Abstract
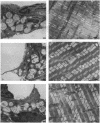
Glutathione deficiency in adult mice leads to lung type 2 cell lamellar body and mitochondrial damage; as reported here, these effects are associated with marked decrease of the levels of phosphatidylcholine (the main component of lung surfactant) in the lung and the bronchoalveolar lining fluid. Severe mitochondrial damage was also found in skeletal muscle. Treatment with ascorbate (1-2 mmol per kg of body weight per day), which led to greatly increased (approximately 2-fold) levels of lung and muscle mitochondrial glutathione, prevented damage to lamellar bodies and mitochondria as well as the decline of phosphatidylcholine levels in lung and alveolar lining fluid. The findings indicate that glutathione deficiency leads to depletion of lung surfactant and that this can be prevented with ascorbate. Administration of ascorbate spares glutathione and prevents cellular damage. Lamellar body degeneration in glutathione deficiency appears to be associated with oxidative damage to the perilamellar membrane, which contains the enzymes required for phosphatidylcholine synthesis. It is notable that although severe glutathione deficiency is lethal to newborn rats, which apparently do not synthesize ascorbate, adult mice are better able to survive such a deficiency because they can synthesize ascorbate. The present studies, which suggest that high doses of ascorbate may be of therapeutic value, emphasize that ascorbate and glutathione have actions in common and that they function together in a physiologically significant antioxidant system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams F. H., Fujiwara T., Emmanouilides G. C., Räihä N. Lung phospholipids of human fetuses and infants with and without hyaline membrane disease. J Pediatr. 1970 Nov;77(5):833–841. doi: 10.1016/s0022-3476(70)80244-x. [DOI] [PubMed] [Google Scholar]
- Adamson I. Y., Bowden D. H. The intracellular site of surfactant synthesis. Autoradiographic studies on murine and avian lung explants. Exp Mol Pathol. 1973 Feb;18(1):112–124. doi: 10.1016/0014-4800(73)90011-7. [DOI] [PubMed] [Google Scholar]
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Anderson M. E., Meister A. Glutathione monoesters. Anal Biochem. 1989 Nov 15;183(1):16–20. doi: 10.1016/0003-2697(89)90164-4. [DOI] [PubMed] [Google Scholar]
- BROWN E. S. ISOLATION AND ASSAY OF DIPALMITYL LECITHIN IN LUNG EXTRACTS. Am J Physiol. 1964 Aug;207:402–406. doi: 10.1152/ajplegacy.1964.207.2.402. [DOI] [PubMed] [Google Scholar]
- Bakewell W. E., Viviano C. J., Dixon D., Smith G. J., Hook G. E. Confocal laser scanning immunofluorescence microscopy of lamellar bodies and pulmonary surfactant protein A in isolated alveolar type II cells. Lab Invest. 1991 Jul;65(1):87–95. [PubMed] [Google Scholar]
- Bigley R., Stankova L., Roos D., Loos J. Glutathione-dependent dehydroascorbate reduction: a determinant of dehydroascorbate uptake by human polymorphonuclear leukocytes. Enzyme. 1980;25(3):200–204. doi: 10.1159/000459248. [DOI] [PubMed] [Google Scholar]
- Brumley G. W., Tuggle B., Luxner L., Crapo J. D. Disaturated phosphatidylcholine in rat lungs with altered numbers of type II alveolar epithelial cells. Am Rev Respir Dis. 1979 Mar;119(3):461–470. doi: 10.1164/arrd.1979.119.3.461. [DOI] [PubMed] [Google Scholar]
- Buckingham S., Heinemann H. O., Sommers S. C., McNary W. F. Phospholipid synthesis in the large pulmonary alveolar cell. Its relation to lung surfactants. Am J Pathol. 1966 Jun;48(6):1027–1041. [PMC free article] [PubMed] [Google Scholar]
- Cantin A. M., North S. L., Hubbard R. C., Crystal R. G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol (1985) 1987 Jul;63(1):152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- Crook E. M., Hopkins F. G. Further observations on the system ascorbic acid-glutathione-ascorbic acid-oxidase. Biochem J. 1938 Aug;32(8):1356–1363. doi: 10.1042/bj0321356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook E. M. The system dehydroascorbic acid-glutathione. Biochem J. 1941 Mar;35(3):226–236. doi: 10.1042/bj0350226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. A., Crapo J. D. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981 Nov 10;256(21):10986–10992. [PubMed] [Google Scholar]
- Griffith O. W., Anderson M. E., Meister A. Inhibition of glutathione biosynthesis by prothionine sulfoximine (S-n-propyl homocysteine sulfoximine), a selective inhibitor of gamma-glutamylcysteine synthetase. J Biol Chem. 1979 Feb 25;254(4):1205–1210. [PubMed] [Google Scholar]
- Griffith O. W. Mechanism of action, metabolism, and toxicity of buthionine sulfoximine and its higher homologs, potent inhibitors of glutathione synthesis. J Biol Chem. 1982 Nov 25;257(22):13704–13712. [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- HENDRY J. M., EASSON L. H., OWEN J. A. THE UPTAKE AND REDUCTION OF DEHYDROASCORBIC ACID BY HUMAN LEUCOCYTES. Clin Chim Acta. 1964 May;9:498–499. doi: 10.1016/0009-8981(64)90089-0. [DOI] [PubMed] [Google Scholar]
- Hopkins F. G., Morgan E. J. Some relations between ascorbic acid and glutathione. Biochem J. 1936 Aug;30(8):1446–1462. doi: 10.1042/bj0301446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A., Mårtensson J., Stole E., Auld P. A., Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1913–1917. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa Y., Spitzer R. Inclusion bodies of type II alveolar cells: species differences and morphogenesis. Anat Rec. 1969 Apr;163(4):525–541. doi: 10.1002/ar.1091630405. [DOI] [PubMed] [Google Scholar]
- King R. J. Composition and metabolism of the apolipoproteins of pulmonary surfactant. Annu Rev Physiol. 1985;47:775–788. doi: 10.1146/annurev.ph.47.030185.004015. [DOI] [PubMed] [Google Scholar]
- Mãrtensson J., Meister A., Mrtensson J. Glutathione deficiency decreases tissue ascorbate levels in newborn rats: ascorbate spares glutathione and protects. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4656–4660. doi: 10.1073/pnas.88.11.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Frayer W., Meister A. Glutathione metabolism in the lung: inhibition of its synthesis leads to lamellar body and mitochondrial defects. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5296–5300. doi: 10.1073/pnas.86.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Meister A. Glutathione is required for intestinal function. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1715–1719. doi: 10.1073/pnas.87.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Stole E., Frayer W., Auld P. A., Meister A. Inhibition of glutathione synthesis in the newborn rat: a model for endogenously produced oxidative stress. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9360–9364. doi: 10.1073/pnas.88.20.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Meister A. Mitochondrial damage in muscle occurs after marked depletion of glutathione and is prevented by giving glutathione monoester. Proc Natl Acad Sci U S A. 1989 Jan;86(2):471–475. doi: 10.1073/pnas.86.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J., Cannon B. Overview--preparation and properties of mitochondria from different sources. Methods Enzymol. 1979;55:3–28. doi: 10.1016/0076-6879(79)55003-4. [DOI] [PubMed] [Google Scholar]
- Omaye S. T., Turnbull J. D., Sauberlich H. E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- Puri R. N., Meister A. Transport of glutathione, as gamma-glutamylcysteinylglycyl ester, into liver and kidney. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5258–5260. doi: 10.1073/pnas.80.17.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redinbaugh M. G., Turley R. B. Adaptation of the bicinchoninic acid protein assay for use with microtiter plates and sucrose gradient fractions. Anal Biochem. 1986 Mar;153(2):267–271. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Basset G., Lecossier D., O'Donnell K. M., Pinkston P., Martin P. G., Crystal R. G. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J Appl Physiol (1985) 1986 Feb;60(2):532–538. doi: 10.1152/jappl.1986.60.2.532. [DOI] [PubMed] [Google Scholar]
- Robinson J. B., Jr, Srere P. A. Organization of Krebs tricarboxylic acid cycle enzymes in mitochondria. J Biol Chem. 1985 Sep 5;260(19):10800–10805. [PubMed] [Google Scholar]
- Rooney S. A., Wai-Lee T. S., Gobran L., Motoyama E. K. Phospholipid content, composition and biosynthesis during fetal lung development in the rabbit. Biochim Biophys Acta. 1976 Jun 22;431(3):447–458. doi: 10.1016/0005-2760(76)90211-3. [DOI] [PubMed] [Google Scholar]
- Rose R. C. Renal metabolism of the oxidized form of ascorbic acid (dehydro-L-ascorbic acid). Am J Physiol. 1989 Jan;256(1 Pt 2):F52–F56. doi: 10.1152/ajprenal.1989.256.1.F52. [DOI] [PubMed] [Google Scholar]
- Spitzer H. L., Rice J. M., MacDonald P. C., Johnston J. M. Phospholipid biosynthesis in lung lamellar bodies. Biochem Biophys Res Commun. 1975 Sep 2;66(1):17–23. doi: 10.1016/s0006-291x(75)80288-9. [DOI] [PubMed] [Google Scholar]
- Sturman J. A., Gaull G., Raiha N. C. Absence of cystathionase in human fetal liver: is cystine essential? Science. 1970 Jul 3;169(3940):74–76. doi: 10.1126/science.169.3940.74. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. Observations on the function of peroxidase systems and the chemistry of the adrenal cortex: Description of a new carbohydrate derivative. Biochem J. 1928;22(6):1387–1409. doi: 10.1042/bj0221387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama M., Itoh S., Nagasaki T., Tanimizu I. A new enzymatic method for determination of serum choline-containing phospholipids. Clin Chim Acta. 1977 Aug 15;79(1):93–98. doi: 10.1016/0009-8981(77)90465-x. [DOI] [PubMed] [Google Scholar]
- Van Golde L. M. Metabolism of phospholipids in the lung. Am Rev Respir Dis. 1976 Nov;114(5):977–1000. doi: 10.1164/arrd.1976.114.5.977. [DOI] [PubMed] [Google Scholar]
- Wells W. W., Xu D. P., Yang Y. F., Rocque P. A. Mammalian thioltransferase (glutaredoxin) and protein disulfide isomerase have dehydroascorbate reductase activity. J Biol Chem. 1990 Sep 15;265(26):15361–15364. [PubMed] [Google Scholar]
- Williams M. C. Conversion of lamellar body membranes into tubular myelin in alveoli of fetal rat lungs. J Cell Biol. 1977 Feb;72(2):260–277. doi: 10.1083/jcb.72.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]