Introduction
Limb-body wall complex (LBWC) is a rare, complicated, polymalformative fetal malformation syndrome with the essential features of: a) exencephaly/encephalocele with facial clefts, b) thoraco-and/or abdominoschisis and c) limb defect [1]. Generally, the diagnosis is based on two of three of the above features. Two phenotypes have been described, the “placenta-cranial” and “placento-abdominal” adhesion phenotypes. This complex malformation, has no sex or familial predilection and is invariably fatal. The poor prognosis of LBWC necessitates an early antenatal diagnosis. Serum alpha-fetoprotein measurement and ultrasonographic examination is the key to prenatal diagnosis. The sonographic hallmark of LBWC is characterized by thoraco- and/or abdominoschisis, neural-tube abnormalities, severe scoliosis, positional deformities and abnormalities of fetal membranes [2]. In this case report we highlight the sonographic and colour flow imaging findings in LBWC presenting on routine antenatal scanning, along with the plain radiographs and the photographs of the aborted fetus.
Case Report
A 31 year old wife of a serving sailor presented to this hospital for routine antenatal ultrasonography scanning on 29 Sep 01. She was G1 P0 A0, at the time of scanning. She gave a history of a non-consanguineous marriage, did not take any drugs or chronic medication and was a booked case.
Ultrasound scans revealed a male fetus with the derived gestation age as 18 weeks 5 days (using her last menstrual period on 21 Jun 2001) and biparietal diameter as 16 weeks 2 days. The scans also revealed a large ill-defined abdominal wall defect through which the abdominal contents herniated into the extra embryonic coelom. The eviscerated organs formed a complex, bizarre appearing mass entangled with membranes (Fig 1). The diaphragm was absent. There was evidence to suggest bowel atresia, renal agenesis, anal atresia and lack of external genitalia. There was a lack of developed pelvic organs and perineum. There was evidence of scoliosis. While the fetal head diameter was normal for gestational age, the thoracic and abdominal diameters were disproportionately reduced. No anomalies were seen at the fetal eyes, facial profile, palate, lips, neck, or fetal cardiac and lung structures. Placenta was fundal and right lateral in position, showing grade 1 changes. Colour flow imaging showed evidence of a single umbilical artery within a short umbilical cord connecting the fetus (Fig 2). Colour flow imaging also clearly delineated the abnormal fetoplacental attachment. A provisional diagnosis of LBWC was made, based on the above sonographic findings.
Fig. 1.
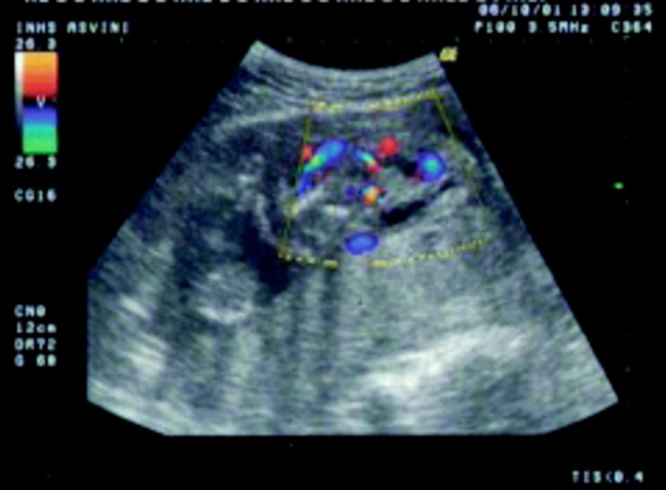
Colour flow imaging scans of the fetus showing a large ill defined abdominal wall defect through which the abdominal contents herniate into the extra embryonic coelom. The eviscerated organs form a complex, bizarre appearing mass entangled with membranes. The diaphragm is absent
Fig. 2.
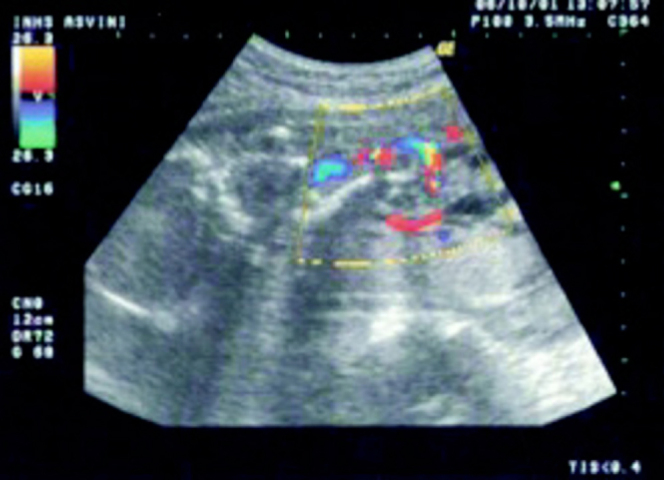
Colour flow imaging showing a single umbilical artery within a short umbilical cord connecting the fetus
The patient was informed of the poor prognosis and, after counselling, the patient elected to terminate the pregnancy. She was admitted as a high-risk pregnancy on 1 October 2001. The pregnancy was terminated with mesoprostol in lignocaine (400 mg), administered intravaginally in three doses five hours apart. Subsequently on 2 October 2001, she aborted a male fetus completely along with placenta and membranes.
The diagnosis of LBWC was confirmed after delivery. The severity of the defects and a distorted appearance made recognition of the lower half of the fetus difficult. The detailed examination of the fetus (Fig 3 A and B) revealed herniation of abdominal contents through a large defect. The abdominal circumference was markedly diminished. The eviscerated organs formed a complex, bizarre appearing mass entangled with membranes. An extracorporeal liver was present. The right lower limb was abnormally hyperflexed at hip joint and clubfoot was present at right foot. There was evidence of marked scoliosis. The umbilical cord was short, straight, incompletely covered by amnion and adherent to the placental membranes as well as the eviscerated mass. The umbilical vessels were embedded in an amniotic sheet, which connected the skin margin of the anterior body wall defect to the placenta. The cut section of the umbilical cord revealed a single umbilical artery. Plain radiographs of the aborted fetus (Fig 4A and B) revealed evidence of marked scoliosis. The thoracic circumference was markedly diminished. After delivery she was advised that there was no familial predilection or no known recurrence risk. After counselling to report for regular screening in her future pregnancies to the hospital, she was discharged.
Fig. 3.
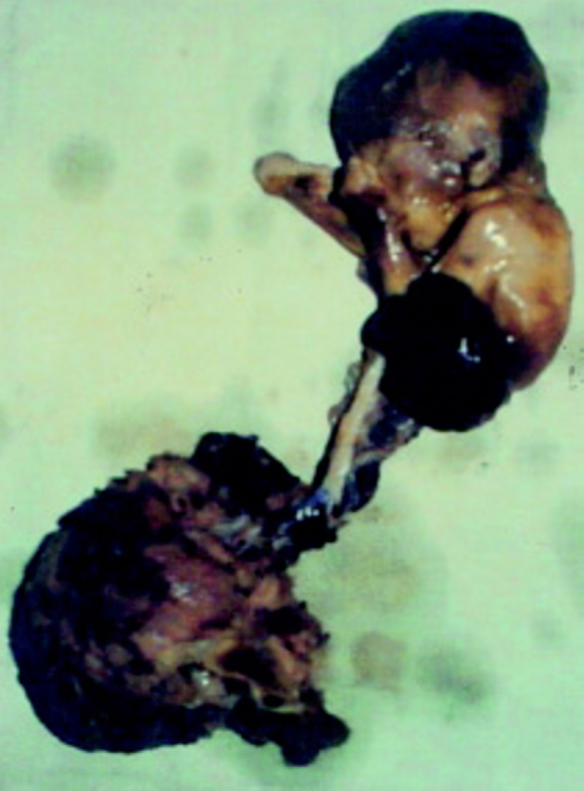
Photographs of the aborted fetus swhows herniation of abdominal contents through a large defect with the eviscerated organs form a compled bizarre appearing mass entangled with membranes. An extracorporeal liver is present. The umbilical cord is short, straight, incompletely covered by amnion and adherent to the placental membranes as well as the eviscerated mass
Fig. 4.
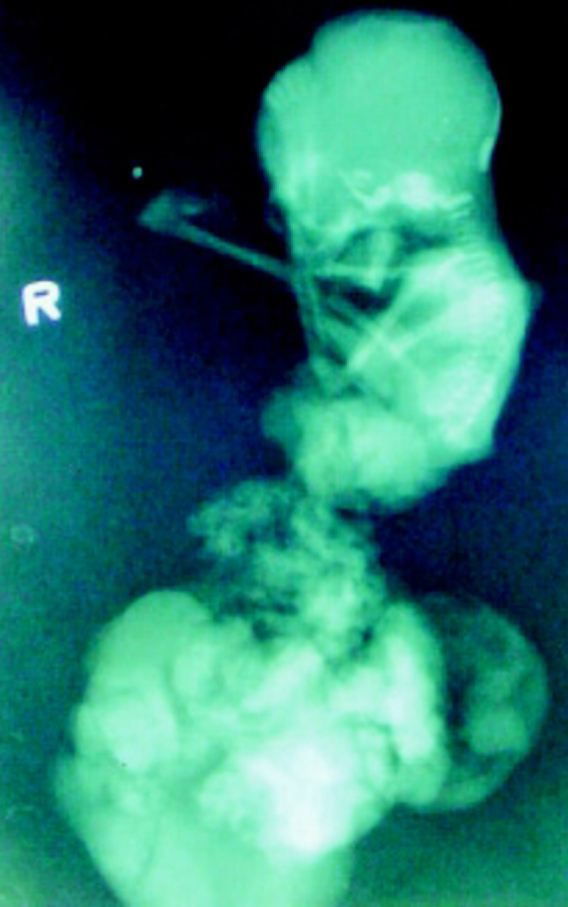
Plain radiographs of the aborted fetus display markedly diminished thoracic and abdominal circumference. The eviscerated organs form a compled, bizarre appearing mass. Clubfoot is present at right side
Discussion
LBWC is a rare complicated, polymalformative fetal malformation syndrome with the essential features of : a) exencephaly/encephalocele with facial clefts, b) thoraco- and/or abdominoschisis, and c) limb defect. Generally the diagnosis is based on two or three of the above features [1]. Coelosomia is found in all cases, it variably coexists with encephalic, vertebral, visceral or limb anomalies. This congenital anomaly has no sex or familial predilection or known recurrence risk. Karyotype study has been reported to be normal in all cases of LBWC. LBWC is invariably fatal [2, 3, 4, 5]. The incidence of this uncommon disorder is 1 in 4000.
The pathogenesis of LBWC is unclear and uncertain. Three pathogenetic mechanisms have been proposed namely, the early amnion rupture theory, vascular disruption theory and embryonic dysgenesis. Similarly, Russo et al have proposed that there are two clearly distinguishable phenotypes : the placento-cranial” and “placento-abdominal” adhesion phenotype [6, 7]. The first phenotype shows craniofacial defects and amniotic bands and/or adhesion, while the second is without craniofacial defects and presents urogenital anomalies, anal atresia, abdominal placental attachment, together with a persistence of the extra-embryonic celom. In addition, the authors attribute the two phenotypes as consequences of different pathogenetic mechanisms : firs type related to an early vascular disruption, while the second type is attributable to an intrinsic embryonal maldevelopment. While the first two theories can explain the form with “placento-cranial” adhesion, the “defective folding process” allows for a better explanation of the form with “palacento-cranial” adhesion. Also, Smith et al have suggested that LBWC results from amnion rupture caused by vascular or mechanical compression occurring between third and fifth weeks [3, 4]. In our case report, the pattern of presentation resembled the “placento-abdominal” adhesion phenotype.
Some consider that LBWC simply represents a severe form of amniotic band syndrome. This is reinforced by the presence of amniotic bands in nearly 40% cases of LBWC. Moreover, some of the limb defects can be explained by the presence of amniotic bands. More recently, Van Allen [4] suggests that the presence of amniotic bands is a consequence of early vascular disruption, but is not the primary abnormality itself. Allen, in a study of a series of fetuses with LBWC found that the major structural defects included limb defects (95%); marked scoliosis (77%), internal organ malformation (95%), craniofacial defects (56%) and limb defects including club foot (32%). The internal organ malformation present in 95% cases was cardiac defects, absent diaphragm, bowel atresia, renal agenesis and renal dysplasia. Body defects was a central feature of LBWC. Similarly, Patten et al reported trunk defects in 92% of the 13 fetuses, which exhibited features of LBWC [2]. Another large series by Van Allen et al found body defects in 96% of cases. The involvement of both abdomen and thorax was a more common feature as compared to involvement of either abdomen or thorax solely. The abdominal and thoracic contents herniate through a large defect into the extra embryonic celom. Typically, the eviscerated organs form a complex, bizarre appearing mass entangled with membranes. In our case the body defect was limited to the abdomen, even though the diaphragm was not identified.
Van Allen et al have divided the limb defects into 3 pathogenetic groups : a) secondary to disruption of embryonic vessels and surrounding tissue (84%), b) secondary to amniotic bands or adhesions (16%), and c) deformation versus haemorrhage (44% with club feet), with some fetuses having more than one pathogenetic mechanism causing limb defects, based on a study of limb defects from 25 fetuses with LBWC [6]. Majority of limb defects were hypothesized to have resulted from disruption of embryonic vessels. Importantly, in the specimens with amniotic band related limb defects, the most likely pathogenesis was identified as mechanical rupture through the amnion in the presence of a persistent extraembryonic celom or from adhesion of the amnion to necrotic embryonic tissue after the initial disruptive event. Clubfeet were present in 44% and was due either to disruption of embryonic vessels or to deformation.
The poor prognosis of LBWC necessitates an early antenatal diagnosis. Ultrasonographic detection of abdominoschisis, scoliosis, abnormalities of the lower extremities, a single umbilical artery, a short umbilical cord and an extremely elevated level of MSAFP is the key to early diagnosis. Ultrasonographically, the principal findings are the thoracoabdominal defect, limb anomalies, spinal and cord abnormalities [6]. All these groups of defects were present in our case. The severity of the defects and a distorted appearance of the fetus make recognition of the fetal parts difficult in almost all cases of LBWC. Spinal abnormalities range from scoliosis (usually severe) to spinal dysraphic defects. The umbilical cord is short and adherent to the placental membranes. A single umbilical artery is very commonly present, similar to our case presentation.
Because LBWC is incompatible with life, it is important to diagnose the lesions prenatally and to differentiate them from other anterior abdominal wall defects [7]. LBWC should be differentiated from common abdominal wall defects such as gastroschisis, omphalocele and uncommon entities like ectopia cordis, amniotic band syndrome, cloacal dystrophy and urachal cyst. One of the principal parameters that assist this differentiation is the site. While ectopia cordis is typically located at anterior aspect of thorax, gastroschisis and omphalocele are localized to the umbilical and paraumbilical areas. Similarly, cloacal dystrophy, urachal cyst are entities involving the lower abdominal wall. Other useful parameters that aid in deriving a specific diagnosis include the presence of membranes covering, the contents of herniated sac, any associated bowel abnormalities, the presence or absence of urinary bladder, scoliosis and limb defects, the presence or absence of other random defects such as facial cleft. Lastly, a combination of omphalocele and scoliosis should raise the suspicion of LBWC.
References
- 1.Kamudhamas A, Manusook S. Limb-body wall complex, report of 2 cases with their quintessence is prenatal diagnosis. J Med Assoc Thai. 2001;84(4):602–608. [PubMed] [Google Scholar]
- 2.Patten RM, Van Allen M, Mack LA. Limb-body wall complex: in utero sonographic diagnosis of a complicated fetal malformation. Am J Roentgenol. 1986;146(5):1019–1024. doi: 10.2214/ajr.146.5.1019. [DOI] [PubMed] [Google Scholar]
- 3.Smith DW. Recognizable Patterns of Human Malformation. 3rd ed. WB Saunders Co; Philadelphia: 1981. pp. 488–496. [Google Scholar]
- 4.Van Allen MI, Curry C, Gallgher Limb-body-wall complex I: Pathogenesis. Am J Med Genet. 1987;28:529–548. doi: 10.1002/ajmg.1320280302. [DOI] [PubMed] [Google Scholar]
- 5.Van Allen MI, Curry C, Gallagher Limb-body-wall complex II: Limb and spine defects. Am J Med Genet. 1987;28:549–565. doi: 10.1002/ajmg.1320280303. [DOI] [PubMed] [Google Scholar]
- 6.Deruelle P, Hay R, Subtil D. Antenatal diagnosis of limb body wall complex. Gynecol Obstet Biol Reprod (Paris) 2000;29(4):385–391. [PubMed] [Google Scholar]
- 7.Russo R, D'Armiento M, Angrisani P, Vecchione R. Limb body wall complex: a critical review and a nosological proposal. Am J Med Genet. 1993;47(6):893–900. doi: 10.1002/ajmg.1320470617. 1. [DOI] [PubMed] [Google Scholar]


