Abstract
Objective(s):
Our recent report indicates that breviscapine play a protective role of the kidney by down-regulating transforming growth factor-β1(TGF-β1), α-smooth muscle actin (α-SMA) and alleviating interstitial fibrosis following unilateral ureteral obstruction (UUO). In this study, we investigate the effect of breviscapine on changes of renal water and sodium transport proteins in response to UUO.
Materials and Methods:
Male Sprague-Dawley rats were divided into 3 groups, sham group, UUO group and UUO treat with breviscapine. After 4, 7 and 14 days, histologic changes and interstitial collagen were determined microscopically following hematoxylin and eosin (H&E) and Masson’s trichrome staining. The expression of Aquaporins (AQP-2) and γ-epithelial sodium channel (γ-ENaC) were investigated using immunohistochemistry and Western blot in each group.
Results:
Breviscapine treatment decrease the tubular injury index and the degree of interstitial collagen deposition significantly compared with the UUO group (P<0.05). Breviscapine treatment also significantly reduced downregulation of AQP2 and γ-ENaC compared to those subjected to the same time course of obstruction in UUO group (P<0.05).
Conclusion:
These results demonstrate that breviscapine could prevent downregulation of renal water and sodium transport proteins in response to UUO so as to protect obstructed kidney.
Keywords: Breviscapine, Kidney, Ureteral obstruction, Water and sodium trans-, port proteins
Introduction
Renal tubulointerstitial fibrosis (RTF), a typical characteristic of chronic renal disease, is widely recognized as a component of many renal diseases and important contributors to chronic progressive renal disease leading to end-stage renal disease (ESRD). Unilateral ureteral obstruction (UUO), a well-characterized experimental hydronephrosis model exhibiting tubular dilatation and interstitial inflammatory-cell infiltration followed by the obstructed kidney, is a representative model of renal tubulointerstitial fibrosis. UUO has many readily quantifiable cellular and molecular events, such as inflammation, apoptosis (1) and dysregulation of key renal electrolyte transporters (2), which play important roles in the tubulointerstitial damage in UUO kidney.
Electrolyte disturbances is one of the common pathophysiologic features and procedures of chronic obstructive nephropathy. Currently the redress of electrolyte disturbances becomes a major topic in the therapy of chronic kidney disease. Series of studies have suggested that reduction in renal aquaporins (AQPs) and renal amiloride-sensitive epithelial sodium channels (ENaC) contributes to dysfunction of water-sodium metabolism, which is pivotal in urinary tract obstruction (3, 4).
Breviscapine, a protein kinase C (PKC) inhibitor, is a flavonoid extracted from the Chinese herb Erigeron breviscapus. As a traditional drug usually used in cardiovascular and cerebrovascular diseases (5), it was recently shown that breviscapine could lowering blood pressure effectively, also it could improve the renal function significantly, reduce the urinary micro-albuminuria, hence showing promising effect on renal protection (6). Another study indicates that treatment with breviscapine also attenuated renal injury in the diabetic rats by inhibiting the up-regulation of monocyte chemotactic protein-1(MCP-1) and intercellular adhesion molecule-1(ICAM-1) in the kidney, along with the reduction of macrophage infiltration (7).
Our previous study has demonstrated that breviscapine may alleviate the renal interstitial fibrosis induced by UUO through down-regulating the TGF-β1 and α-SMA expressions, thus showing a renoprotective effect, but whether breviscapine affects renal water and sodium transport proteins expressions after urinary tract obstruction and protects renal function is rarely reported. We set out to determine whether breviscapine affects the expression of AQP2 and γ-ENaC in UUO rats and to investigate the possible mechanism.
Materials and Methods
Animals
Seventy-two male Sprague-Dawley (SD) rats (body weight, 180-200 g) were purchased from WenZhou Medical University Laboratory Animal Center (WenZhou, China). All rats were randomly divided into 3 groups: Sham operation group, UUO group and UUO group treated with breviscapine (breviscapine group). The animal protocols were approved by the board of the Laboratory Animal Center, WenZhou Medicine University, according to the licenses for use of experimental animals issued by the Zhejiang Ministry of Justice. The rats had free access to a standard rodent diet and tap water.
Surgical procedure
The rats were placed on anesthesia with an intra-peritoneal (IP) injection of 60 mg/kg sodium pentobarbital (Merial, Hallbergmoos, Germany), Through a midline abdominal incision, the left ureters of the rats in both UUO and breviscapine treatment group were exposed and then a 5-0 silk ligature occluded the midportion of the ureter at 2 points, and made an incision between the points of ligation, while in sham group the left ureters were simply isolated without further manipulation. breviscapine (100 mg/kg·d, Shenwei Pharmaceu-tical Limited Company, Hebei, China) was administra-ted to the rats in breviscapine treatment group by intraperitoneally (2.5 ml once, twice a day) one day before operation till the rats were sacrificed humanly. Rats in sham group and UUO group were given same amount of saline by IP route. Each eight rats in three groups were sacrificed 4, 7 and 14 day after obstruction, The obstructed kidneys were removed and sliced transversely into two portions, the upper ones continuously transected into 3 mm thick sections and fixed in 4% paraformldehyde for histological examination and immunohistochemistry staining, and the subjacent ones frozen in -80 °C for Western blot procedure.
Tissue processing
The upper section of renal tissue was fixed in 4% paraformldehyde for at least 6 hr and paraffin embedded. 3 μm sections were utilized. The slides were deparaffinized with xylol and subsequently hydrated with gradient ethyl alcohol and running water.
Histopathology
For HE and Masson’s trichrome staining were used to stain 3 μm- thick paraffin sections. Histological sections were deparaffinized and hydrated as described above. The sections were then immersed in saturated Harris hematoxylin solution for 3 min and then in eosin for 1 more min.
Renal injury index including inflammatory, cell infiltration, interstitial fibrosis, interstitial edema, cell vacuolar degeneration, tubular atrophy, and tubular expansion were measured to assess the renal interstitial lesions. Ten different fields were selected to estimate the level of renal injury index with HE staining using bio-image analysis system (Bio-Profile). Each parameter was evaluated and given a score from 0 to 4+, (0, no changes; 1+, changes affecting 5-25% of the sample; 2+, changes affecting 25-50%; 3+, changes affecting 50-75%; 4+, changes affecting 75-100%).
The immunostaining antioxipositive area in the interstitium and tubular basement membrane stained in brown were picked up on the image analysis system (Bio-Profile), and the percentage of the immunostaining positive area relative to the whole area of the field was measured. glomeruli and large vessels were not included for image analysis. The scores of the 5 fields of each kidney were averaged. The scores of eight separate animals were then averaged.
Immunohistochemistry
Localization of AQP2 (diluted 1:300; Abcam, USA) and γ-ENaC (diluted 1:300; Abcam, USA) were assessed in paraffin-embedded tissue sections. The slides were deparaffinized, rehydrated and subjected to antigen retrieval solution at 95 °C for 15 min. The endogenous peroxidase activity was blocked with 3% hydrogen peroxide, and sections were then blocked with Protein Block Solution (Arrayit). Slides were incubated with a primary antibody or isotype non-specific IgG as negative control reagent overnight at 4 °C, followed by incubation with the labeled polymer (Envision, DAKO) at room temperature for 30 min. Staining was completed with 3,3’-diaminobenzidine (DAB) to produce a brown color. Finally, the sections were counterstained with hematoxylin.
The interstitial area positive detected by immunohistochemical was analyzed and quantitied as described previously. The expressions of renal tubular AQP2 and γ-ENaC were assessed with Image-Pro Plus 6.0 program according to staining intensity that compensated with the background staning intensity. (intensity of staining) × (% of interstitium with that intensity)= staining score.
Western blot Analysis
The subjacent kidneys were completely homogenated and lysed in RIPA buffer. Protein concentrations were electrophoresed at 50 μg using a BCA kit (PIERCE). Then the proteins were lane in a SDS-polyacrylamide gel and transferred onto a nitrocellulose membrane. The membrane was incubated in a blocking buffer A (PBS, 5% nonfat milk and 0.1% Tween-20) and incubated overnight at 4 °C with primary rabbit anti-rat AQP2 (diluted 1:300; Abcam, USA) and γ-ENaC (diluted 1:300; Abcam, USA) antibody. Then the membrane was washed once for 15 min and twice for five min in PBS, followed by a peroxidase-conjugated sheep anti-rabbit IgG (Santa Cruz Biotechnology) at a 1:10000 dilution. At last, the membrane was developed with a chemiluminescent agent (ECL). Each membrane was stripped and probed with mouse primary anti-β-actin antibody (Sigma, USA) to against a loading control. We used a bio-image analysis system (Bio-Rad, USA). to analyze the bands.
Statistical analysis
All values are expressed as the mean ± standard. Statistical analysis was performed using the statistical package SPSS for Windows Version 13.0 (SPSS, Inc., Chicago, IL, USA). The results were analyzed using a one-way analysis of variance (ANOVA) at the 0.05 level of significance for multiple groups comparisons. P-value of less than 0.05 was considered significant.
Results
Breviscapine attenuated the histological changes in the obstructed kidney induced by UUO
Microscopic examination of kidney sections in control group showed normal morphology. Following UUO, the rats demonstrated a progressive increase in the level of interstitial fibrosis, cellular infiltration, tubular ectasia, atrophy, necrosis and epithelial cell degeneration which reached a maximum at day 14 (Figure 1a). However, exposure to breviscapine significantly suppressed the level of interstitial fibrosis, cellular infiltration, tubular ectasia, atrophy, necrosis and epithelial cell degeneration compared with that seen in UUO group at the same time point (Figure 1a). Pathological changes were evaluated by renal tubular injury index. Histological analysis showed a higher tubular injury index in the rats subjected only to UUO when compared to breviscapine -treated rats (Figure 1a) (P<0.05).
Figure 1.
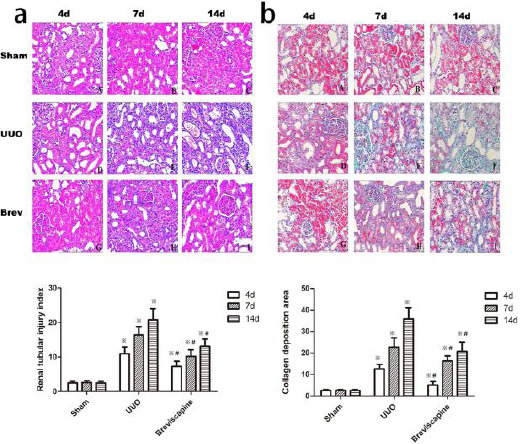
a: Breviscapine attenuated the histological changes in the obstructed kidney induced by UUO. Representative hematoxylin–eosin staining micrographs of (A, B, C) sham group, (D, E, F) UUO group and (G, H, I) breviscapine group. b: Breviscapine attenuated the interstitial collagen deposition in the obstructed kidney induced by UUO. Representative Masson’s trichrome staining micrographs of (A, B, C) sham group, (D, E, F) UUO group and (G, H, I) breviscapine group. Original magnification×200. ※P<0.05 in comparison with Sham group. ※P<0.05 in comparison with UUO group
Breviscapine attenuated the interstitial collagen deposition in the obstructed kidney induced by UUO
Masson’s trichrome stain demonstrates increased interstitial collagen deposition in the tubulointers-titium 4, 7 and 14 days after undergoing ureteral obstruction (Figure 2). However, exposure to breviscapine suppressed the interstitial collagen deposition at the same time point after UUO (Figure 1b). No gross alterations were observed in sham-operated rats. Indeed, semi-quantitative score of the degree of interstitial collagen revealed higher levels in obstructed kidneys of UUO rats compared to breviscapine treated rats at the same time point. (Figure 1b) (P<0.05)
Figure 2.
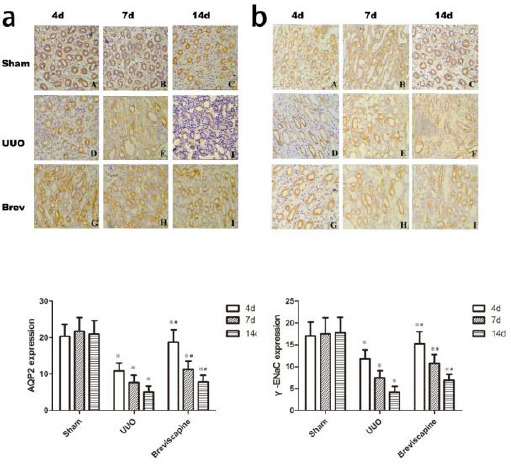
a: Breviscapine suppressed the downregulation of AQP2 in the obstructed kidney induced by UUO. Representative micrographs of (A, B, C) sham group, (D, E, F) UUO group and (G, H, I) Breviscapine group. b: Breviscapine suppressed the downregulation of γ-ENaC in the obstructed kidney induced by UUO. Representative Masson’s trichrome staining micrographs of (A, B, C) sham group, (D, E, F) UUO group and (G, H, I) breviscapine group. Original magnification×200. ※P<0.05 in comparison with Sham group at the same time point. ※P<0.05 in comparison with UUO group at the same time point
Breviscapine suppressed the downregulation of AQP2 and ENaC in the obstructed kidney induced by UUO
We next examined the effect of breviscapine on the expression of AQP2 and γ-ENaC in the obstructed kidney induced by UUO. AQP2 was essentially expressed in the subapical vesicles of collecting duct principal cells and apical plasma membrane (Figure 2a). γ-ENaC was located in the cytoplasm of principal cells of cortex (Figure 2b). Immunohistochemestry staining showed that a progressive decrease in the expression of AQP2 and γ-ENaC after undergoing UUO. exposure to breviscapine obviously retarded this progression as shown in Figure 2. Semi-quantitative score revealed the expression of AQP2 and γ-ENaC decreased in the kidneys of the UUO group compared with the sham group. However, exposure to breviscapine suppressed the downregulation of AQP2 and γ-ENaC at the same time point after undergoing UUO (Figure 2) (P<0.05).
Western blot analyses also revealed that the expression of AQP2 and γ-ENaC in the obstructed kidney was significantly decreased in a time-dependent manner (Figure 3). The administration of breviscapine significantly attenuated the decrease of AQP2 and γ-ENaC expression compared to UUO rats at the same time point (Figure 3) (P<0.05).
Figure 3.
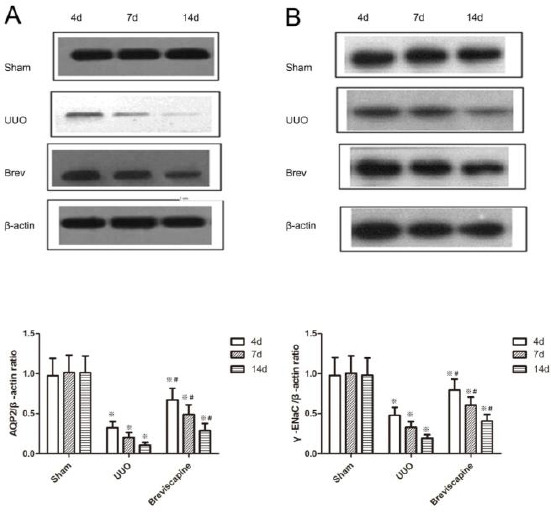
A. Breviscapine suppressed the down-regulation of AQP2 in the obstructed kidney assessed by Western blot assay. B. Breviscapine suppressed the down-regulation of γ-ENaC in the obstructed kidney assessed by Western blot assay. ※P<0.05 in comparison with Sham group. ※P<0.05 in comparison with UUO group
Discussion
The kidney plays a crucial role in regulating body salt and water balance so as to preserve the sodium and water homeostasis in vivo, which depends on various channel proteins located at nephron and collecting tubule. Urinary tract obstruction, a common clinical disease which impairs urinary concentrating capacity and reabsorption of sodium and water, is a serious condition in both children and adults, and it has marked effects on renal blood flow (7), glomerular filtrationrate (GFR) and renal tubular function (8). The pathophysiology behind the loss of the urinary concentration and dilution ability is complex and involves all nephron segments.
Chronic unilateral ureteral obstruction is a typical experimental rodent model of renal tubulointerstitial fibrosis which was pioneered by Klahr (9). Because of its distinguishing features-normotensive, nonproteinuric, and nonhyperlipidemic without any apparent immune or toxic renal insult, the UUO model is widely used in the investigation of the molecular and cellular mechanisms of interstitial fibrosis and impairment in UUO kidney. Previous studies have confirmed that urinary tract obstruction is associated with a significant water channels and sodium transporters reduction that are paralleled by water and electrolyte losses (4). These findings suggested that reduced expression of the renal aquaporins and sodium transporters contributed to the impairment in salt wasting and urinary concentrating capacity in response to ureteral obstruction. The mechanisms responsible for the dysregulation of these channels and transport proteins remain incompletely understood.
Regulation of water and salt excretion depends on an array of water and solute transporters in renal tubules and glomeruli in the various areas of the kidney. During and after unilateral ureteral obstruction (UUO), Major renal sodium transporters and AQPs located along all nephron segments were significantly reduced (8-10).
AQPs, the water channels located at the collecting duct, descending thin limb, proximal tubule, which regulate the excretion of water and sodium, belongs to a membranin family. As far as we know, at least seven aquaporins (AQP 1, 2, 3, 4, 6, 7 and 8) are expressed in the kidney. AQP 2, 3 and 4 are expressed in the cortical collecting duct, and play a major role in the reabsorption of water (11). AQP3 and AQP4 are abundantly expressed and persistent actived in the basolateral plasma membranes of collecting duct principal cell, however, AQP2 is expressed at lower abundance in the connecting tubules, and serves as the crucial factor of water permeability in collecting duct in rats (12-14), and play a key role in urinary concentration (10, 15, 16).
Renal amiloride-sensitive epithelial sodium channel (ENaC) (17) is an apically located channel expressed primarily in polarized epithelia in the distal nephron. It is composed of three homologous subunits: α, β, and γ (18, 19). Renal amiloride-sensitive epithelial sodium channel mediates Na+ entry across the apical membrane of cells in the cortical collecting duct (CCD), the connecting tubule (CNT), and the distal convoluted tubule (DCT), and primarily primparticipates in the regulation of sodium balance in the whole body. Although it presides over less than 5 percent reabsorption of sodium in the initial urine, γ-ENaC is the rate-limiting step for sodium absorption in the kidney (18), Series studies have shown the important roles of γ-ENaC in several physiological and pathophysiological conditions (19-21).
According to the immunohistochemistry and Western blotting result, we demonstrate that the expression of γ-ENaC and AQP2 in the kidney clearly decreased in the apical plasma membrane and cytoplasm after UUO for 4, 7 and 14 days compared with that in sham-operated rats (Figure 2, 3). Our findings are consistent with previous investigations (4, 22).
Breviscapine injection is isolated from Asteraceae plant Erigeron breviscapus and prepared into a Chinese patent medicine (23). The essential active ingredient is total flavones, and its pharmacologic action is dilating micro-blood vessel, reducing blood viscosity, and improving microcirculation (24). It possesses an anti-platelet, anti-thrombus action and lowers plasma fibrin content and promotes fibrinolytic activity (25, 26). A study recently showed that breviscapine could prevent early liver injury in streptozotocin-induced diabetic rats. (27), and another research found that breviscapin can protect hepatic cells, improve hepatic function, and alleviate hepatic injury by inhibiting PKC-mRNA and its protein expression in brain-dead BA-Ma mini pigs (28).
Our previous study confirm that breviscapine markedly alleviate the renal tubulointerstitial fibrosis in rats at 4, 7, 14 days after UUO, and the mechanism may be partially associated with down-regulating the expressions of TGF-β1 andα-SMA to restrain the phenotypic transition from renal tubular epithelial cells into myofibroblasts. Furthermore, in this study, we demonstrated that breviscapine effectively suppressed the loss of AQP2 and γ-ENaC induced by unilateral ureteral obstruction, showing a renoprotective effect in UUO rats.
Conclusion
Our results showed that, breviscapine, a traditional Chinese medicine, may play a positive role in the prevention of down-regulating the expressions of AQP2 and γ-ENaC in response to ureteral obstruction. Our study describes a novel mechanism on which breviscapine provides a renoprotective effect.
Acknowledgment
This project was supported by Zhejiang Provincial Natural Science Foundation (LQ16H100002, LQ13H100003, Y2110944).
References
- 1.Nath KA. Heme oxygenase-1 and acute kidney injury. Curr Opin Nephrol Hypertens. 2014;23:17–24. doi: 10.1097/01.mnh.0000437613.88158.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muto S, Asano Y. Electrical properties of the rabbit cortical collecting duct from obstructed kidneys after unilateral ureteral obstruction. Effects of renal decapsulation. J Clin Invest. 1994;94:1846–1854. doi: 10.1172/JCI117534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang G, Yuan W, Kwon TH, Li Z, Wen J, Topcu SO, et al. Age-related changes in expression in renal AQPs in response to congenital, partial, unilateral ureteral obstruction in rats. Pediatr Nephrol. 2012;27:83–94. doi: 10.1007/s00467-011-1878-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li C, Wang W, Norregaard R, Knepper MA, Nielsen S, Frokiaer J. Altered expression of epithelial sodium channel in rats with bilateral or unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2007;293:F333–341. doi: 10.1152/ajprenal.00372.2006. [DOI] [PubMed] [Google Scholar]
- 5.Xiong F, Wang H, Geng KK, Gu N, Zhu JB. Optimized preparation, characterization and biodistribution in heart of breviscapine lipid emulsion. Chem Pharm Bull. 2010;581:1455–1460. doi: 10.1248/cpb.58.1455. [DOI] [PubMed] [Google Scholar]
- 6.Wei L, Tan J. Clinical observation on Breviscapine in treating hypertension patients complicated with micro-albuminuria of renal impairment. Chin J Integr Med. 2005;11:31–33. doi: 10.1007/BF02835745. [DOI] [PubMed] [Google Scholar]
- 7.Qi XM, Wu GZ, Wu YG, Lin H, Shen JJ, Lin SY. Renoprotective effect of breviscapine through suppression of renal macrophage recruitment in streptozotocin-induced diabetic rats. Nephron Exp Nephrol. 2006;104:e147–157. doi: 10.1159/000094966. [DOI] [PubMed] [Google Scholar]
- 8.Pelaez LI, Juncos LA, Stulak JM, Lerman LO, Romero JC. Non-invasive evaluation of bilateral renal regional blood flow and tubular dynamics during acute unilateral ureteral obstruction. Nephrol Dial Transplant. 2005;20:83–88. doi: 10.1093/ndt/gfh556. [DOI] [PubMed] [Google Scholar]
- 9.Klahr S. New insights into the consequences and mechanisms of renal impairment in obstructive nephropathy. Am J kidney Dis. 1991;18:689–699. doi: 10.1016/s0272-6386(12)80611-1. [DOI] [PubMed] [Google Scholar]
- 10.Lee MD, King LS, Agre P. The aquaporin family of water channel proteins in clinical medicine. Medicine. 1997;76:141–156. doi: 10.1097/00005792-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen S. Renal aquaporins: an overview. BJU Int. 2002;90:1–6. doi: 10.1046/j.1464-410x.90.s3.1.x. [DOI] [PubMed] [Google Scholar]
- 12.Wood-Bradley RJ, Barrand S, Giot A, Armitage JA. Understanding the role of maternal diet on kidney development;an opportunity to improve cardiovascular and renal health for future generations. Nutrients. 2015;7:1881–1905. doi: 10.3390/nu7031881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiGiovanni SR, Nielsen S, Christensen EI, Knepper MA. Regulation of collecting duct water channel expression by vasopressin in Brattleboro rat. Proc Natl Acad Sci U S A. 1994;91:8984–8988. doi: 10.1073/pnas.91.19.8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nielsen S, Kwon TH, Frokiaer J, Knepper MA. Key roles of renal aquaporins in water balance and water-balance disorders. News Physiol Sci. 2000;15:136–143. doi: 10.1152/physiologyonline.2000.15.3.136. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa S. Urinary excretion of aquaporin-2 in pathological states of water metabolism. Ann Med. 2000;32:90–93. doi: 10.3109/07853890009011757. [DOI] [PubMed] [Google Scholar]
- 16.Jensen JM, Mose FH, Kulik AE, Bech JN, Fenton RA, Pedersen EB. Changes in urinary excretion of water and sodium transporters during amiloride and bendroflumethiazide treatment. World J Nephrol. 2015;4:423–437. doi: 10.5527/wjn.v4.i3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, et al. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- 18.Inagaki A, Yamaguchi S, Ishikawa T. Amiloride-sensitive epithelial Na+ channel currents in surface cells of rat rectal colon. Am J Physiol Cell Physiol. 2004;286:C380–390. doi: 10.1152/ajpcell.00373.2003. [DOI] [PubMed] [Google Scholar]
- 19.Pochynyuk O, Bugaj V, Rieg T, Insel PA, Mironova E, Vallon V, et al. Paracrine regulation of the epithelial Na+ channel in the mammalian collecting duct by purinergic P2Y2 receptor tone. J Biol Chem. 2008;283:36599–36607. doi: 10.1074/jbc.M807129200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Wang W, Kwon TH, Knepper MA, Nielsen S, Frokiaer J. Altered expression of major renal Na transporters in rats with unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2003;284:F155–166. doi: 10.1152/ajprenal.00272.2002. [DOI] [PubMed] [Google Scholar]
- 21.Konstas AA, Korbmacher C. The gamma-subunit of ENaC is more important for channel surface expression than the beta-subunit. Am J Physiol Cell Physiol. 2003;284:C447–456. doi: 10.1152/ajpcell.00385.2002. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Wang W, Kwon TH, Isikay L, Wen JG, Marples D, et al. Downregulation of AQP1, -2, and -3 after ureteral obstruction is associated with a long-term urine-concentrating defect. Am J Physiol Renal Physiol. 2001;281:F163–171. doi: 10.1152/ajprenal.2001.281.1.F163. [DOI] [PubMed] [Google Scholar]
- 23.Lin LL, Liu AJ, Liu JG, Yu XH, Qin LP, Su DF. Protective effects of scutellarin and breviscapine on brain and heart ischemia in rats. J Cardiovasc Pharm. 2007;50:327–332. doi: 10.1097/FJC.0b013e3180cbd0e7. [DOI] [PubMed] [Google Scholar]
- 24.Yan L, Huang H, Tang QZ, Zhu LH, Wang L, Liu C, et al. Breviscapine protects against cardiac hypertrophy through blocking PKC-alpha-dependent signaling. J Cell Biochem. 2010;109:1158–1171. doi: 10.1002/jcb.22495. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Zhang WB, Zhu JH, Fu GS, Zhou BQ. Breviscapine ameliorates cardiac dysfunction and regulates the myocardial Ca(2+)-cycling proteins in streptozotocin-induced diabetic rats. Acta Diabetol. 2010;47:209–218. doi: 10.1007/s00592-009-0164-x. [DOI] [PubMed] [Google Scholar]
- 26.Wang C, Li Y, Gao S, Cheng D, Zhao S, Liu E. Breviscapine injection improves the therapeutic effect of western medicine on angina pectoris patients. PloS One. 2015;10:e0129969. doi: 10.1371/journal.pone.0129969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu YG, Xia LL, Lin H, Zhou D, Qian H, Lin ST. Prevention of early liver injury by breviscapine in streptozotocin-induced diabetic rats. Planta Med. 2007;73:433–438. doi: 10.1055/s-2007-967182. [DOI] [PubMed] [Google Scholar]
- 28.Zhang SJ, Song Y, Zhai WL, Shi JH, Feng LS, Zhao YF, et al. Breviscapine alleviates hepatic injury and inhibits PKC-mRNA and its protein expression in brain-dead BA-Ma mini pigs. Hepatobiliary Pancreat. Dis Int. 2007;6:604–609. [PubMed] [Google Scholar]


