown adipose tissue (BAT) is profoundly involved in the regulation of energy balance and the control of body weight, which are mediated by non-shivering thermogenesis in mammals [1,2]. As a main mediator of adaptive thermogenesis, BAT is highly dependent on the activity of uncoupling protein 1 (UCP-1), which represents the mitochondrial activity [3]. Previous studies have demonstrated that BAT is also important for human metabolism [4,5].
Resveratrol (RSV), which has biological activities similar to those of estrogen [6], is a polyphenolic phytoalexin that is known to have several effects on metabolic diseases, cardiovascular disease, and malignancies.
With respect to energy metabolism, RSV increased heat production in mice, which was accompanied by a prolonged life span and improved insulin sensitivity [7]. Furthermore, RSV had inhibitory effects on adipokine expression and secretion in human white adipose tissue [8]. However, there have been few reports demonstrating the exact role of RSV on BAT. Although Miranda et al. [9] reported the apoptotic role of RSV on BAT, Andrade et al. [10] demonstrated that RSV promoted the biological function of BAT through increasing SIRT1 and energy expenditure.
Although white adipose tissue has major functions in the development of obesity, recent studies have demonstrated that BAT has an important role as a modulator of metabolic disease. However, there are few reports on agents which could modulate the activity of BAT, especially in patients with type 2 diabetes. With this background, we evaluated the role of RSV on the mitochondria of BAT, both in vivo and in vitro. The protein levels of UCP-1, which represents the mitochondrial activity of BAT, were evaluated in this study.
METHODS
Ethics statement
This study was conducted in accordance with the Institutional Animal Care and Use Committee of Yonsei University Health System based on the Laboratory Animal Manual and the "Guide for the Care and Use of Laboratory Animals" edited by the National Research Council of the National Academies. All animal studies were performed in facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Yonsei Laboratory Animal Research Center (permit no.: 2010-0268).
Primary cell isolation and culture
Brown preadipocytes were isolated from the interscapular BAT of 4-week-old male Sprague Dawley rats (Orient Bio Inc., Seongnam, Korea) and differentiated as described previously [9,11]. Isolated brown preadipocytes were incubated in high glucose Dulbecco's Modified Eagle's Medium containing a 1% antibiotic solution and 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA) at 37℃ in a humidified atmosphere with 5% CO2. For differentiation, the immortalized brown adipocytes were grown in Dulbecco's Modified Eagle's Medium containing 10% fetal bovine serum, 1 nM T3, and 20 nM insulin (Boehringer-Mannheim, Mannheim, Germany) until 70% confluent; thereafter, it was called differentiation medium (DM). Then, the cells were cultured in DM supplemented with 0.5 mM isobutylmethylxanthine, 0.125 µM indomethacin (Sigma-Aldrich Inc., St. Louis, MO, USA), and 0.5 µM dexamethasone (Sigma-Aldrich Inc.) for 2 days; thereafter, it was called induction medium (IM). Next, the cells were cultured in DM until they exhibited a fully differentiated phenotype with multiple multilobular lipid droplets in the cytoplasm. For the in vitro study, RSV (Sigma-Aldrich Inc.) was provided during the IM supplementation period and maintained thereafter. RSV was dissolved in dimethyl sulfoxide (Sigma-Aldrich Inc.) for stock solution with concentration of 100 mM.
Animals and treatment management
Male Otsuka Long Evans Tokushima Fatty (OLETF) rats (Otsuka Pharmaceutical Co. Ltd., Tokushima, Japan), 9 weeks of age and weighing 290 to 320 g, were housed in an animal room controlled at 23℃±2℃ and 55%±5% room humidity under a 12-hour light/12-hour dark cycle, and given tap water ad libitum. Animals were separated into four groups: (1) standard chow (standard diet [SD]) diet-fed with saline treatment (n=7); (2) SD diet-fed with RSV (Federal Laboratories Corp., Alden, NY, USA) treatment (n=8); (3) high fat diet (HFD)-fed with saline treatment (n=7); and (4) HFD-fed with RSV treatment (n=8). RSV at a dose of 10 mg/kg dissolved in 1.5 mL saline, or the same volume of saline, was administered via oral gavage once daily for 27 weeks. SD diet was consisted of 24% protein, 65% carbohydrate, and 12% fat (PicoLab Rodent Diet 20, LabDiet, St. Louis, MO, USA). The caloric content of the HFD was 17% protein, 43% carbohydrate, and 41% fat (RD Western Diet, D12079B, Research Diets Inc., New Brunswick, NJ, USA). Body weights and fasting glucose were checked weekly. Insulin tolerance tests were conducted every month. After 27 weeks of treatment with RSV or saline, OLETF rats were sacrificed and their plasma was collected. Furthermore, each organ was weighed, including the heart, liver, kidney, epididymal fat, and BAT. BAT was isolated from each rat and prepared for Western blotting analysis and histopathological evaluation as described previously [12]. Plasma leptin and adiponectin levels were measured with an enzyme immunoassay (Mouse/Rat Adiponectin enzyme-linked immunoassay kit, BioTrader Inc., Seoul, Korea).
Immunoblotting
Cell lysates were prepared and subjected to Western blot analysis. Membranes were immunoblotted with primary antibodies for UCP-1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), AMP-activated protein kinase (AMPK, Cell Signaling Technologies, Danvers, MA, USA), and estrogen receptor α (ER-α, Santa Cruz Biotechnology). Peroxidase-conjugated anti-rabbit or anti-mouse antibodies were used as secondary antibodies (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Oil-Red-O staining
Dishes were washed with phosphate-buffered saline and fixed with 10% buffered formalin for 16 hours at 4℃. Then, cells were stained for 4 hours at room temperature with Oil-Red-O solution (5 g/L in isopropyl alcohol), washed five times with water, and visualized.
Statistical analysis
Data were analyzed using Mann-Whitney tests. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). All statistical tests were two-tailed, and P<0.05 were considered significant.
RESULTS
Resveratrol promotes the differentiation of brown preadipocytes in the early phase
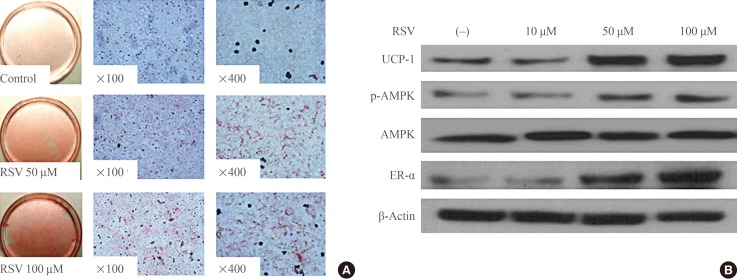
To evaluate the effect of RSV on the differentiation of brown preadipocytes, RSV was provided together with the IM and maintained thereafter. Oil-Red-O staining revealed that RSV promoted the differentiation of preadipocytes in a dose-dependent manner (Fig. 1A). In a separate experiment to evaluate the role of RSV in brown adipogenesis, RSV treatment was performed at each step promoting the differentiation of brown preadipocytes at a fixed concentration of 100 µM. However, there was no effect of RSV on brown adipogenesis when RSV treatment was performed after the period of IM supplementation. When RSV was administered simultaneously with IM and maintained thereafter, Western blotting revealed that the expression of UCP-1, phospho-AMPK, and ER-α had increased (Fig. 1B). Similar to the results of the Oil-Red-O staining, no effect of RSV treatment on the expression of these proteins was found after IM supplementation.
Fig. 1. Effects of resveratrol (RSV) on the differentiation of brown adipocytes. (A) Oil-red-O staining of primary cultured brown preadipocytes. RSV treatment was performed with induction medium at doses of 50 and 100 µM and maintained thereafter. Adipogenesis was significantly increased with RSV treatment. (B) Western blotting for mitochondrial activity and estrogen receptor α (ER-α) in brown adipocytes. Brown preadipocytes were cultured and differentiated with or without RSV treatment. The protein lysates were collected after the differentiation of brown adipocytes was confirmed. UCP-1, uncoupling protein 1; p-AMPK, phospho-AMP-activated protein kinase; AMPK, AMP-activated protein kinase.
Resveratrol improves insulin sensitivity in OLETF rats, without affecting white adipocytes
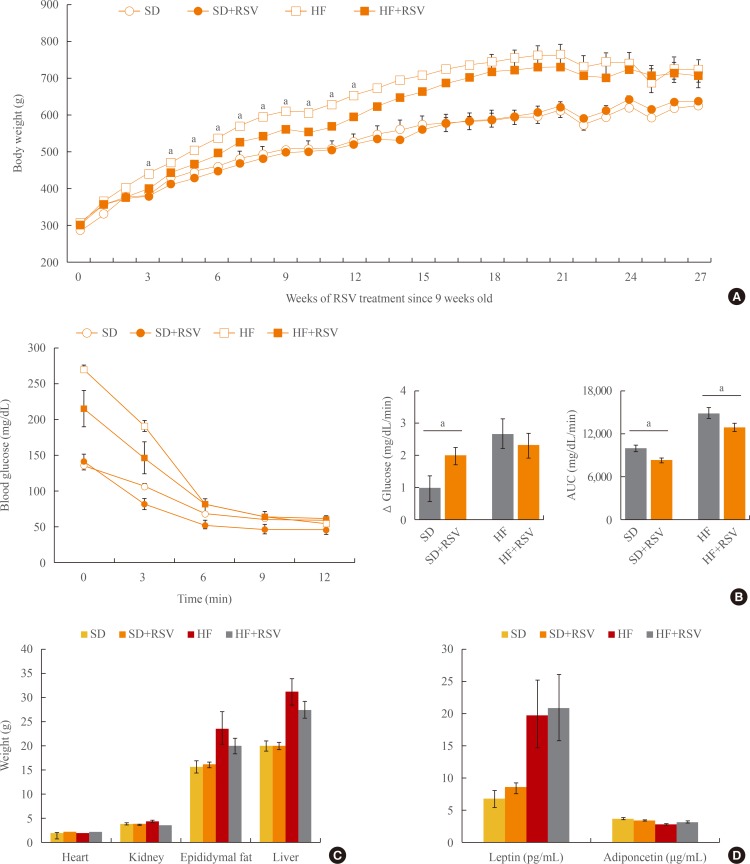
OLETF rats were treated with RSV and fed the SD or HF diet for 27 weeks. Although a significant reduction in body weight was observed after 12 weeks of RSV treatment, the difference diminished thereafter (Fig. 2A). However, insulin tolerance tests revealed that RSV treatment improved insulin sensitivity in both diet groups (Fig. 2B). There were no differences in the histologies and organ weights of the heart, liver, epididymal fat, retroperitoneal fat, gastrocnemius muscle, and soleus muscle after the RSV treatment (Fig. 2C, data not shown). Furthermore, RSV treatment did not alter the serum levels of leptin and adiponectin in either diet group (Fig. 2D).
Fig. 2. Effects of resveratrol (RSV) on metabolic parameters in Otsuka Long Evans Tokushima Fatty (OLETF) rats. (A) Body weight changes after RSV treatment of OLETF rats. RSV had been provided since the age of 9 weeks and maintained for 27 weeks. (B) Insulin tolerance test of OLETF rats at 35 weeks of age. (C) Organ weights at 36 weeks of age. (D) Changes in leptin and adiponectin levels. Serum samples from 36-week-old rats were collected and evaluated with an enzyme-linked immunoassay kit. SD, standard diet; HF, high fat; AUC, area under the curve. aP value below 0.05 with treatment of RSV in each diet group (Mann-Whitney U test).
Resveratrol accelerates the activity of brown adipocytes in obese rats
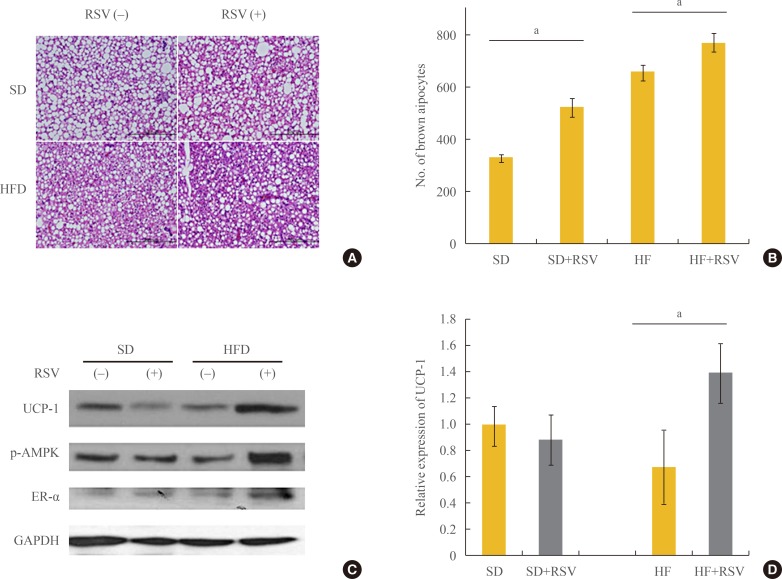
Histological analysis revealed that RSV prevented the deposit formation of lipid droplets in all of the spaces between the brown adipocytes of OLETF rats. The OLEFT rats fed the HFD had more brown adipocytes than those fed the SD diet. Furthermore, RSV treatment of OLETF rats fed the HF diet increased the number of brown adipocytes, as confirmed by H&E stain (Fig. 3A, B). However, analysis of protein lysates from the BAT of OLETF rats indicated that RSV increased the expression of UCP-1 and ER-α only in the HFD-fed group (Fig. 3C). Although the histology of BAT revealed an increased number of brown adipocytes in HFD-fed OLETF rats, the protein expression of UCP-1 was reduced, whereas it was restored in HFD-fed rats treated with RSV (Fig. 3D).
Fig. 3. Effects of resveratrol (RSV) on the differentiation and activation of brown adipocytes in Otsuka Long Evans Tokushima Fatty (OLETF) rats. (A) Hematoxylin and eosin staining for brown adipocytes. Brown adipose tissue was collected from OLETF rats at 36 weeks of age. With the treatment of RSV and feeding of the high fat diet (HFD), the differentiation of brown adipocytes increased. (B) The number of brown adipocytes visible in the high-power field increased significantly with RSV treatment and HFD feeding. The average number of brown adipocytes was calculated at four sites of each section. The number of rats in each group is described in the Methods. (C) Western blotting for mitochondrial activity and estrogen receptor α (ER-α). (D) Relative expression in Western blotting, determined using a densitometer. All protein lysates were evaluated and compared with those of rats on the standard diet. SD, standard diet; HF, high fat; UCP-1, uncoupling protein 1; p-AMPK, phospho-AMP-activated protein kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. aP value below 0.05 with treatment of RSV in each diet group (Mann-Whitney U test).
DISCUSSION
Adipose tissues, both white and brown, play pivotal roles in energy homeostasis. While white adipocytes store energy and cause obesity, BAT induces non-shivering thermogenesis and consumes excess calories [13]. Given the role of BAT in energy expenditure, several studies have suggested BAT as a new therapeutic target for obesity. For thermogenesis, BAT utilizes a unique protein, UCP-1, which is located in the inner mitochondrial membrane and uncouples mitochondrial respiration, leading to the release of energy as heat [14]. Together with UCP-1, peroxisome proliferator-activated receptor coactivator 1α (PGC-1α) has been show to express multiple physiologic roles for mitochondrial biogenesis and activity [15]. Several studies have demonstrated that PGC-1α were expressed in BAT and were dysregulated in obesity, diabetes and neurodegeneration.
There are several physiologic regulators of the differentiation and activity of BAT, including cold exposure, the sympathetic nervous system, the β-adrenergic system, catecholamines, thyroid hormones, nutrition, and sex hormones [13]. In this study, we confirmed that over-nutrition and RSV play important roles in the mitochondrial differentiation of brown adipocytes. The HFD itself augmented the number of brown adipocytes in OLETF rats. There have been debates about the changes in brown adipocytes during the development of obesity. In some studies, both impaired mitochondrial activity and a reduced amount of brown adipocytes were observed in obese animal models and human patients [4,16,17]. On the other hand, other studies have found that the differentiation of brown adipocytes correlated with increased supplement of nutrition [18]. In this study, we used OLETF rats to confirm the role of brown adipocytes in type 2 diabetes combined with obesity. OLETF rats were generated with inactive cholecystokinin-1 receptors on the background of Long-Evans Tokushima Otsuka rats, leading to the phenotype of type 2 diabetes with mild obesity. To eliminate the genetic influence of OLETF rats on brown adipocyte, the brown preadipocytes were isolated from Sprague Dawley rats for in vitro study. As described in this study, the HFD led to a significant increase in body weight and induced the differentiation of brown adipocytes. However, the HFD itself did not affect the mitochondrial activity of brown adipocytes. Although we could not find the exact mechanism, impaired biogenesis could have reduced mitochondrial activity, despite the increased differentiation of BAT after HFD intake [19].
Many studies have reported the effects of RSV on obesity [20,21,22]. Although most of them have focused on changes in white adipocyte, a few recent studies focused on the role of BAT or the 'browning' of white adipocyte [23,24]. They evaluated the development of brown-adipocyte-like characteristics in white adipocytes, through the main mechanism of SIRT1 activator combined with phytoestrogen activity [25]. Furthermore, Miranda et al. [9] reported that RSV leaded Akt/protein kinase B and foxo1 dephosphorylation/deacetylation and induced apoptosis of brown adipocyte. In the present study, RSV restored both the differentiation and activation of BAT and increased the expression of ER-α, especially in the HFD group. Considering that a sex-dependent difference in BAT expression was reported even in human subjects, and RSV treatment of postmenopausal women had favourable effects on estrogen-associated metabolism [26,27,28], RSV might modulate the activity of brown adipocytes through the estrogen pathway. However, RSV had no effects on white adipocytes, which were suggested by previous studies to be the target of the anti-obesity effects of RSV [29,30]. In our obesity-related inflammatory marker analysis, there were no differences in the levels of leptin and adiponectin following RSV treatment. Considering that the histologies and organ weights of the heart, liver, epididymal fat, retroperitoneal fat, gastrocnemius muscle, and soleus muscle did not differ with the RSV treatment, improved insulin sensitivity may have been achieved through the increased differentiation and activation of BAT. The differences in body weight were diminished after 19 weeks of age in the OLETF rats, possibly due to the relatively long study duration, the high dose of the HFD treatment, and differences in animal species for type 2 diabetes.
Andrade et al. [10] demonstrated that early treatment of RSV on mice fed with SD increased BAT thermogenesis markers by increasing SIRT1 and energy expenditure. However, Miranda et al. [9] reported that RSV induced apoptosis of apoptosis. The differences might be resulted from the time-point of RSV treatment. In our study, the effect of RSV on brown adipogenesis was confirmed at each step during in vitro study. Although RSV treatment was performed at different stages of adipogenesis, only the early treatment before the application of IM significantly affected the differentiation and activity of BAT. This suggests that RSV treatment might have a role in the early development of brown adipocytes with more prominent mitochondrial activities than those induced by obesity.
There are several limitations of current study. Although mitochondrial activities were evaluated by UCP-1, the role of RSV on mitochondrial biogenesis of BAT, such as PGC-1α, was not demonstrated. Furthermore, the direct mechanism of RSV involving mitochondrial activation of BAT was not clarified. Finally, the effect of RSV on BAT thermogenesis was not defined in in vivo condition using obesity animal model. However, we identified the relationship between RSV and mitochondrial activities of BAT in current study, both from in vitro and in vivo conditions.
In conclusion, we confirmed that RSV might have a role on promotion of mitochondrial activity in BAT, both in vitro and in vivo. In the in vivo study, RSV restored the impaired mitochondrial activity induced by the HFD, increased the expression of ER-α, and improved insulin sensitivity. Further studies evaluating the relationship between mitochondrial activity and ER-α could be helpful in understanding the anti-obesity effects of RSV.
ACKNOWLEDGMENTS
This work was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI13C0423 [to EJL]).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984;64:1–64. doi: 10.1152/physrev.1984.64.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Trayhurn P, Milner RE. A commentary on the interpretation of in vitro biochemical measures of brown adipose tissue thermogenesis. Can J Physiol Pharmacol. 1989;67:811–819. doi: 10.1139/y89-128. [DOI] [PubMed] [Google Scholar]
- 3.Nedergaard J, Golozoubova V, Matthias A, Asadi A, Jacobsson A, Cannon B. UCP1: the only protein able to mediate adaptive non-shivering thermogenesis and metabolic inefficiency. Biochim Biophys Acta. 2001;1504:82–106. doi: 10.1016/s0005-2728(00)00247-4. [DOI] [PubMed] [Google Scholar]
- 4.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cos P, De Bruyne T, Apers S, Vanden Berghe D, Pieters L, Vlietinck AJ. Phytoestrogens: recent developments. Planta Med. 2003;69:589–599. doi: 10.1055/s-2003-41122. [DOI] [PubMed] [Google Scholar]
- 7.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olholm J, Paulsen SK, Cullberg KB, Richelsen B, Pedersen SB. Anti-inflammatory effect of resveratrol on adipokine expression and secretion in human adipose tissue explants. Int J Obes (Lond) 2010;34:1546–1553. doi: 10.1038/ijo.2010.98. [DOI] [PubMed] [Google Scholar]
- 9.Miranda S, Gonzalez-Rodriguez A, Revuelta-Cervantes J, Rondinone CM, Valverde AM. Beneficial effects of PTP1B deficiency on brown adipocyte differentiation and protection against apoptosis induced by pro- and anti-inflammatory stimuli. Cell Signal. 2010;22:645–659. doi: 10.1016/j.cellsig.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Andrade JM, Frade AC, Guimaraes JB, Freitas KM, Lopes MT, Guimaraes AL, et al. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur J Nutr. 2014;53:1503–1510. doi: 10.1007/s00394-014-0655-6. [DOI] [PubMed] [Google Scholar]
- 11.Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR. Beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem. 1999;274:34795–34802. doi: 10.1074/jbc.274.49.34795. [DOI] [PubMed] [Google Scholar]
- 12.Kim WK, Choi HR, Park SG, Ko Y, Bae KH, Lee SC. Myostatin inhibits brown adipocyte differentiation via regulation of Smad3-mediated beta-catenin stabilization. Int J Biochem Cell Biol. 2012;44:327–334. doi: 10.1016/j.biocel.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Lee P, Swarbrick MM, Ho KK. Brown adipose tissue in adult humans: a metabolic renaissance. Endocr Rev. 2013;34:413–438. doi: 10.1210/er.2012-1081. [DOI] [PubMed] [Google Scholar]
- 14.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 15.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–735. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 16.Vegiopoulos A, Muller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
- 17.Lowell BB, S-Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 18.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carmona MC, Hondares E, Rodriguez de la Concepcion ML, Rodriguez-Sureda V, Peinado-Onsurbe J, Poli V, et al. Defective thermoregulation, impaired lipid metabolism, but preserved adrenergic induction of gene expression in brown fat of mice lacking C/EBPbeta. Biochem J. 2005;389(Pt 1):47–56. doi: 10.1042/BJ20050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, et al. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int J Obes (Lond) 2014;38:470–473. doi: 10.1038/ijo.2013.155. [DOI] [PubMed] [Google Scholar]
- 21.Beaudoin MS, Snook LA, Arkell AM, Simpson JA, Holloway GP, Wright DC. Resveratrol supplementation improves white adipose tissue function in a depot-specific manner in Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R542–R551. doi: 10.1152/ajpregu.00200.2013. [DOI] [PubMed] [Google Scholar]
- 22.Cho SJ, Jung UJ, Choi MS. Differential effects of low-dose resveratrol on adiposity and hepatic steatosis in diet-induced obese mice. Br J Nutr. 2012;108:2166–2175. doi: 10.1017/S0007114512000347. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC, et al. Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology. 2014;146:539–549.e7. doi: 10.1053/j.gastro.2013.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 26.Chow HH, Garland LL, Heckman-Stoddard BM, Hsu CH, Butler VD, Cordova CA, et al. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: effects on systemic sex steroid hormones. J Transl Med. 2014;12:223. doi: 10.1186/s12967-014-0223-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi DK, Oh TS, Choi JW, Mukherjee R, Wang X, Liu H, et al. Gender difference in proteome of brown adipose tissues between male and female rats exposed to a high fat diet. Cell Physiol Biochem. 2011;28:933–948. doi: 10.1159/000335807. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez-Cuenca S, Monjo M, Frontera M, Gianotti M, Proenza AM, Roca P. Sex steroid receptor expression profile in brown adipose tissue. Effects of hormonal status. Cell Physiol Biochem. 2007;20:877–886. doi: 10.1159/000110448. [DOI] [PubMed] [Google Scholar]
- 29.Franco JG, Lisboa PC, da Silva Lima N, Peixoto-Silva N, Maia LA, Oliveira E, et al. Resveratrol prevents hyperleptinemia and central leptin resistance in adult rats programmed by early weaning. Horm Metab Res. 2014;46:728–735. doi: 10.1055/s-0034-1375688. [DOI] [PubMed] [Google Scholar]
- 30.Gómez-Zorita S, Fernandez-Quintela A, Lasa A, Hijona E, Bujanda L, Portillo MP. Effects of resveratrol on obesity-related inflammation markers in adipose tissue of genetically obese rats. Nutrition. 2013;29:1374–1380. doi: 10.1016/j.nut.2013.04.014. [DOI] [PubMed] [Google Scholar]