Abstract
Objectives
To identify symptom clusters among patients with advanced heart failure (HF) and the independent relationships with their quality of life (QoL).
Methods
This is the secondary data analysis of a cross-sectional study which interviewed 119 patients with advanced HF in the geriatric unit of a regional hospital in Hong Kong. The symptom profile and QoL were assessed by using the Edmonton Symptom Assessment Scale (ESAS) and the McGill QoL Questionnaire. Exploratory factor analysis was used to identify the symptom clusters. Hierarchical regression analysis was used to examine the independent relationships with their QoL, after adjusting the effects of age, gender, and comorbidities.
Results
The patients were at an advanced age (82.9 ± 6.5 years). Three distinct symptom clusters were identified: they were the distress cluster (including shortness of breath, anxiety, and depression), the decondition cluster (fatigue, drowsiness, nausea, and reduced appetite), and the discomfort cluster (pain, and sense of generalized discomfort). These three symptom clusters accounted for 63.25% of variance of the patients' symptom experience. The small to moderate correlations between these symptom clusters indicated that they were rather independent of one another. After adjusting the age, gender and comorbidities, the distress (β = −0.635, P < 0.001), the decondition (β = −0.148, P = 0.01), and the discomfort (β = −0.258, P < 0.001) symptom clusters independently predicted their QoL.
Conclusions
This study identified the distinctive symptom clusters among patients with advanced HF. The results shed light on the need to develop palliative care interventions for optimizing the symptom control for this life-limiting disease.
Keywords: Advanced heart failure, Palliative care model, Quality of life, Symptom clusters
1. Introduction
Heart failure (HF) is an important public health issue among older people; the condition has a prevalence of over 23 million persons worldwide.[1] The aging of the population and medical advances have increased the number of people living with this progressively deteriorating condition at a more advanced disease stage. Advanced HF is a very debilitating condition, for which patients are less responsive to the conventional treatment therapy, HF's annual mortality rate is reported to be as high as 50%.[2] Palliative care approaches which place greater emphasis on symptom management more than on active treatment have been broadly advocated as the most appropriate model of care for older people with advanced HF.[3] This is especially true because patients with advanced HF are characterized by a more complicated symptom profile. The increased variety and severity of their symptoms not only result from the failure of myocardial pumping, but also from the debilitating effects caused by skeletal and muscular abnormalities, comorbidities, and medical treatment.[4]
Symptom clusters have emerged as an important concept for promoting the effective management of multiple symptoms. They are defined as a constellation of two or more co-occurring symptoms which relate to each other, but which do not necessarily share the same etiology.[5] Such co-occurring symptoms would have a synergistic effect to complicate the symptom perception. Patients and healthcare professionals will, therefore, have greater difficulty in accurately recognizing the underlying pathologies of the symptoms and this hinders effective responses. Consequently, identifying the symptom clusters will help to set the direction for the development of palliative care interventions for more accurate symptom recognition and management for older patients with advanced HF. Besides, the co-occurring symptoms would interact to potentiate the detrimental effects of each other on the patients' well-being.[6] Tackling symptom clusters would hence bring about greater benefits in enhancing the health outcomes of patients with advanced HF as compared to handling the discrete individual symptoms separately.
Previous studies have indicated that the symptoms are presented in clusters among HF patients and that such symptom clusters are correlated significantly to poorer health outcomes; these include functional limitation, hospital readmission, and mortality.[7]–[10] Two studies have identified the physical symptom clusters and the emotional–cognitive symptom cluster among HF patients.[8],[9] Whereas Moser, et al.[9] found that the physical symptoms are co-occurring as one cluster, Jurgens, et al.[8] showed that such symptoms were clustered in accordance with the etiologies into the acute fluid overload cluster and the chronic fluid overload cluster. Song, et al.[10] also identified two physical symptoms clusters, but the symptoms were co-occurring in accordance with their nature (i.e., the weary symptom cluster and the dyspnea-related symptom cluster). On the other hand, Herr, et al.[7] indicated that the typical symptoms of fatigue and sleepiness are co-occurring with the emotional-cognitive symptoms to form the sickness behavior cluster. They also identified the fluid overload symptom cluster and the gastro-intestinal symptom cluster. The inconsistent findings between these studies may be related to the variation in the number and types of symptoms being included in the analysis. However, none of these studies focused on patients with advanced HF. Knowing that advanced HF is an end-stage disease and that patients would present with more debilitating and deconditioning symptoms, the existing knowledge identified from the HF patients would have limitations in informing the development of palliative care for those at the advanced disease stage. The aim of this study was, therefore, to examine the symptom clusters among patients with advanced HF, and to investigate its relationship with their health-related quality of life (QoL).
2. Methods
2.1. Setting and sample
This is the secondary data analysis of a cross-sectional survey which examined the palliative care needs of older people with advanced HF. The subjects were recruited from the geriatric unit of a sub-acute hospital in Hong Kong. It is a 650-bed regional hospital which provides rehabilitative service to the patients after their conditions have been stabilized in the cardiac unit of an acute hospital. Eligible subjects have to be admitted with an index diagnosis of advanced HF (as defined by the New York Heart Association Grade III or IV[11]), cognitively competent to report their symptom experience, and willing to participate. Because exploratory factor analysis was used to identify the symptom cluster, a priori sample size was determined by the rule-of-thumb that at least 10 subjects for each included symptom would be required.[12]
2.2. Data collection method
Ethics approval was obtained from the New Territories East Cluster Clinical Research Ethics Committee. A research nurse identified the eligible patients by screening the medical records. She invited them to participate and obtained their informed consent. Demographic and clinical data of the subjects were collected by carrying out the medical record review. This was followed by a face-to-face interview for administering the following instruments.
2.3. Edmonton Symptom Assessment Scale (ESAS, Chinese version)
The ESAS (Chinese version) was used to solicit the symptom profile of patients receiving palliative care.[13] The 9-item ESAS assesses nine symptoms which are common in patients with end-stage disease. These symptoms are fatigue, shortness of breath, anxiety, depression, poor appetite, nausea, dizziness, generalized discomfort, and pain. All these symptoms are highly relevant and specific to patients with advanced HF.[14] Besides, it has an additional open-ended question by which patients are asked to identify additional symptoms not included in the list. Patients were asked to rate each symptom on a 0 to 10 numeric scale, with a higher score indicating a more severe symptom. The ESAS has satisfactory test-retest reliability[15] and good concurrent validity when used in a palliative care setting.[16]
2.4. McGill Quality of Life Questionnaire (MQoL; Chinese version)
The MQoL was used to measure the QoL of patients at the advanced disease stage.[17] The original 16-item MQoL was developed in Canada to measure the QoL of patients receiving palliative care. When it was adopted to the Chinese culture in Hong Kong, three cultural-sensitive items including patients' satisfaction on the food, sex and feeling of being respected were added. The MQoL Chinese version consists of four subscales to measure the physical, psychological, spiritual and support domains of life. The physical domain (5 items) measures patients' perception on physical well-being, symptom burden and quality of eating. The psychological domain (6 items) covers emotional well-being including feelings of depression, nervousness, worry, sadness, fear and being respected. The spiritual domain (five items) assesses patients' meaning of existence, life goals, feeling of life worthiness, self-content, and feeling life as burdensome. The support domain (two items) measure feeling of being supported and a caring world. Also, there is one single item to measure sex. Each item is rated on a 1 to 10 numeric scale, with higher scores indicating a better QoL. The mean of item scores of each domain gives the domain score. The average of the domain scores gives an overall QoL score. The Chinese version of the MQoL has been widely used in patients with advanced disease in Hong Kong.[17] The Cronbach's alpha was 0.83 when used in patients with end-stage disease, and good construct and concurrent validity have been reported.[17]
2.5. Data analysis
Descriptive statistics were used to summarize the subjects' characteristics, their symptom profile, and their QoL. Pearson's produce–moment correlation was used to examine the relationship between the symptoms. Only those with significant correlation with at least one of the other symptom were included in the identification of symptom clusters. Exploratory factor analysis was used to examine the symptom clusters among patients with advanced HF. Principal component analysis was conducted and the clusters with the initial eigenvalues set at > 0.80 were extracted.[12] The factor solution was obtained by using the direct oblimin rotation method. The Kaiser-Myer-Olkin (KMO) Index (> 0.5) was used to determine the factorability of the data.[12] The commonalities of each item need to be above 0.40 with a factor loading of not less than 0.32.[18]
Hierarchical regression analysis was used to examine the independent relationship between the identified symptom clusters and QoL. The normality, linearity, and homoscedasticity of the data were examined by residual plot. Multi-collinearity was excluded if the variance inflation factor was less than 10 and the tolerance level was < 0.10.[18] Demographic data, including age, gender, and the comorbidity index, were firstly entered to the model as a control variable, whereas the symptom clusters were entered in step 2 as a predictive variable. All statistical analysis was performed by using SPSS version 22.0 (Chicago, IL, USA), with the level of significance set at 0.05.
3. Results
3.1. Characteristics of the subjects
A total of 133 patients were invited to participate and 119 of them completed the questionnaire, with a response rate of 89.5%. Table 1 summarizes their demographic and clinical characteristics. Around 60% of the subjects were male. They were at an advanced age with a mean of 82.9 ± 6.5 years. Around 75% were community-dwelling whereas the others were living in residential care homes. Almost all of them (95.8%) had more than three chronic diseases, with 26.1% of them having a weighted Charlson's comorbidity Index of 5.0 or over, indicating that their one-year mortality rate after hospitalization would be as high as 0.78.[19] Almost 50% of the subjects had repeated hospital readmission for three or more times during the previous year. As for the QoL as measured by the MQoL, the physical health was most affected.
Table 1. Clinical characteristics of the patients with advanced heart failure (n = 119).
| Characteristics | Values |
| Age, yrs | 82.9 ± 6.5 |
| Male | 62 (59.0%) |
| Living arrangements | |
| Alone | 11 (13.4%) |
| With family | 90 (75.6%) |
| With friend | 2 (1.7%) |
| Residential care home | 16 (13.4%) |
| Charlson's comorbidity index | |
| ≤ 2 | 39 (32.8%) |
| 2–5 | 49 (41.2%) |
| ≥ 5 | 31 (26.1%) |
| New York Heart Association Classification | |
| Grade III | 74 (62.2%) |
| Grade IV | 45 (37.8%) |
| Types of comorbidity | |
| Coronary heart disease | 103 (86.6%) |
| Myocardial infarction | 23 (19.3%) |
| Diabetic mellitus | 58 (48.7%) |
| Cerebrovascular disease | 35 (29.4%) |
| Number of hospitalizations in the previous year | |
| 1–2 | 63 (52.9%) |
| 3–4 | 46 (38.7%) |
| ≥ 5 | 10 (8.4%) |
| McGill Quality of Life questionnaire | |
| Overall score (range: 0–10) | 4.83 ± 1.44 |
| Physical subscale (0–10) | 3.78 ± 1.60 |
| Psychological subscale (0–10) | 7.18 ± 2.37 |
| Existential subscale (0–10) | 6.08 ± 1.80 |
| Support subscale (0–10) | 6.27 ± 2.06 |
| Sex (0–10) | 3.88 ± 3.90 |
Data are presented as mean ± SD or n (%).
3.2. Symptom profile
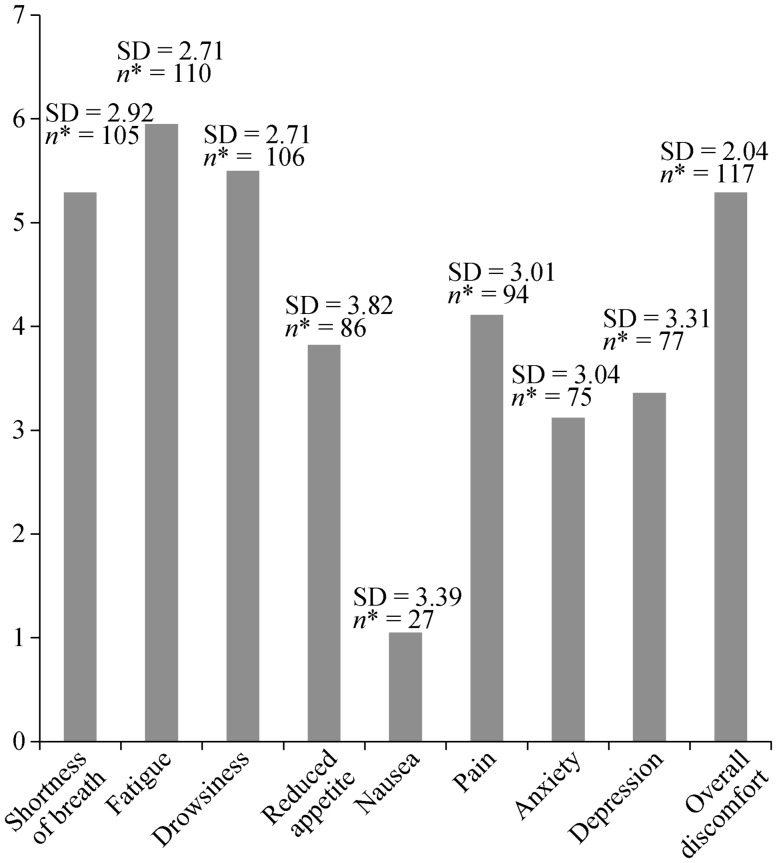
Figure 1 shows the symptom profile of the subjects. The majority of the subjects (95.0%) reported more than three of the symptoms on the ESAS, and they were more affected by the typical HF symptoms including shortness of breath and fatigue. Other symptoms representing the debilitation associated with end-stage disease, including drowsiness, generalized discomfort, pain, and reduced appetite, were also dominant. For the open-ended question on the ESAS, other symptoms including edema (10.1%) and poor sleep quality (4.2%) were also reported.
Figure 1. Mean rating on Edmonton Symptom Assessment Scale among of the patients with advanced heart failure (n = 119).
*Number of subjects reported the referred symptoms. SD: standard deviation.
3.3. Correlations between the symptoms
The majority of the symptoms were found to be significantly correlated to at least two other symptoms. The highest correlations were observed between fatigue and drowsiness (r = 0.772, P < 0.001), depression and anxiety (r = 0.858, P < 0.001), and fatigue and reduced appetite (r = 0.414, P < 0.001). Because edema and poor sleep quality were not related to any other symptom, these two symptoms were excluded from the analysis of symptom clusters.
3.4. Symptom clusters
Table 2 shows the results of exploratory factor analysis. The KMO Index was > 0.5 to indicate the factorability of the data. Based on the rotated factor solution, three unique symptom clusters were identified. They were the distress symptom cluster (shortness of breath, anxiety, and depression), the decondition symptom cluster (fatigue, drowsiness, reduced appetite and nausea), and the discomfort symptom cluster (generalized discomfort and pain). The distress symptom cluster explained most variance (37.08%) for the symptom impact of advanced HF, and the three clusters explained a total of 63.25% of variance. There were small to moderate correlations between the three symptom clusters, with Pearson's correlation coefficients ranging from 0.275– 0.385, (P = 0.002 to < 0.001), thereby indicating that the symptom clusters were rather manifested independently of one another.
Table 2. Factor loadings of each symptom on the symptom cluster.
| Component 1 (distressing symptom cluster) | Component 2 (deconditioning symptom cluster) | Component 3 (discomforting symptom cluster) | |
| Shortness of breath | 0.622* | 0.165 | 0.330 |
| Depression | 0.907* | 0.307 | 0.004 |
| Anxiety | 0.906* | 0.365 | 0.081 |
| Fatigue | 0.325 | 0.876* | 0.026 |
| Nausea | 0.130 | 0.586* | 0.382 |
| Drowsiness | 0.303 | 0.857* | 0.064 |
| Reduced appetite | 0.258 | 0.654* | 0.364 |
| Pain | 0.220 | 0.207 | 0.785* |
| Overalldiscomfort | 0.385 | 0.194 | 0.458* |
*Factor loading > 0.4.
The three identified symptom clusters also independently predicted QoL among patients with advanced HF. Table 3 shows the results of the hierarchical regression analysis. After adjusting the effects of age, gender, and the comorbidity index (CCI), the distress symptom cluster (β = −0.635, P < 0.001), the decondition symptom cluster (β = −0.148, P = 0.01), and the discomfort symptom cluster (β = −0.258, P < 0.001) made a significant contribution to explaining the QoL among the subjects. The influence of the distressing symptom was found to be the most prominent.
Table 3. Predictive relationships between symptom clusters and the McGill Quality of Life Questionnaire (n = 119).
| Unstandardized coefficient B (SE) | Standardized coefficients (β) | t | P | |
| Model 1 | ||||
| Age | −0.013 (0.016) | −0.075 | −0.790 | 0.431 |
| Gender | −0.315 (0.274) | −0.108 | −1.148 | 0.253 |
| Model 2 | ||||
| Age | −0.012 (0.016) | −0.073 | −0.766 | 0.445 |
| Gender | −0.326 (0.279) | −0.112 | −1.168 | 0.245 |
| Number of comorbidities | −0.016 (0.051) | −0.023 | −0.250 | 0.803 |
| Model 3 | ||||
| Age | −0.003 (0.009) | −0.021 | −0.388 | 0.699 |
| Gender | −0.124 (0.154) | −0.043 | −0.808 | 0.421 |
| Number of comorbidities | −0.023 (0.028) | −0.043 | −0.832 | 0.407 |
| Distressing symptom cluster | −0.119 (0.011) | −0.635 | −11.029 | < 0.001 |
| Deconditioning symptom cluster | −0.025 (0.010) | −0.148 | −2.630 | 0.010 |
| Discomforting symptom cluster | −0.094 (0.021) | −0.258 | −4.566 | < 0.001 |
SE: standard error.
4. Discussion
Effective symptom management is a highly important agenda in the care planning for life-limiting disease. This is the first study to examine symptom clusters among patients with advanced HF, and to investigate its influence on their QoL. By using the ESAS, this study took into account the typical symptom profile of HF as well as the prevalent symptoms of life-limiting diseases. Three major symptom clusters, namely, the distressing, the deconditioning, and the discomforting clusters, were identified. Such findings indicate that the symptoms of advanced HF were not only related to cardiac decompensation but also to the general debilitating effects of this advanced disease. Effective symptom management for this vulnerable group of patients needs to adequately address such complexities in the symptom manifestation. This is especially true because all three symptom clusters independently predict the patients' QoL.
The current study findings about symptom clusters are different from those reported for HF patients. Instead of clustering the symptoms into the physical and emotional domains,[8],[9] the two prominent emotional symptoms, namely, anxiety and depression, are linked to shortness of breath to form the distressing symptom cluster. This may be related to the fact that shortness of breath in advanced HF is more persistent and less responsive to treatment.[20] Such a pattern of symptom manifestation, together with their perception of a life-limiting disease, may potentiate the feeling of impending death during the dyspnea attack, and result in heightened negative emotional arousal.
As for the decondition symptom cluster, it includes a list of symptoms which are common across the various advanced life-limiting diseases such as cancer, renal failure, chronic respiratory obstructive disease, etc.[21] The clustering of these symptoms signifies that patients with advanced HF have a common pathway in terms of symptom experience as do the patients with other life-limiting diseases. In particular, this decondition symptom cluster represents the wearing of the bodily functions, activity level, and vitality. Different from the previous studies, which indicated that fatigue was clustered with shortness of breath to represent the acute volume retention and cardiac decompensation in HF patients,[8],[9] this study found that fatigue is clustered with other debilitating symptoms of life-limiting disease to indicate a weary condition. Such findings may reflect the more complex and multiple etiologies underlying the HF symptoms when the disease has developed to an advanced stage. This observation deserves more attention when developing effective symptom management for this vulnerable group of patients.
The discomfort symptom cluster is unique to HF patients when the disease develops to an advanced stage. The involved symptoms, including pain and generalized discomfort, have seldom been included in studies on symptom clusters for HF patients. Although such symptoms are not specific to the etiology of HF, pain was found to be a prominent symptom which affects two-thirds of patients with advanced HF.[22] Our study findings further indicate that this symptom was disturbing and intertwined with a non-specific sense of generalized discomfort to hamper the well-being of the sufferers.
The significant relationship between the three identified symptom clusters and QoL provides further evidence to suggest the importance of adopting a palliative care model, with an emphasis on holistic symptom management,[21] in supporting the patients who are living with advanced HF. Indeed, the findings about how the symptoms are clustered provide important insights into effective symptom management. Firstly, shortness of breath is a prominent symptom of advanced HF. In addition to relieving cardiac decompensation, prompt attention should be given to the patient's concurrent psychological needs. Identifying the associated symptom perception and encouraging the patients to ventilate their feelings are crucial to understanding the patients' emotional responses to this symptom experience. Further counseling would be indicated to promote effective psychological adaptation and coping with this life-limiting disease. Secondly, effective symptom management for advanced HF needs to look beyond the pathophysiology of HF and to consider the generalized debilitating symptoms associated with an end-stage disease. In particular, efforts should be given to promote the patients' vitality. Nutritional management, tailored exercise to increase physical capacity, and continuous effort to optimize social engagement within the patients' physical limitations are identified as relevant strategies to achieve this care goal.[23],[24] Finally, the uniqueness of the discomforting symptom cluster implies that managing advanced HF requires a comprehensive pain assessment and management protocol. This is especially true because pain is always being under-treated among patients with advanced HF.[22]
This study is not without limitations. Firstly, we used the ESAS to solicit the symptom profile of patients with advanced HF. Although it covers the typical symptoms of HF as well as those of life-limiting diseases, it does not include edema and poor sleeping which are also prevalent in HF patients. Even if there is an open-ended question to individualize the symptom profile of the respondent, there was a possibility that such symptoms were under-reported, and this resulted in a lack of correlation of such symptoms with other symptoms. Secondly, this study recruited only those patients with advanced heart failure who were competent enough to report their symptom experience. The findings could not be generalized to those who have compromised cognitive functions or those who are not at the advanced disease stage. Besides, by using the Charlson's comorbidity index to measure the burden from comorbidity, this study did not collect more precise information about the prevalence of hypertension, arrhythmia and atrial fibrillation, which all may influence the symptom presentation of the subjects. Finally, the use of a cross-sectional study design also fails to preclude the influence of the identified symptom clusters on their QoL.
In conclusion, this study has identified three unique symptom clusters, namely, the distress, the decondition, and the discomfort clusters, for advanced HF. Higher symptom distress from these clusters was associated with a poorer QoL among the patients. These findings reinforce the complexities of the symptom experience of patients with advanced HF. A palliative care model would, therefore, be most crucial to optimize symptom management. Instead of monitoring and managing each individual symptom as a separate problem, care planning needs to consider the additive and synergistic effects of the symptoms in each symptom cluster in affecting each patient's well-being.
Acknowledgments
This study was supported by the Departmental Fund, the Nethersole School of Nursing the Chinese University of Hong Kong (G<C02/2013).
References
- 1.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. doi: 10.1038/nrcardio.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee DS, Austin PC, Rouleau JL, et al. Predicting mortality among patients hospitalized for heart failure. JAMA. 2003;290:2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 3.Hemani S, Letizia MJ. Providing palliative care in end-stage heart failure. J Hospice Palliative Nurs. 2008;10:100–105. [Google Scholar]
- 4.Blinderman CD, Homel P, Billings A, et al. Symptom distress and quality of life in patients with advanced congestive heart failure. J Pain Symptom Manage. 2008;35:594–603. doi: 10.1016/j.jpainsymman.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. J Adv Nurs. 2001;33:668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- 6.Lenz ER, Pugh LC, Miligan RA, et al. The middle-range theory of unpleasant symptoms: an update. Adv Nurs Sci. 1997;19:14–27. doi: 10.1097/00012272-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Herr JK, Salyer J, Flattery M, et al. Heart failure symptom clusters and functional status: a cross-sectional study. J Adv Nurs. 2015;71:1274–1287. doi: 10.1111/jan.12596. [DOI] [PubMed] [Google Scholar]
- 8.Jurgens CY, Moser DK, Armola R, et al. Symptom clusters of heart failure. Res Nurs Health. 2009;32:551–560. doi: 10.1002/nur.20343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moser DK, Lee KS, Wu JR, et al. Identification of symptom cluster among patients with heart failure: An international observational study. Int J Nurs Stud. 2014;51:1366–1372. doi: 10.1016/j.ijnurstu.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song EK, Moser DK, Rayens MK, et al. Symptom clusters predict event-free survival in patients with heart failure. J Cardiovas Nurs. 2010;25:284–291. doi: 10.1097/JCN.0b013e3181cfbcbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedrich EB, Bohm M. Management of end stage heart failure. Heart. 2007;93:626–631. doi: 10.1136/hrt.2006.098814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costello AB, Osborne JW. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pract Ass Res Eval. 2005;10:1–9. [Google Scholar]
- 13.Bruera E, Kuehn N, Miller MJ, et al. The Edmonton Symptom Assessment System (ESAS): A simple method for the assessment of palliative care patients. J Palliative Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 14.Nordgren L, Sorensen S. Symptoms experienced in the last six months of life in patients with end-stage heart failure. Eur J Cardiovas Nurs. 2003;2:213–217. doi: 10.1016/S1474-5151(03)00059-8. [DOI] [PubMed] [Google Scholar]
- 15.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88:2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Philip J, Smith WB, Craft P, et al. Concurrent validity of the modified Edmonton Symptom Assessment System with Rotterdam Symptom Checklist and the Brief Pain Inventory. Support Care Cancer. 1998;6:539–541. doi: 10.1007/s005200050212. [DOI] [PubMed] [Google Scholar]
- 17.Lo RSK, Woo J, Zhoc KCH, et al. Cross-cultural validation of the McGill Quality of Life questionnaire in Hong Kong Chinese. Palliative Med. 2001;15:387–397. doi: 10.1191/026921601680419438. [DOI] [PubMed] [Google Scholar]
- 18.Tabachnick BG, Fidell LS. Using multivariate statistics. 4th Edition. Boston, USA: Allyn & Bacon; 2001. [Google Scholar]
- 19.Radovanovic D, Seifert B, Urban P, et al. Validity of Charlson comorbidity index in patients hospitalized with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002-2012. Heart. 2014;100:288–294. doi: 10.1136/heartjnl-2013-304588. [DOI] [PubMed] [Google Scholar]
- 20.Adler ED, Goldfinger JZ, Kalman J, et al. Contemporary reviews in Cardiovascular Medicine: Palliative care in the treatment of advanced heart failure. Circulation. 2009;120:2597–2606. doi: 10.1161/CIRCULATIONAHA.109.869123. [DOI] [PubMed] [Google Scholar]
- 21.Solano JP, Gomes B. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Evangelista LS, Sackett E, Dracup K. Pain and heart failure: unrecognized and untreated. Eur J Cardiovas Nurs. 2009;3:169–173. doi: 10.1016/j.ejcnurse.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuhr MJ, Pette D, Berger R, et al. Beneficial effects of chronic low-frequency stimulation of thigh muscles in patients with advanced chronic heart failure. Eur Heart J. 2004;25:136–143. doi: 10.1016/j.ehj.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 24.Sandek A, Doehner W, Anker S, et al. Nutrition in heart failure: An update. Clin Nutr Metabolic Care. 2009;12:384–391. doi: 10.1097/MCO.0b013e32832cdb0f. [DOI] [PubMed] [Google Scholar]