Abstract
Objective(s):
Multiple sclerosis (MS) is a serious neurological autoimmune disease, it commonly affects young adults. Vitamin E (Vit E) is an important component of human diet with antioxidant activity, which protects the body’s biological systems. In order to assess the effect of Vit E treatment on this autoimmune disease, we established experimental autoimmune encephalomyelitis (EAE), the animal model of MS, and treated EAE with α-tocopherol (AT) which is the main content of Vit E.
Materials and Methods:
Twenty C57BL/6 adult female mice were used and divided into two groups randomly. EAE was induced with myelin oligodendrocyte glycoprotein (MOG), and one group was treated with AT, at a dose of 100 mg/kg on the 3th day post-immunization with MOG, the other group was treated with 1% alcohol. Mice were euthanized on day 14, post-immunization, spleens were removed for assessing splenocytes proliferation and cytokine profile, and spinal cords were dissected to assess the infiltration of inflammatory cells in spinal cord.
Results:
AT was able to attenuate the severity of EAE and delay the disease progression. H&E staining and fast blue staining indicated that AT reduced the inflammation and the demyelination reaction in the spinal cord. Treatment with AT significantly decreased the proliferation of splenocytes. AT also inhibited the production of IFN-γ (Th1 cytokine), though the other cytokines were only affected slightly.
Conclusion:
According to the results, AT ameliorated EAE, through suppressing the proliferation of T cells and the Th1 response. AT may be used as a potential treatment for MS.
Keywords: Alpha-tocopherol, Autoimmunity, Inflammation, Multiple sclerosis, Vitamin E
Introduction
Multiple sclerosis (MS) is an idiopathic autoimmune disorder of the central nervous system (CNS), which is prevalent among young adults (1). The disease starts with increased migration of inflammatory cells across the blood-brain barrier, and induces the inflammatory reaction in the brain and spinal cord (2). However, the precise pathogenic mechanism of MS remains unknown. Experimental autoimmune encephalomyelitis (EAE) is a widely used animal model for MS. It is mediated by activated T cells specific for myelin autoantigens, such as myelin oligodendrocyte glycoprotein (MOG) (3). EAE can be induced in laboratory through immunized animals with myelin antigens or by the adoptive transfer of myelin-specific CD4+ T cells (3-5). Based on their secreted cytokines and transcription factor expression, CD4+ T cells were classified into four subsets, such as Th1 cells which produce interferon-γ (IFN-γ), Th2 cells which produce interleukin-4 (IL-4), Th17 cells which produce interleukin-17 (IL-17), and Treg cells which produce transforming growth factor-β (TGF-β) (6, 7). Th1 and Th17 cells play an important role in EAE pathogenesis, whereas Treg cells mediate immunological tolerance and limit inflammation and prevent autoimmune diseases (8).
Vitamin E (Vit E) is a natural antioxidant found in palm oil (9). It was used as a necessary phytonutrient for reproduction in rats, by Evans et al in 1922 (10). Except the antioxidant activity, Vit E suppresses the peroxidation of membrane lipids by scavenging peroxyl, oxygen, and superoxide anion radicals (11, 12). It has been reported that Vit E also regulates immunological response of the organism, both humoral and cellular (13). Vit E is a generic name for a complex mixture of homologues. The two main components of Vit E are tocopherols and tocotrienols. It has been reported that δ-tocotrienol could effectively ameliorate the collagen-induced arthritis (14), and α-tocopherol (AT) regulates the progression of allergic disease through inhibiting the generation of CD11c+CD11b+ dendritic cells (15). Furthermore, TFA-12, the derivative of tocopherol can modulate the development of MS through myelin repair (16).
This study was undertaken to evaluate the ability of AT to improve clinical recovery in EAE and explore the underlying mechanism of the disease with respect to the regulation of signaling events in inflammation and T cell response in EAE.
Materials and Methods
Animals and reagents
8-12 weeks of age, 18-22 g, female C57BL/6 mice, were purchased from Jackson Immune Research Laboratories, and were housed in a specific pathogen free facility in the animal center of the Zhengzhou University School of Medicine (Zhengzhou, China).
AT was purchased from Sigma-Aldrich (St. Louis, MO), myelin oligodendrocyte glycoprotein (MOG35-55, MEVGWYRSPFSRVVHLYR- NGK) from GL Biochem (Shanghai, China), incomplete Freund’s adjuvant (IFA) from Sigma-Aldrich (St. Louis, MO), Mycobacterium tuberculosis (Mtb) from Difco (strain H37Ra; Lawrence, KS Inc. Franklin Lakes, NJ, USA.), pertussis toxin (PT) from List Biochemical (Campbell, CA), and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) from Beyotime (Nantong, China).
Induction of experimental autoimmune encephalomyelitis and clinical evaluation
Mice were immunized subcutaneously (SC) with a peptide of MOG (300 μg/mouse) under the rostral part of the flanks at the base of the tail. MOG peptide was mixed with CFA, which contains IFA and 5 mg/ml Mtb. On days 0 and 2, PT (200 ng) in PBS was administered, IV. Mice were examined daily and disease severity was scored using the standard scale by researchers blinded to mouse identity: 0, normal mouse with no overt signs of disease; 1, complete paralysis of the tail; 2, weakness in both hind limbs; 3, complete hind limb paralysis; 4, complete forelimb paralysis; 5, death by EAE. After the onset of EAE, food and water were provided on the floor of the cage.
Treatment of α-tocopherol
AT was dissolved in 1% alcohol which was diluted by isotonic saline. All mice were weighed and randomly divided into two groups (10 mice per group). The experimental animals were given AT through IP injection daily, with the dose of 100 mg/kg of the body weight on the 3th day post-immunization with MOG. Mice were subjected to the same procedure in the control group; except that, AT was substituted with 1% alcohol.
Histopathology
On day 14 post-immunization, mice were transcardially perfused with 4% paraformaldehyde and killed by cervical dislocation, spinal cords were removed, postfixed overnight, and embedded in paraffin. 7 μm coronal spinal cord sections were dissected, the sections were stained with haematoxylin and eosin (H&E) and fast blue, and then examined by light microscopy for histological analysis.
Cell proliferation and cytokine assay
On the 14th day post-immunization, when disease peaked in the control animals, splenocytes were isolated from EAE mice, and then were cultured at 2×105/well in complete culture medium which contained DMEM, 10% FCS, 100 U/ml penicillin plus 100 mg/ml streptomycin, 10 mM Hepes, 2 mM L-glutamine, and 50 mM 2-ME (all from Life Technologies, Carlsbad, CA) in 5% CO2, at 37 °C. These cells were stimulated with MOG peptide (20 μg/ml) or antibodies to CD3 and CD28 (5 μg/ml; Life Technologies, Carlsbad, CA) with the presence or absence of AT (2 μg/ml).
Cell proliferation was measured by MTT assay. MTT was dissolved in MTT solvent to a concentration of 5 mg/ml. After splenocytes were cultured for 72 hr, 20 µl MTT solutions were added to each well of 96-well plate. After 4 hr incubation, the MTT-containing medium was removed, 200 µl DMSO was added to each well, and incubated at 37 °C to dissolve formazan product. Finally, the plate was read at 570 nm with a microplate reader (Thermo Fisher, Shanghai, China).
Cytokines were detected in culture supernatants using mouse ELISA kits (Peprotech, Shanghai, China), according to the manufacturer instructions. Absorbance was detected at 475 nm using a microplate reader (Thermo Fisher, Shanghai, China). A standard curve was described for each plate. The absolute concentrations of the indicated cytokines could be calculated from the standard curve.
Statistical analysis
Data were analyzed by one-way ANOVA followed by Newman-Keul’s multiple comparison tests. Analyses were performed using Graphpad Prism software.
Results
Alpha-tocopherol ameliorated development of clinical signs of experimental autoimmune encephalomyelitis
To analyze the biological activity of AT (Figure 1A), the effectiveness of this agent at modifying the course of disease in EAE was tested. EAE was induced with MOG. Treatment with AT was initiated from the 3th day post-immunization, and disease progression was monitored daily. The analysis revealed that, AT was able to attenuate the severity of EAE and also delay the disease progression compared to the control group (Figure 1B, Table 1). On day 11 post-immunization, all mice developed EAE in the control group, whereas only 25% of mice showed EAE appearance in the AT treated group (Table 1).
Figure 1.
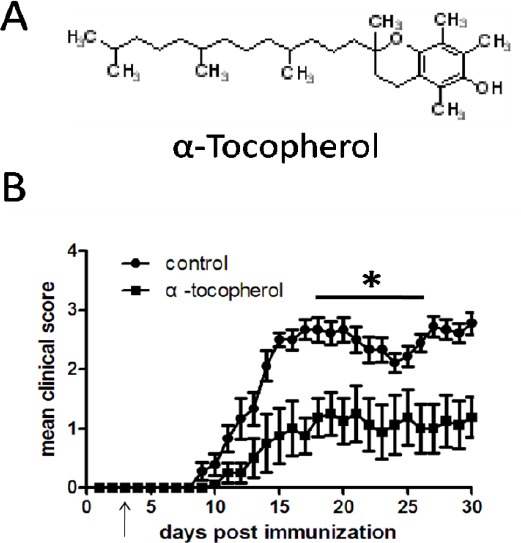
Severity and clinical course of experimental autoimmune encephalomyelitis in mice treated with α-tocopherol.
Table 1.
Effects of treatment with α-tocopherol on disease progression of experimental autoimmune encephalomyelitis
| Mean time of onset (d) | Mean maximum score | D11 incidence | |
|---|---|---|---|
| Control | 10.2±1.09 | 3.1±0.30 | 100% |
| AT | 16.6±6.60* | 2.6±1.10* | 25%* |
EAE was induced with MOG immunized in C57BL/6 mice. Mice were treated with daily IP. Injection of AT (100 mg/kg) or 1% alcohol, on day 3 post-immunization with MOG.
P<0.05 comparison between control and AT treated groups.
(A) Chemical structure of AT.
(B) EAE was induced with MOG immunized in C57BL/6 mice. Mice were treated with daily IP injection of AT (100 mg/kg) or 1% alcohol, on day 3 post-immunization with MOG. Results are shown with the means and SEM of individual mice. *P<0.05 by two-way ANOVA (AT vs control) compared. Each group was composed of 10 mice and the clinical course was monitored and scored daily as described in Materials and Methods. Data are representative of three independent experiments.
Alphaα-tocopherol reduced inflammation in the spinal cord
As we all know, the pathological changes of CNS in EAE are associated with the blood-brain barrier breakdown and infiltration of inflammatory cells. To further analyze the immunosuppressive properties of AT, on day 14 post-immunization, we stained spinal cord sections from EAE mice with H&E staining for routine histological examination and fast blue staining for demyelination assessment. Histological analysis revealed that, treatment with AT markedly decreased dense inflammatory cells infiltration and reduced the demyelination, as compared to those from the control group (Figure 2).
Figure 2.
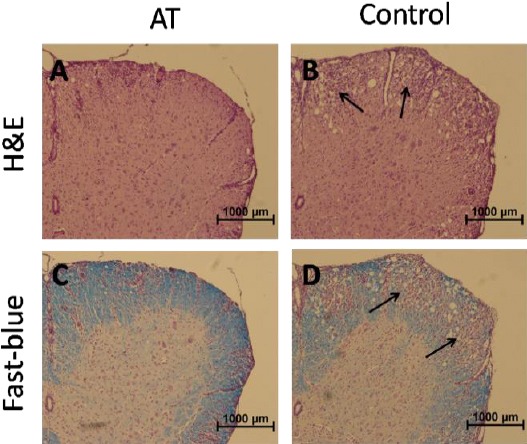
Histological changes of spinal cord tissue from experimental autoimmune encephalomyelitis mice treated with α-tocopherol.
(A-B) H&E staining of spinal cord tissue sections from AT-treated mice and control.
(C-D) Fast blue staining of spinal cord tissue sections from AT-treated mice and control.
Arrows indicate the infiltration of inflammatory cells and demyelination by loss of blue staining.
Effects of α-tocopherol treatment on proliferation of splenocytes
Spleen and lymph nodes are the main sites of T cell activation and play an important role in the development of EAE. We examined the effect of AT on the populations of splenocytes and lymphocytes. Spleen cells and lymphocytes were collected on day 14 post-immunization, when disease peaked in control group; we observed that AT significantly reduced the total number of cells from spleen and lymph nodes (Figure 3A). To further analysis the effect of AT on the proliferation of splenocytes, we isolated splenocytes from MOG-immunized mice and activated the cells in vitro with antibodies to the TCR CD3 and co-stimulatory receptor CD28 or with MOG peptide, AT was added into the culture system or not. After 72 hr, the proliferation responses were assessed by MTT assay. As was shown in Figure 3B, cell proliferations were reduced by AT, whether stimulators were antibodies to CD3 and CD28 or MOG peptide.
Figure 3.
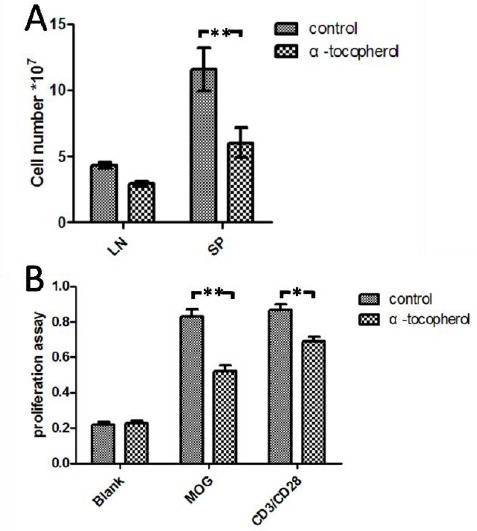
Analyses of the effect of α-tocopherol on T cell response in the stimulation with MOG peptide or antibodies to CD3/CD28.
(A) EAE was induced and treated with AT as shown in Figure 1, spleen (SP) and lymph nodes (LN) were removed, the mononuclear cells were isolated on 14 days post-immunization, and cell number were counted.
(B) The proliferation of splenocytes were analyzed ex vivo following stimulation with MOG peptide or antibodies to CD3/CD28. AT (2 µg/ml) was added or not, as indicated, into the culture system. The proliferation assay was determined by MTT. Results are shown with the means and SEM of individual mice. (n = 10 per group).
**P<0.01 and *P<0.05 by one-way ANOVA followed by Newman-Keul’s multiple comparison test.
Alpha-tocopherol treatment decreased Th1 responses
In order to better elucidate the role of AT treatment on the amelioration of disease response, we examined the cytokine profile influenced by AT. To this end, splenocytes were isolated from MOG-immunized mice and treated with MOG peptide (Figure 3), AT was added or not into the culture system as the protocol. Supernatants were collected and cytokines were detected by enzyme-linked immunosorbent assay (ELISA). The results revealed that the production of IFN-γ was significantly decreased as compared with that of untreated controls (P<0.05; Figure 4A). The production of IL-17 and TNF-α was decreased and the production of IL-10 was increased by AT, though the statistical evaluations were not significant (Figure 4B, C, and D). Taken together, these findings indicated that AT could inhibit Th1 responses.
Figure 4.
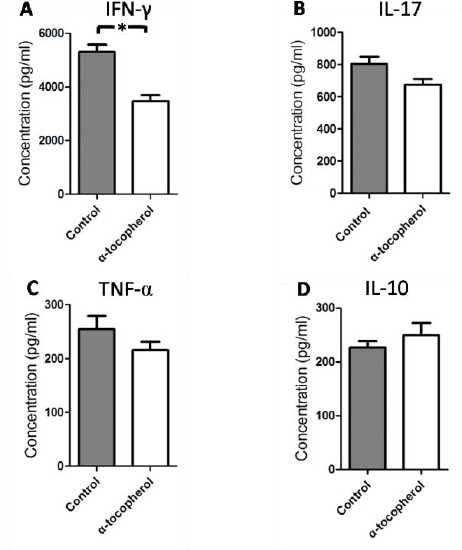
α-tocopherol treatment inhibited Th1 responses
EAE was induced, splenocytes were isolated on 14th day post-immunization and were stimulated with MOG peptide with or without AT (Figure 3), after 48 hr of incubation, we collected the supernatants and detected the cytokines with ELISA. The values shown, represent mean concentration (picograms per milliliter SEM) of triplicate samples. The results were reproducible in three independent experiments. *P<0.05 by one-way ANOVA followed by Newman-Keul’s multiple comparison test
Discussion
Multiple sclerosis (MS) is an idiopathic autoimmune disorder of the CNS, which is prevalent among young adults. It always starts with increased infiltration of autoreactive lymphocytes into CNS across the blood-brain barrier (17, 18). However, the pathogenesis and etiology of MS remains unclear. There are only a few drugs used to treat MS, which have little therapeutic effect and some drugs are associated with significant side effects or toxicity (19, 20). Thus, alternative therapies with greater efficacy are needed.
Vit E is one of the natural antioxidants which are produced by plants alone; many studies have shown its anti-inflammatory effect (21,22). The term “Vit E” contains eight different forms of the vitamin with similar chromanol structures, including trimethyl (α-), dimethyl (β- or γ-) and monomethyl (δ-) tocopherol, and the corresponding tocotrienols (T3) (23); only α-tocopherol meets human Vit E requirements. There has been reported that, AT protects the white matter lipids from damage changes during moderate hypoxia in rats (24). Vit E plays a critical role in the migration of “neuroprotective” CD8+IFN-γ+ T cells into the CNS (25).
In this study, we found that AT has a significant treatment effect on the clinical course of EAE (a commonly used and well-established animal model to human MS), and delays the onset of the disease. Only 25% of mice developed EAE in the AT-treated group, whereas all mice in the control group developed EAE on day 11 post-immunization. EAE involves many pro-inflammatory factors that interact with each other, these molecular mechanisms perpetuate and amplify the inflammatory processes in EAE.
It has been reported that TFA-12, the derivative of AT, reduces the severity and improves clinical recovery in EAE mice by promoting myelin repair (16). There have been reported that AT could also influence the expression of some important chemotactic molecules such as MCP-1 (26). Yang CS et al found that tocopherols can inhibit inflammation and carcinogenesis in lung and colon (21). Here we showed that, AT decreased the migration of inflammatory cells into the spinal cord, by H&E staining, and reduced the demyelination, by luxol fast blue staining. This give us a hypothesis that the effect of AT in ameliorating the severity of EAE is mediated by reducing cell migration into the spinal cord.
Tahan et al found that Vit E suppresses oxidant damage and inflammatory cytokines, and inhibits the colonic inflammation induced by acetic acid (AA) in rats; It also decreases the circulating level of IL-1β, IL-6, and TNF-α (22). Supplementation of all-rac AT, down-regulated the production of IL-1, TNF-α; AT suppressed PMA-induced IL-1β expression in vitro in human monocyte leukemic cell line THP-1 (26). In our study, AT not only decreased the number of inflammatory cells in lymph nodes and spleen in vivo, but also inhibited the proliferation of splenocytes stimulated by MOG or anti-CD3/CD28 in vitro. Then we collected the supernatant of cells culture and examined the cytokines (IL-10, IFN-γ, TNF-α, IL-17, and TGF-β) affected by AT; AT significantly inhibited the production of IFN-γ (Th1 cytokine), the other cytokines were only affected slightly. From these results we found that, AT influenced the process of EAE through the regulation of Th1 cells.
The natural Vit E exist in some vegetable oils, such as safflower seed oil, soy oil, and palm oil (27). Hence, diet therapy, especially with anti-inflammatory and antioxidant agents (e.g. AT) could have potential beneficial effects on treatment of autoimmune disease such as MS.
Conclusion
We found that AT significantly ameliorates EAE. According to the results, AT influences the process of EAE through the regulation of Th1 cells. Dietary micronutrients (e.g. AT) may have potential beneficial effects with regard to autoimmune disease such as MS.
Acknowledgments
This work was supported by the Youth Fund of the First Affiliated Hospital of Zhengzhou University (Code: ZDYFY3426) and the Key Project on Science and Technology Research of Henan Province, China (Code: 152102410067).
References
- 1.Dyment DA, Ebers GC, Sadovnick AD. Genetics of multiple sclerosis. Lancet Neurol. 2004;3:104–110. doi: 10.1016/s1474-4422(03)00663-x. [DOI] [PubMed] [Google Scholar]
- 2.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–15017. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 3.Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- 4.Stromnes IM, Goverman JM. Passive induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1952–1960. doi: 10.1038/nprot.2006.284. [DOI] [PubMed] [Google Scholar]
- 5.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Paul WE. Heterogeneity and plasticity of T helper cells. Cell Res. 2010;20:4–12. doi: 10.1038/cr.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan YY, Flavell RA. How diverse--CD4 effector T cells and their functions. J Mol Cell Biol. 2009;1:20–36. doi: 10.1093/jmcb/mjp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korn T, Anderson AC, Bettelli E, Oukka M. The dynamics of effector T cells and Foxp3+ regulatory T cells in the promotion and regulation of autoimmune encephalomyelitis. J Neuroimmunol. 2007;191:51–60. doi: 10.1016/j.jneuroim.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundram K, Sambanthamurthi R, Tan YA. Palm fruit chemistry and nutrition. Asia Pac J clin Nutr. 2003;12:355–362. [PubMed] [Google Scholar]
- 10.Evans HM, Bishop KS. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 1922;56:650–651. doi: 10.1126/science.56.1458.650. [DOI] [PubMed] [Google Scholar]
- 11.Mirbagheri SA, Nezami BG, Assa S, Hajimahmoodi M. Rectal administration of d-alpha tocopherol for active ulcerative colitis: a preliminary report. World J Gastroenterol. 2008;14:5990–5995. doi: 10.3748/wjg.14.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isozaki Y, Yoshida N, Kuroda M, Takagi T, Handa O, Kokura S, et al. Effect of a novel water-soluble vitamin E derivative as a cure for TNBS-induced colitis in rats. Int J Mol Med. 2006;17:497–502. [PubMed] [Google Scholar]
- 13.Meydani M, Macauley JB, Blumberg JB. Effect of dietary vitamin E and selenium on susceptibility of brain regions to lipid peroxidation. Lipids. 1988;23:405–409. doi: 10.1007/BF02535510. [DOI] [PubMed] [Google Scholar]
- 14.Haleagrahara N, Swaminathan M, Chakravarthi S, Radhakrishnan A. Therapeutic efficacy of vitamin E delta-tocotrienol in collagen-induced rat model of arthritis. Bio Med Res Int. 2014;2014:539540. doi: 10.1155/2014/539540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdala-Valencia H, Berdnikovs S, Soveg FW, Cook-Mills JM. alpha-Tocopherol supplementation of allergic female mice inhibits development of CD11c+ CD11b+ dendritic cells in utero and allergic inflammation in neonates. Am J Physiol Lung Cell Mol Physiol. 2014;307:L482–496. doi: 10.1152/ajplung.00132.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blanchard B, Heurtaux T, Garcia C, Moll NM, Caillava C, Grandbarbe L, et al. Tocopherol derivative TFA-12 promotes myelin repair in experimental models of multiple sclerosis. J Neurosci. 2013;33:11633–11642. doi: 10.1523/JNEUROSCI.0774-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasper LH, Shoemaker J. Multiple sclerosis immunology: The healthy immune system vs the MS immune system. Neurology. 2010;74:S2–8. doi: 10.1212/WNL.0b013e3181c97c8f. [DOI] [PubMed] [Google Scholar]
- 19.Sorensen PS, Koch-Henriksen N, Bendtzen K. Are ex vivo neutralising antibodies against IFN-beta always detrimental to therapeutic efficacy in multiple sclerosis? Mult Scler. 2007;13:616–621. doi: 10.1177/1352458506072344. [DOI] [PubMed] [Google Scholar]
- 20.DeAngelis T, Lublin F. Multiple sclerosis: new treatment trials and emerging therapeutic targets. Curr Opin Neurol. 2008;21:261–271. doi: 10.1097/WCO.0b013e328300c70d. [DOI] [PubMed] [Google Scholar]
- 21.Yang CS, Lu G, Ju J, Li GX. Inhibition of inflammation and carcinogenesis in the lung and colon by tocopherols. Ann N Y Acad Sci. 2010;1203:29–34. doi: 10.1111/j.1749-6632.2010.05561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tahan G, Aytac E, Aytekin H, Gunduz F, Dogusoy G, Aydin S, et al. Vitamin E has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can J Surg. 2011;54:333–338. doi: 10.1503/cjs.013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nazrun AS, Norazlina M, Norliza M, Nirwana SI. The anti-inflammatory role of vitamin e in prevention of osteoporosis. Advances in pharmacological sciences. 2012;2012:142702. doi: 10.1155/2012/142702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapelusiak-Pielok M, Adamczewska-Goncarzewicz Z, Dorszewska J, Grochowalska A. The protective action of alpha-tocopherol on the white matter lipids during moderate hypoxia in rats. Folia Neuropathol. 2005;43:103–108. [PubMed] [Google Scholar]
- 25.Sheridan PA, Beck MA. The dendritic and T cell responses to herpes simplex virus-1 are modulated by dietary vitamin E. Free Radic Biol Med. 2009;46:1581–1588. doi: 10.1016/j.freeradbiomed.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 27.Munteanu A, Zingg JM, Azzi A. Anti-atherosclerotic effects of vitamin E--myth or reality? J Cell Mol Med. 2004;8:59–76. doi: 10.1111/j.1582-4934.2004.tb00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


