Abstract
Objective(s):
The alteration of glucose transporters is closely related with the pathogenesis of brain edema. We compared neuronal damage/death in the hippocampus between adult and young gerbils following transient cerebral ischemia/reperfusion and changes of glucose transporter-1(GLUT-1)-immunoreactive microvessels in their ischemic hippocampal CA1 region.
Materials and Methods:
Transient cerebral ischemia was developed by 5-min occlusion of both common carotid arteries. Neuronal damage was examined by cresyl violet staining, NeuN immunohistochemistry and Fluoro-Jade B histofluorescence staining and changes in GLUT-1 expression was carried out by immunohistochemistry.
Results:
About 90% of pyramidal neurons only in the adult CA1 region were damaged after ischemia/reperfusion; in the young, about 53 % of pyramidal neurons were damaged from 7 days after ischemia/reperfusion. The density of GLUT-1-immunoreactive microvessels was significantly higher in the young sham-group than that in the adult sham-group. In the ischemia-operated-groups, the density of GLUT-1-immunoreactive microvessels was significantly decreased in the adult and young at 1 and 4 days post-ischemia, respectively, thereafter, the density of GLUT-1-immunoreactive microvessels was gradually increased in both groups after ischemia/reperfusion.
Conclusion:
CA1 pyramidal neurons of the young gerbil were damaged much later than that in the adult and that GLUT-1-immunoreactive microvessels were significantly decreased later in the young. These data indicate that GLUT-1 might differently contribute to neuronal damage according to age after ischemic insults.
Keywords: CA1 region, Delayed neuronal death, Ischemia/reperfusion injury Pyramidal neurons, Young gerbil
Introduction
Although ischemic stroke occurs mainly in elderly individuals aged 65 years or older, nowadays, there are growing evidences of an increasing trend of childhood ischemic stroke (1, 2). Childhood ischemic stroke is generally considered as a rare and benign occurrence, with an incidence of at least 3.3/100,000 (3). However, childhood ischemic stroke is increasingly recognized as an important cause of disability and lifelong morbidity and it is among the top 10 causes of death in children (2).
Reperfusion following ischemia causes a wide range of pathophysiological process that leads to further damage, and the process may be defined as ischemia/reperfusion injury (4, 5). Limited oxygen availability is associated with impaired endothelial cell barrier function (6) and a concomitant increase in vascular permeability and leakage (7). In addition, ischemia/reperfusion leads to the activation of cell death programs, including apoptosis, autophagy-associated cell death and necrosis (8). Some studies using ischemic animal models have shown that young animals are resistant to ischemia/reperfusion injury (9, 10). Recently, we also compared neuronal damage in the ischemic CA1 region between the young and adult gerbils after 5 min of transient cerebral ischemia/reperfusion and showed that the neuronal death in the hippocampal CA1 region of the young was more delayed and less than that in the adult (11-13). However, the precise mechanisms of the more delayed neuronal death in the young remain unclear.
Glucose is a major energy substrate of energy metabolism for the central nervous system (CNS). Neurons in adult brains have a higher energy demand a continuous supply of glucose from blood (14). Glucose transporters (GLUTs) play critical roles in regulating glucose transportation and controlling the level of glucose in the brain (15, 16). Many studies have investigated that GLUT-1 is highly enriched in the endothelial cells of the blood brain barrier (BBB) (17-19).
A disruption of glucose uptake and utilization in the brain is anticipated to cause negative effects on the function and survival of the brain cells. It has been reported that the alteration of GLUT-1 is closely related with the pathogenesis of cerebral edema (20). GLUT-1 expression increases in response to focal and global ischemia in the adult rat brain (21, 22). Therefore, regulating GLUT-1 expression may ensure more glucose supply to ischemic areas. However, few studies regarding GLUT-1 changes in ischemic brains between the young and adult have been demonstrated. Therefore, in the present study, we compared chronological change of GLUT-1 immunoreactivity in the hippocampus of the young gerbil with that in the adult following 5 min of transient cerebral ischemia reperfusion.
Materials and Methods
Induction of transient cerebral ischemia
We used male Mongolian gerbils (Meriones unguiculatus) obtained from the Experimental Animal Center, Kangwon National University, Chunchon, South Korea. Gerbils were used at 1 (BW 25-30 g) and 6 months (BW 65-75 g) of age for the young and adult group. Experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) at Kangwon University and adhered to guidelines that are in compliance with the current international laws and policies (Guide for the Care and Use of Laboratory Animals, The National Academies Press, 8th Ed., 2011). The animals were divided into four groups: adult sham-operated-group, young sham-operated-group, adult ischemia-operated-group, and young ischemia-operated-group (n = 7 at each point in time in each group).
Transient cerebral ischemia was developed according to a previously published method by us (13). In brief, the animals were anesthetized with a mixture of 2.5% isoflurane in 33% oxygen and 67% nitrous oxide. Ischemia/reperfusion was induced by occluding both common carotid arteries for 5 min. The body (rectal) temperature under free-regulating or normothermic (37±0.5 °C) conditions was monitored with a rectal temperature probe (TR-100; Fine Science Tools, Foster City, CA) and maintained using a thermometric blanket before, during and after the surgery until the animals completely recovered from anesthesia. Sham-operated animals were subjected to the same surgical procedures except that the common carotid arteries were not occluded.
Tissue processing for histology
As described previously method (13), in brief, gerbils (n= 7 at each point in time) in each group were sacrificed under anesthesia at designated times (1, 2, 4, 7 and 15 days after reperfusion) and perfused transcardially with 0.1 M phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde in 0.1 M phosphate-buffer (PB, pH 7.4). The brains were serially sectioned into 30 µm coronal sections on a cryostat (Leica, Wetzlar, Germany).
Cresyl violet (CV) staining and Fluoro-Jade B (F-J B) histofluorescence
To investigate neuronal death in the hippocampus after ischemia-reperfusion, CV staining for normal cells and F-J B histofluorescence for dying or dead cells were performed. As described (13), in brief, the sections were stained with 1.0% (w/v) cresyl violet acetate (Sigma–Aldrich, St. Louis, MO, USA) and dehydrated by immersing in serial ethanol bath. For F-J B histofluorescence, the sections were immersed in a 0.0004% F-J B (Histochem, Jefferson, AR, USA) staining solution and examined using an epifluorescent microscope (Carl Zeiss, Göttingen, Germany) with blue (450-490 nm) excitation light and a barrier filter.
Immunohistochemistry for neuronal nuclei (NeuN) and GLUT-1
Immunohistochemistry was carried out as described (13). In brief, the brain sections were blocked with 10% normal goat serum in 0.05 M PBS followed by staining with primary mouse anti-NeuN (a neuron-specific soluble nuclear antigen) (diluted 1:1,000, Chemicon International, Temecula, CA, USA) and rabbit anti-GLUT-1 (diluted 1:200, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4 °C. The sections were next incubated with the secondary antibodies (Vector Laboratories Inc., Burlingame, CA, USA) and were developed using Vectastain ABC (Vector Laboratories Inc.). And they were visualized with 3,3’-diaminobenzidine in 0.1 M Tris-HCl buffer. In order to establish the specificity of the immunostaining, a negative control test was carried out with pre-immune serum instead of primary antibody. The negative control resulted in the absence of immunoreactivity in any structures.
Data analysis
As applied (13), the sections were selected according to anatomical landmarks corresponding to AP from −1.4 to −1.8 mm of gerbil brain atlas. The number of NeuN-immunoreactive and F-J B-positive cells was counted in a 250×250 µm square, applied approximately at the center of the stratum pyramidale (SP) of the hippocampal CA1 region. Cell counts were obtained by averaging the total cell numbers from each animal per group: A ratio of the count was calibrated as %.
In order to quantitatively analyze GLUT-1 immunoreactivity, as described (23), briefly, the density of GLUT-1-immunoreactive structures was evaluated on the basis of a relative optical density (ROD), which was obtained after the transformation of the mean gray level using the formula: ROD = log (256/mean gray level). A ratio of the ROD was calibrated as %, with the sham-group designated as 100 %.
Statistical analysis
Data are expressed as the mean ± SEM. The data were evaluated by a Tukey test for post-hoc multiple comparisons following one-way ANOVA. Statistical significance was considered at P<0.05.
Results
CV-positive (CV+) cells
In the adult and young sham-operated-groups, CV+ cells were well distributed in the hippocampus (Figures 1A, 1a, 1B and 1b). In the adult ischemia-operated-groups, a significant loss of CV+ cells was observed in the stratum pyramidale (SP) of the CA1 region, not the other subregions, 4 days after ischemia/reperfusion (Figures 1C and 1c). Thereafter, the distribution pattern of CV+ cells in the SP of the CA1 region was similar to that at 4 days post-ischemia (Figures 1E, 1e, 1G and 1g).
Figure 1.
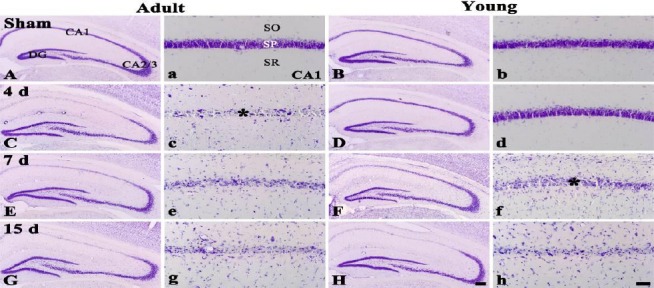
CV staining in the hippocampus of the adult (left two columns) and young (right two columns) sham- (A, a, B and b) and ischemia-operated- (C – H and c – h) groups. In the adult ischemia-operated-groups, CV+ cells are damaegd in the stratum pyramidale (SP) from 4 days after ischemia/reperfusion (asterisk). However, in the young ischemia-operated-groups, CV+ cells are damaged from 7 days post-ischemia (asterisk). SO, stratum oriens; SR, stratum radiatum. Scale bar = 800 (A – H) and 50 (a –h) μm
In the young ischemia-operated-groups, the distribution pattern of CV+ cells in the SP of the CA1 region was not changed 4 days after ischemia/reperfusion (Figures 1D and 1d). However, at 7 days post-ischemia, the morphological damage of CV+ cells was shown in the SP of the CA1 region (Figures 1F and 1f), thereafter, similar damage was found in the SP (Figures 1H and 1h).
NeuN+ and F-J B+ cells
In the adult sham-group, pyramidal neurons in the CA1 region were well immuno-stained with NeuN and no F-J B+ pyramidal neurons were found (Table 1, Figures 2A and 2a). In the young sham-group, the distribution pattern of NeuN+ and F-J B+ neurons in the CA1 region was similar to that in the adult sham-group (Table 1, Figures 2B and 2b).
Table 1.
Changes in the mean average number of pyramidal neurons of the ischemic hippocampal CA1 region in the adult and young gerbils
| Time after I-R | NeuN-immunoreactive neurons | F-J B-positive cells | ||
|---|---|---|---|---|
| Adult | Young | Adult | Young | |
| Sham | 90 ± 4.32 | 85 ± 3.65 | 0 | 0 |
| 4 days | 9 ± 1.71* | 86 ± 2.01+ | 50 ± 3.79* | 0 |
| 7 days | 10 ± 3.65* | 40 ± 1.76*#+ | 54 ± 1.24* | 16 ± 1.11*#+ |
| 15 days | 13 ± 1.56* | 42 ± 3.42*+ | 57 ± 3.41* | 21 ± 3.03*+ |
The mean number of NeuN-immunoreactive neurons and F-J B-positive cells was counted in a 250 X 250 μm square of the stratum pyramidale of the CA1 region after ischemia-reperfusion (I-R) (n=7 per group;
P<0.05, significantly different from the corresponding sham-group;
P<0.05, significantly different from the respective pre-time point group;
P<0.05, significantly different from the corresponding adult-group)
Figure 2.
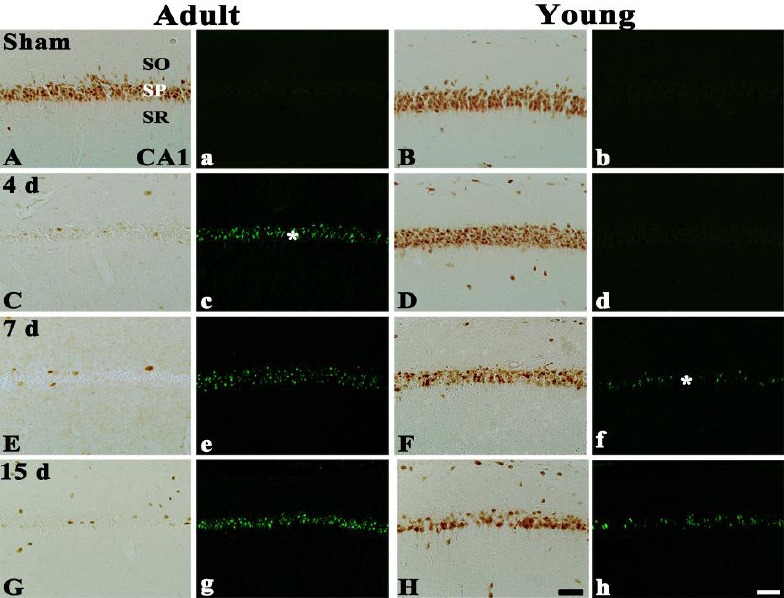
NeuN immunohistochemistry and F-J B histofluorescence staining in the CA1 region of the adult (left two columns) and young (right two columns) sham- (A, a, B, b) and ischemia-operated- (C – H and c – h) groups. In the adult ischemia-operated-groups, a few NeuN+ and many F-J B+ cells (asterisk) are shown in the stratum pyramidale (SP) from 4 days post-ischemia; however, NeuN+ cells are significantly decreased and F-J B+ cells (asterisk) are observed in the young ischemia-operated-groups from 7 days post-ischemia. SO, stratum oriens; SR, stratum radiatum. Scale bar = 50 µm
In the adult ischemia-operated-groups, a signi-ficant loss of NeuN+ neurons (about 90% of the adult sham-group) was observed in the SP of the CA1 region at 4 days post-ischemia (Table 1, Figure 2C), and, at this point in time, many F-J B+ cells were observed in the SP of the CA1 region (Table 1, Figure 2c). Thereafter, the distribution pattern of NeuN+ and F-J B+ pyramidal neurons in the ischemic CA1 region was similar to that at 4 days post-ischemia (Table 1, Figures 2E, 2e, 2G and 2g).
In the young ischemia-operated-groups, NeuN+ pyramidal neurons in the CA1 region at 4 days post-ischemia were similar to those in the sham-group (Table 1, Figure 2D), and no F-J B+ cells were detected in the SP (Table 1, Fig. 2d). However, at 7 days post-ischemia, NeuN+ neurons were significantly decreased (about 52 % of the sham-group) in the SP of the CA1 region (Table 1, Figure 2F), and, at this point in time, many F-J B+ cells were found in the SP (Table 1, Figure 2f). Thereafter, distribution patterns of NeuN+ and F-J B+ pyramidal neurons were similar to those at 7 days post-ischemia (Table 1, Figures 2H and 2h).
GLUT-1 immunoreactivity
CA1 region: GLUT-1-immunoreactive micro-vessels in the adult sham-group were easily observed in all layers of the CA1 region (Figure 3A).
Figure 3.
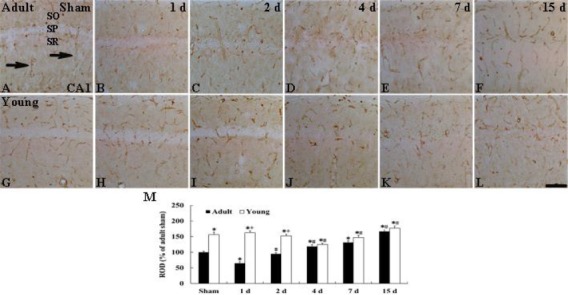
Immunohistochemistry for GLUT-1 in the CA1 region of the adult (upper column) and young (lower column) of the sham- (A and G) and ischemia-operated- (B-F, H-L) groups. GLUT-1-immunoreactive microvessels (arrows) in the young sham-group are higher in density than to those in the adult sham-group. In the ischemia-operated-groups, the density of GLUT-1-immunoreactive microvessels is distinctively decreased at 1 and 4 days post-ischemia, respectively, in the adult and young, and, thereafter, the density in both groups is gradually increased. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. Scale bar= 50 µm. M: ROD as % of GLUT-1-immunoreactive structures in the adult and young groups (n= 7 per group; *P<0.05, significantly different from the young sham-group, #P<0.05, significantly different from the respective pre-time point group; +P<0.05, significantly different from the corresponding adult ischemia-group). The bars indicate the means ± SEM
In the young sham-group, the relative optical density (ROD) of GLUT-1-immunoreactive microvessels in the CA1 region was significantly higher (about 56% of the adult sham-group) than that in the adult sham-group (Figures 3B and 3M). In the adult ischemia-operated-groups, the ROD of GLUT-1-immunoreactive microvessels in the CA1 region was significantly decreased (about 35% of the adult sham-group) 1 day after ischemia/reperfusion compared with that in the adult sham-group (Figures 3B and 3M). At 2 days post-ischemia, the ROD of GLUT-1-immunoreactive microvessels was recovered to the ROD of the sham-group, thereafter, the ROD of GLUT-1-immunoreactive microvessels was gradually increased with time after ischemia/reperfusion (Figures 3C–3F and 3M).
In the young ischemia-operated-groups, the ROD of GLUT-1-immunoreactive microvessels in the CA1 region was not changed until 2 days post-ischemia (Figures 3H, 3I and 3M). Four days after ischemia-reperfusion, the ROD of GLUT-1-immunoreactive microvessels was decreased (about 20% of the young sham-group) (Figures 3J and 3M), thereafter, the ROD of GLUT-1-immunoreactive microvessels in the CA1 region was increased with time (Figures 3K, 3L and 3M).
CA2/3 region: The ROD of GLUT-1-immuno-reactive microvessels in the CA2/3 region of the adult sham-group was similar to that in the CA1 region (Figure 4A). In the young sham-group, The ROD of GLUT-1-immunoreactive microvessels was also higher (about 24% of the adult sham-group) than that in the adult sham-group (Figures 4G and 4M). In the adult ischemia-groups, the ROD of GLUT-1-immunoreactive microvessels in the CA2/3 region as not changed after ischemia/reperfusion (Figures 4B–4F and 4M). In addition, the pattern of GLUT-1 immunoreactivity in the young-ischemia groups was not changed after ischemia/reperfusion (Figures 4H–4L and 4M).
Figure 4.
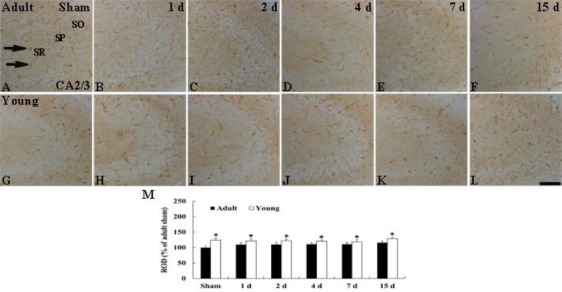
Immunohistochemistry for GLUT-1 in the CA2/3 region of the adult (upper column) and young (lower column) groups of the sham- (A and G) and ischemia-operated- (B-F, H-L) groups. GLUT-1-immunoreactive microvessels (arrows) in the young sham-group are is higher in density than to those in the adult sham-group. In both ischemia-operated-groups, distribution patterns of GLUT-1-immunoreactive microvessels in the CA2/3 region are not significantly changed after ischemia-reperfusion. SO, stratum oriens; SP, stratum pyramidale; SR, stratum radiatum. Scale bar = 50 µm. M: ROD as % of GLUT-1-immunoreactive structures in the adult and young groups (n= 7 per group; *P < 0.05, significantly different from the young sham-group). The bars indicate the means ± SEM
Discussion
The Mongolian gerbil has been commonly used for making a good animal model to investigate mechanisms of neuronal death following transient global cerebral ischemia/reperfusion (24, 25), because about 90% of the gerbils lack the communicating arteries between the carotid and vertebral arteries. Thus, the bilateral occlusion of the common carotid arteries essentially and completely eliminates blood flow to the telencephalon while completely sparing the vegetative centers of the brain stem (26). Among the brain regions, pyramidal neurons of the hippocampal CA1 region are the most vulnerable to transient cerebral ischemic insult (27), and this neuronal death is called “delayed neuronal death” since the neuronal death occurs very slowly after ischemia/reperfusion (28-30). Therefore, we have chosen the gerbil as an animal model of transient cerebral ischemia to study this subject.
Among risk factors for ischemic stroke, age is an important in determining the outcome of cerebral ischemic injury. Until now, age-related studies have been focused on neuronal death using adult gerbils (31, 32), and some reports have demonstrated that neuronal death induced by transient cerebral ischemia occurs much later in the aged than in the adult (31-33). On the other hand, childhood ischemic stroke is increasingly recognized as an important cause of disability and lifelong morbidity, although cerebral ischemia occurs mainly in the older (2). A previous study showed a greater resistance to various periods of transient cerebral ischemia using from 2 week-old to 12 week-old gerbils (10). Some researchers have also reported that young animals are less vulnerable to brain ischemic insult (9, 34), and we recently reported that resistance to cerebral ischemic insults was different according to age; young and aged gerbils are more resistant to cerebral ischemia than the adult under the same condition (11, 32, 35). In the present study, using CV staining, NeuN immunohistochemistry and F-J B histofluorescence staining, we observed the neuronal death in the young gerbil was much more delayed and less severe than that in the adult. This result is similar to previous studies that showed that the young gerbil was resistant to ischemic damage (9, 10). In addition, we recently reported that endogenous anti-oxidants and anti-inflammatory cytokines were markedly increased and they might be related with much more delayed and lesser neuronal death in the young gerbil hippocampus following transient cerebral ischemia/reperfusion (13, 36, 37).
It is well known that transient cerebral ischemia leads in oxygen-glucose deprivation and energy failure, which is associated with the development of neuronal cell damage/death in the hippocampal CA1 region (38). Because glucose is a major source of energy metabolism for the CNS, it is essential to keep adequate glucose supply to the brain (14). GLUT-1 plays a critical role in regulating glucose transportation and controlling the level of glucose in the brain (17, 39), and its expression is modulated in concert with metabolic demand and regional rates of cerebral glucose utilization (16). GLUT-1 is specifically localized to capillary endothelial cells of the brain (18, 19). Several studies have examined effects of global or focal ischemia on brain GLUT-1 expression in adult animal models (21, 40-42). A recent research demonstrated that the expression of GLUT-1 was increased in the hippocampus and cerebral cortex after ischemia/reperfusion injury in diabetic rats (43). On the other hand, Li et al (44) reporetd that the accumulation of GLUT-1 induced by progesterone treatment showed neuroprotective effects against cerebral ischemic insults. In the present study, we found that the density of GLUT-1-immunoreactive microvessels was significantly higher in the young than that in the adult and that, in the ischemia-operated-groups, the density of GLUT-1-immunoreactive microvessels in the young were changed later and significantly higher than that in the adult. This finding is supported by previous studies that showed that cerebral hypoxia-ischemia significantly increased the expression of GLUT-1 in the immature rat brain (45, 46). Based on these findings, high GLUT-1 expression seems to supply more glucose for ischemic brain, which may be an effective protection against cerebral ischemic insults. Although it was recently reported that hypoxia stimulated GLUT-1 expression in endothelial cells in vitro and in vivo (47, 48) and that GLUT-1 contributed to maintaining the integrity of BBB (49), it needs to study the precise mechanism of the ischemia-induced change of GLUT-1 expression in brain microvessels.
Conclusion
Our present findings demonstrate that GLUT-1-immunoreactive microvessels in the hippocampal CA1 region of the young were more than those in the adult and that the density of the microvessels in the young following ischemic insult was significantly increased much later than that in the adult. We suggest that the ischemia-mediated increase of GLUT-1-immunoreactive microvessels in the young may contribute to less and more delayed neuronal death in the young gerbil.
Acknowledgment
The authors would like to thank Mr Seung Uk Lee for his technical help in this study. This work was supported by 2013 Research Grant from Kangwon National University (No. 120131472), and by Hallym University Research Fund 2014 (HURF-2014-25).
Conflict of interest
The authors have no financial conflict of interest.
References
- 1.George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults 1995-2008. Ann Neurol. 2011;70:713–721. doi: 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 2.Amlie-Lefond C, Sebire G, Fullerton HJ. Recent developments in childhood arterial ischaemic stroke. Lancet Neurol. 2008;7:425–35. doi: 10.1016/S1474-4422(08)70086-3. [DOI] [PubMed] [Google Scholar]
- 3.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 4.Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology. 2007;49:93–102. doi: 10.1007/s00234-006-0183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa S, Gerlach H, Esposito C, Pasagian-Macaulay A, Brett J, Stern D. Hypoxia modulates the barrier and coagulant function of cultured bovine endothelium. Increased monolayer permeability and induction of procoagulant properties. J Clin Invest. 1990;85:1090–1998. doi: 10.1172/JCI114540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao Y, Li Z, Wang R, Wei J, Li G, Zhao H. Angiopoietin 1 counteracts vascular endothelial growth factor-induced blood-brain barrier permeability and alleviates ischemic injury in the early stages of transient focal cerebral ischemia in rats. Neurol Res. 2010;32:748–755. doi: 10.1179/016164109X12445616596562. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;15(361):1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oguro K, Miyawaki T, Yokota H, Kato K, Kamiya T, Katayama Y, et al. Upregulation of GluR2 decreases intracellular Ca2+ following ischemia in developing gerbils. Neurosci Lett. 2004;364:101–105. doi: 10.1016/j.neulet.2004.03.091. [DOI] [PubMed] [Google Scholar]
- 10.Kusumoto M, Arai H, Mori K, Sato K. Resistance to cerebral ischemia in developing gerbils. J Cereb Blood Flow Metab. 1995;15:886–891. doi: 10.1038/jcbfm.1995.110. [DOI] [PubMed] [Google Scholar]
- 11.Seo JY, Yan BC, Park JH, Ahn JH, Kim IH, Lee JC, et al. Comparison of the immunoreactivities of NMDA receptors between the young and adult hippocampal CA1 region induced by experimentally transient cerebral ischemia. J Neurol Sci. 2013;325:108–114. doi: 10.1016/j.jns.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 12.Yan BC, Park JH, Ahn JH, Choi JH, Yoo KY, Lee CH, et al. Comparison of glial activation in the hippocampal CA1 region between the young and adult gerbils after transient cerebral ischemia. Cell Mol Neurobiol. 2012;32:1127–1138. doi: 10.1007/s10571-012-9837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan BC, Kim SK, Park JH, Ahn JH, Lee CH, Yoo KY, et al. Comparison of inflammatory cytokines changes in the hippocampal CA1 region between the young and adult gerbil after transient cerebral ischemia. Brain Res. 2012;1461:64–75. doi: 10.1016/j.brainres.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Shestov AA, Emir UE, Kumar A, Henry PG, Seaquist ER, Oz G. Simultaneous measurement of glucose transport and utilization in the human brain. Am J Physiol Endocrinol Metab. 2011;301:E1040–1049. doi: 10.1152/ajpendo.00110.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrell CL, Pardridge WM. Blood-brain barrier glucose transporter is asymmetrically distributed on brain capillary endothelial lumenal and ablumenal membranes: an electron microscopic immunogold study. Proc Natl Acad Sci U S A. 1991;88:5779–5783. doi: 10.1073/pnas.88.13.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerhart DZ, LeVasseur RJ, Broderius MA, Drewes LR. Glucose transporter localization in brain using light and electron immunocytochemistry. J Neurosci Res. 1989;22:464–472. doi: 10.1002/jnr.490220413. [DOI] [PubMed] [Google Scholar]
- 20.Dwyer DS, Vannucci SJ, Simpson IA. Expression, regulation, and functional role of glucose transporters (GLUTs) in brain. Int Rev Neurobiol. 2002;51:159–188. doi: 10.1016/s0074-7742(02)51005-9. [DOI] [PubMed] [Google Scholar]
- 21.McCall AL, Van Bueren AM, Nipper V, Moholt-Siebert M, Downes H, Lessov N. Forebrain ischemia increases GLUT1 protein in brain microvessels and parenchyma. J Cereb Blood Flow Metab. 1996;16:69–76. doi: 10.1097/00004647-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Urabe T, Hattori N, Nagamatsu S, Sawa H, Mizuno Y. Expression of glucose transporters in rat brain following transient focal ischemic injury. J Neurochem. 1996;67:265–271. doi: 10.1046/j.1471-4159.1996.67010265.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee CH, Park JH, Cho JH, Ahn JH, Yan BC, Lee JC, et al. Changes and expressions of Redd1 in neurons and glial cells in the gerbil hippocampus proper following transient global cerebral ischemia. J Neurol Sci. 2014;344:43–50. doi: 10.1016/j.jns.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Liu YR, Lei RY, Wang CE, Zhang BA, Lu H, Zhu HC, et al. Effects of catalpol on ATPase and amino acids in gerbils with cerebral ischemia/reperfusion injury. Neurol Sci. 2014;35:1229–1233. doi: 10.1007/s10072-014-1687-7. [DOI] [PubMed] [Google Scholar]
- 25.Radenovic L, Selakovic V, Olivan S, Calvo AC, Rando A, Janac B, et al. Neuroprotective efficiency of tetanus toxin C fragment in model of global cerebral ischemia in Mongolian gerbils. Brain Res Bull. 2014;101:37–44. doi: 10.1016/j.brainresbull.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Fukuchi T, Katayama Y, Kamiya T, McKee A, Kashiwagi F, Terashi A. The effect of duration of cerebral ischemia on brain pyruvate dehydrogenase activity, energy metabolites, and blood flow during reperfusion in gerbil brain. Brain Res. 1998;792:59–65. doi: 10.1016/s0006-8993(98)00121-8. [DOI] [PubMed] [Google Scholar]
- 27.Lorrio S, Negredo P, Roda JM, Garcia AG, Lopez MG. Effects of memantine and galantamine given separately or in association, on memory and hippocampal neuronal loss after transient global cerebral ischemia in gerbils. Brain Res. 2009;1254:128–137. doi: 10.1016/j.brainres.2008.11.095. [DOI] [PubMed] [Google Scholar]
- 28.Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984;62:201–208. doi: 10.1007/BF00691853. [DOI] [PubMed] [Google Scholar]
- 29.Ohk TG, Yoo KY, Park SM, Shin BN, Kim IH, Park JH, et al. Neuronal damage using fluoro-jade B histofluorescence and gliosis in the striatum after various durations of transient cerebral ischemia in gerbils. Neurochem Res. 2012;37:826–834. doi: 10.1007/s11064-011-0678-9. [DOI] [PubMed] [Google Scholar]
- 30.Burda J, Matiasova M, Gottlieb M, Danielisova V, Nemethova M, Garcia L, et al. Evidence for a role of second pathophysiological stress in prevention of delayed neuronal death in the hippocampal CA1 region. Neurochem Res. 2005;30:1397–1405. doi: 10.1007/s11064-005-8510-z. [DOI] [PubMed] [Google Scholar]
- 31.Tamagaki C, Murata A, Asai S, Takase K, Gonno K, Sakata T, et al. Age-related changes of cornu ammonis 1 pyramidal neurons in gerbil transient ischemia. Neuropathology. 2000;20:221–227. doi: 10.1046/j.1440-1789.2000.00344.x. [DOI] [PubMed] [Google Scholar]
- 32.Lee CH, Yoo KY, Choi JH, Park OK, Hwang IK, Kim SK, et al. Neuronal damage is much delayed and microgliosis is more severe in the aged hippocampus induced by transient cerebral ischemia compared to the adult hippocampus. J Neurol Sci. 2010;294:1–6. doi: 10.1016/j.jns.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 33.Crain BJ, Westerkam WD, Harrison AH, Nadler JV. Selective neuronal death after transient forebrain ischemia in the Mongolian gerbil: a silver impregnation study. Neuroscience. 1988;27:387–402. doi: 10.1016/0306-4522(88)90276-x. [DOI] [PubMed] [Google Scholar]
- 34.Tortosa A, Ferrer I. Poor correlation between delayed neuronal death induced by transient forebrain ischemia, and immunoreactivity for parvalbumin and calbindin D-28k in developing gerbil hippocampus. Acta Neuropathol. 1994;88:67–74. doi: 10.1007/BF00294361. [DOI] [PubMed] [Google Scholar]
- 35.Yan BC, Park JH, Kim SK, Choi JH, Lee CH, Yoo KY, et al. Comparison of trophic factors changes in the hippocampal CA1 region between the young and adult gerbil induced by transient cerebral ischemia. Cell Mol Neurobiol. 2012;32:1231–1242. doi: 10.1007/s10571-012-9848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan BC, Park JH, Ahn JH, Lee YJ, Lee TH, Lee CH, et al. Comparison of the immunoreactivity of Trx2/Prx3 redox system in the hippocampal CA1 region between the young and adult gerbil induced by transient cerebral ischemia. Neurochem Res. 2012;37:1019–1030. doi: 10.1007/s11064-012-0702-8. [DOI] [PubMed] [Google Scholar]
- 37.Yan BC, Park JH, Lee CH, Yoo KY, Choi JH, Lee YJ, et al. Increases of antioxidants are related to more delayed neuronal death in the hippocampal CA1 region of the young gerbil induced by transient cerebral ischemia. Brain Res. 2011;1425:142–154. doi: 10.1016/j.brainres.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen MB, Wright DC, Diemer NH. Postischemic glucose metabolism is modified in the hippocampal CA1 region depleted of excitatory input or pyramidal cells. J Cereb Blood Flow Metab. 1990;10:243–251. doi: 10.1038/jcbfm.1990.41. [DOI] [PubMed] [Google Scholar]
- 39.Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab. 2005;25:2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- 40.Gerhart DZ, Leino RL, Taylor WE, Borson ND, Drewes LR. GLUT1 and GLUT3 gene expression in gerbil brain following brief ischemia: an in situ hybridization study. Brain Res Mol Brain Res. 1994;25:313–322. doi: 10.1016/0169-328x(94)90167-8. [DOI] [PubMed] [Google Scholar]
- 41.Lee WH, Bondy CA. Ischemic injury induces brain glucose transporter gene expression. Endocrinology. 1993;133:2540–2544. doi: 10.1210/endo.133.6.8243275. [DOI] [PubMed] [Google Scholar]
- 42.Lawrence MS, Sun GH, Kunis DM, Saydam TC, Dash R, Ho DY, et al. Overexpression of the glucose transporter gene with a herpes simplex viral vector protects striatal neurons against stroke. J Cereb Blood Flow Metab. 1996;16:181–185. doi: 10.1097/00004647-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Zhang WW, Zhang L, Hou WK, Xu YX, Xu H, Lou FC, et al. Dynamic expression of glucose transporters 1 and 3 in the brain of diabetic rats with cerebral ischemia reperfusion. Chin Med J (Engl) 2009;122:1996–2001. [PubMed] [Google Scholar]
- 44.Li X, Han H, Hou R, Wei L, Wang G, Li C, et al. Progesterone treatment before experimental hypoxia-ischemia enhances the expression of glucose transporter proteins GLUT1 and GLUT3 in neonatal rats. Neurosci Bull. 2013;29:287–294. doi: 10.1007/s12264-013-1298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vannucci SJ, Seaman LB, Vannucci RC. Effects of hypoxia-ischemia on GLUT1 and GLUT3 glucose transporters in immature rat brain. J Cereb Blood Flow Metab. 1996;16:77–81. doi: 10.1097/00004647-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Vannucci SJ, Reinhart R, Maher F, Bondy CA, Lee WH, Vannucci RC, et al. Alterations in GLUT1 and GLUT3 glucose transporter gene expression following unilateral hypoxia-ischemia in the immature rat brain. Brain Res Dev Brain Res. 1998;107:255–264. doi: 10.1016/s0165-3806(98)00021-2. [DOI] [PubMed] [Google Scholar]
- 47.Harik SI, Behmand RA, LaManna JC. Hypoxia increases glucose transport at blood-brain barrier in rats. J Appl Physiol. 1985-1994;77:896–901. doi: 10.1152/jappl.1994.77.2.896. [DOI] [PubMed] [Google Scholar]
- 48.Loike JD, Cao L, Brett J, Ogawa S, Silverstein SC, Stern D. Hypoxia induces glucose transporter expression in endothelial cells. Am J Physiol. 1992;263:C326–333. doi: 10.1152/ajpcell.1992.263.2.C326. [DOI] [PubMed] [Google Scholar]
- 49.Abdul Muneer PM, Alikunju S, Szlachetka AM, Murrin LC, Haorah J. Impairment of brain endothelial glucose transporter by methamphetamine causes blood-brain barrier dysfunction. Mol Neurodegener. 2011;6:23. doi: 10.1186/1750-1326-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]


