Abstract
Direct and efficient production of ethanol by fermentation from raw corn starch was achieved by using the yeast Saccharomyces cerevisiae codisplaying Rhizopus oryzae glucoamylase and Streptococcus bovis α-amylase by using the C-terminal-half region of α-agglutinin and the flocculation functional domain of Flo1p as the respective anchor proteins. In 72-h fermentation, this strain produced 61.8 g of ethanol/liter, with 86.5% of theoretical yield from raw corn starch.
Recent years have seen the introduction of large-scale processing in the bioconversion of biomass resources, especially starchy materials, to ethanol, which is expected to find a wide range of uses, including as biofuel and as the starting material for various chemicals. However, the present process for ethanol production from starchy materials via fermentation consists of two or three steps and requires improvement if it is to realize efficient production at low cost. There are two main reasons for the present high cost: one is that, as the yeast Saccharomyces cerevisiae cannot utilize starchy materials, large amounts of amylolytic enzymes, namely, glucoamylase (EC 3.2.1.3) and α-amylase (EC 3.2.1.1), need to be added; the other is that the starchy materials need to be cooked at a high temperature (140 to 180°C) to obtain a high ethanol yield. To reduce the energy cost for cooking of starchy materials, previously reported noncooking and low-temperature-cooking fermentation systems (11, 12) have succeeded in reducing energy consumption by approximately 50% (11), but it is still necessary to add large amounts of amylolytic enzymes to hydrolyze the starchy materials to glucose.
Many researchers have reported on attempts to resolve this problem by using recombinant glucoamylase-expressing yeasts with the ability to ferment starch to ethanol directly (1, 3, 7, 9, 14, 16). Recombinant yeasts which coproduce glucoamylase and α-amylase have meanwhile been developed to further improve the efficiency of starch fermentation (2, 4, 6, 15, 21, 22). We also have used a cell surface engineering system based on α-agglutinin to demonstrate the advantages of yeast strains codisplaying amylolytic enzymes and have succeeded in producing ethanol from soluble and low-temperature-cooked corn starch using yeast strains which display Rhizopus oryzae glucoamylase and codisplay or secrete Bacillus stearothermophilus α-amylase (21, 22). These strains cannot, however, ferment raw corn starch to ethanol.
In the present study, instead of the B. stearothermophilus α-amylase, we therefore attempted to express α-amylase from the lactic acid bacterium Streptococcus bovis 148 together with R. oryzae glucoamylase. Extracellular α-amylase secreted from S. bovis 148 is known to have a strong ability to hydrolyze and be adsorbed onto raw corn starch (18, 19). For the development of a novel noncooking fermentation system, direct ethanol production from raw corn starch was investigated with yeast strains that codisplayed R. oryzae glucoamylase and S. bovis 148 α-amylase by using the C-terminal half of α-agglutinin and the flocculation functional domain of Flo1p (13, 26) as anchor proteins.
Strains and media.
Escherichia coli NovaBlue (Novagen Inc., Madison, Wis.) was used for genetic manipulation. The S. cerevisiae strain used was YF207 (MATa ura3-52 trp1Δ2 leu can1-100 FLO8) (9). E. coli was grown in Luria-Bertani medium (10 g of tryptone, 5 g of yeast extract, and 10 g of sodium chloride/liter) containing 100 μg of ampicillin per ml. Synthetic medium (6.7 g of yeast nitrogen base/liter without amino acids [Difco Laboratories, Detroit, Mich.] with appropriate nucleic acids and amino acids, 20 g of glucose per liter, and 20 g of Casamino Acids [Difco] per liter) was used for yeast cultivation under selective conditions (SDC medium).
Construction of plasmids for display of α-amylase from S. bovis.
Figure 1 shows the expression plasmids used for C-terminal immobilization based on the C-terminal half of α-agglutinin and for N-terminal immobilization based on the flocculation functional domain of Flo1p of S. bovis α-amylase. The gene encoding the mature region of α-amylase was amplified by PCR (primers 5′-GGATCCTGCAGATGAACAAGTGTCAA-3′ and 5′-CTCGAGTTTTAGCCCATCTTTATTAT-3′) with S. bovis 148 genomic DNA as the template and inserted into the BamHI-SalI site of plasmid pQE31 (Qiagen, Valencia, Calif.,) to give plasmid pQE31::amyA.
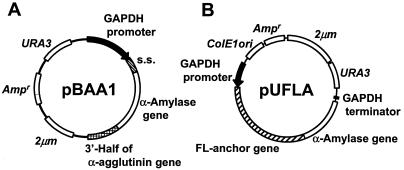
FIG. 1.
Expression plasmids for cell surface display of S. bovis α-amylase. (A) Plasmid pBAA1 for C-terminal immobilization using the α-agglutinin-based surface display system; (B) plasmid pUFLA for N-terminal immobilization using the Flo1p-based surface display system. s.s., secretion signal sequence of R. oryzae glucoamylase gene.
The multicopy plasmid pBAA1 (Fig. 1A) for cell surface display of α-amylase using a surface display system based on α-agglutinin was constructed as follows: the mature region of α-amylase was prepared by PCR (primers 5′-ACGGCCGCGGGGGATGAACAAGTGTCAATGAAAGATGGT-3′ and 5′-CTGCCTCGAGGGTTTTAGCCCATCTTTATTATAGTTTCC-3′) with plasmid pQE31::amyA as the template and inserted into the SacII-XhoI site of plasmid pCAS1 (20) to give plasmid pCAS1::amyA. Next, a 2.2-kb DNA fragment containing the secretion signal sequence of the R. oryzae glucoamylase gene and the mature region of the α-amylase gene was prepared by PCR (primers 5′-AATACTCGAGATGCAACTGTTCAATTTGCCATTGAAAGT-3′ and 5′-CTGCCTCGAGGGTTTTAGCCCATCTTTATTATAGTTTCC-3′) with plasmid pCAS1::amyA as the template and inserted into the XhoI site of the cell surface expression plasmid pUGP12 (21). The resulting plasmid was designated pBAA1.
The multicopy plasmid pUFLA (Fig. 1B) for cell surface display of α-amylase using a surface display system based on the flocculation functional domain of Flo1p was constructed as follows: the gene encoding the mature region of α-amylase was amplified by PCR (primers 5′-CGTTAGATCTGATGAACAAGTGTCAATGAAAGATGGTACG-3′ and 5′-ATAACTCGAGTTATTTTAGCCCATCTTTATTATAGTTTCC-3′) with plasmid pQE31::amyA as the template and inserted into the BglII-XhoI site of plasmid pWIFL (13) to give plasmid pWIFLA. The fusion gene encoding amino acids 1 to 1447 of Flo1p (FL anchor) and the mature region of α-amylase was prepared by PCR (primers 5′-ACATGGATCCATGACAATGCCTCATCGCTATATGTTTTTG-3′ and 5′-ATAACTCGAGTTATTTTAGCCCATCTTTATTATAGTTTCC-3′) with plasmid pWIFLA as the template. This fragment was digested with BamHI and XhoI and inserted into the BamHI-SalI site of plasmid pUGP3 (24). The resulting plasmid was designated pUFLA.
Plasmid pGA11 for cell surface display of R. oryzae glucoamylase was constructed previously (14). The plasmids were transformed into S. cerevisiae YF207 by the lithium acetate method using the YEASTMAKER transformation system (Clontech Laboratories, Inc., Palo Alto, Calif.).
Determination of enzyme activities.
Glucoamylase and α-amylase activities of yeast strains were determined by using the saccharifying-ability assay kit and α-amylase assay kit (Kikkoman Corp., Chiba, Japan), respectively, with 4-nitrophenyl β-maltoside and 2-chloro-4-nitrophenyl 65-azide-65-deoxy-β-maltopentaoside as the substrates. After aerobic cultivation of yeast in SDC medium at 30°C for 60 h, cells were collected by centrifugation for 10 min at 8,000 × g, resuspended in distilled water, and used for enzyme assays.
Experiments on ethanol production via fermentation.
Yeast strains were aerobically grown in SDC medium at 30°C for 60 h and harvested by centrifugation for 10 min at 8,000 × g. The cell pellet was used to inoculate YPS medium, which was prepared by adding raw corn starch (Wako Pure Chemical Industries, Ltd., Osaka, Japan) to sterile YP medium (10 g of yeast extract, 20 g of polypepton, and 0.5 g of potassium disulfite/liter). Ethanol production via fermentation was carried out in 100-ml closed bottles equipped with a bubbling CO2 outlet and containing 50 ml of YPS medium. All fermentations were performed at 30°C with mild agitation at 100 rpm. Potassium disulfite as an antiseptic was added to the YPS medium to prevent contamination with harmful microorganisms. The initial cell density was adjusted to 10 to 100 g [wet weight] of cells per liter. The wet weight of cells was determined by measuring the weight of the cell pellet, which was collected by centrifugation at 8,000 × g for 10 min and careful removal of the supernatant. The dry weight of cells corresponds to 0.15 times the wet weight of cells.
Total sugar and glucose concentrations were measured using the phenol-sulfuric acid method (5) as the glucose equivalent and the Glucose CII test (Wako), respectively. Ethanol concentration was measured by gas chromatography (model GC-8A fitted with a flame-ionization detector; Shimadzu, Kyoto, Japan) using a glass column (3.2 mm by 2.0 m) packed with Thermon-3000 (Shimadzu).
Activities of surface-displayed glucoamylase and α-amylase.
To confirm the coproduction of glucoamylase and α-amylase, their activities were determined (Table 1). The glucoamylase activities of yeast strains YF207/pGA11, YF207/pGA11/pBAA1, and YF207/pGA11/pUFLA harboring the plasmid pGA11 for surface display were 42.5, 42.3, and 57.0 U/g [wet weight] of cells, respectively; no activity was detected in strains YF207/pBAA1 and YF207/pUFLA. α-Amylase activity, meanwhile, was high in strains YF207/pUFLA and YF207/pGA11/pUFLA, which display α-amylase using Flo1p as an anchor (90.1 and 114 U/g [wet weight] of cells), but approximately 40 times lower in strains YF207/pBAA1 and YF207/pGA11/pBAA1, which display α-amylase using α-agglutinin as an anchor (2.52 and 2.38 U/g [wet weight] of cells). It has been reported that several α-amylases have raw-starch-digesting and raw-starch-binding abilities and that the starch binding domain is located in the C-terminal region (8, 10, 17, 23, 25). Immobilization on the cell surface at the N terminus using Flo1p allows α-amylase to be efficiently adsorbed onto, and to degrade, raw corn starch. No glucoamylase or α-amylase activity was detected in the culture supernatant of any of the strains.
TABLE 1.
Glucoamylase and α-amylase activities of yeast strains carrying different plasmids
| Strain | Activity (U/g [wet wt] of cells)a
|
|
|---|---|---|
| Glucoamylase | α-Amylase | |
| YF207 | ND | ND |
| YF207/pGA11 | 42.5 | ND |
| YF207/pBAA1 | ND | 2.52 |
| YF207/pUFLA | ND | 90.1 |
| YF207/pGA11/pBAA1 | 45.9 | 2.38 |
| YF207/pGA11/pUFLA | 57.0 | 114 |
Values are averages of three independent experiments. ND, not detected.
Ethanol production from raw corn starch.
Each of the recombinant yeast strains was used in direct ethanol production via fermentation from raw corn starch. The raw corn starch, which corresponds to 200 g of total sugar/liter, was used as the sole carbon source. As shown in Fig. 2, strain YF207/pGA11, displaying only glucoamylase, and strains YF207/pBAA1 and YF207/pUFLA, displaying only α-amylase, produced almost no ethanol, while soluble sugar accumulated in the fermentation medium of strain YF207/pUFLA due to degradation of corn starch to oligosaccharides by the surface-displayed α-amylase. Although strain YF207/pGA11/pBAA1, codisplaying glucoamylase and α-amylase via α-agglutinin, did produce ethanol from the raw corn starch, the ethanol yield was low (23.5 g/liter) after 72 h of fermentation. On the other hand, the yeast strain codisplaying glucoamylase and α-amylase using α-agglutinin and Flo1p (YF207/pGA11/pUFLA) was able to produce ethanol directly from raw corn starch without addition of commercial enzymes. The concentration of raw corn starch decreased drastically during fermentation, as the ethanol concentration increased to 61.8 g/liter after 72 h of fermentation. A reduction in the particle size and the number of corn starch granules during fermentation was also observed by microscopy (data not shown). The yield in terms of grams of ethanol produced per gram of sugar consumed was 0.44 g/g, which corresponds to 86.5% of theoretical yield (0.51 g of glucose consumed/g). No glucose was detected in the fermentation medium. The yeast strain YF207/pGA11/pUFLA maintained almost the same glucoamylase and α-amylase activities during fermentation. This result suggests that degradation of raw corn starch by α-amylase plays a very important role and that the putative starch binding domain at the C terminus of α-amylase acts on raw corn starch in the yeast strain used (YF207/pGA11/pUFLA), which codisplays glucoamylase and α-amylase using α-agglutinin and Flo1p as anchors.
FIG. 2.
Time course of direct ethanol production via fermentation from raw corn starch, which corresponds to 200 g of total sugar as the sole carbon source/liter using 100 g [wet weight] of cells of yeast strains S. cerevisiae YF207/pGA11 (squares), YF207/pBAA1 (triangles), YF207/pUFLA (inverted triangles), YF207/pGA11/pBAA1 (circles), and YF207/pGA11/pUFLA (diamonds) per liter. Open and closed symbols show ethanol and total sugar concentrations, respectively. Data are averages from three independent experiments.
Effect of initial cell density.
To reduce the number of yeast cells used for direct ethanol production via fermentation, the effect of initial cell density was examined with yeast strain YF207/pGA11/pUFLA and raw corn starch. Direct fermentation was carried out in the range of 10 to 100 g [wet weight] of cells per liter. As shown in Fig. 3, in fermentations using 50, 75, and 100 g of cells/liter, ethanol productivity was almost the same, but it was higher than with 10 and 25 g of cells/liter. Specifically, with 50 g of cells/liter, the maximum ethanol concentration increased gradually and reached 93.3 g/liter in the 132-h fermentation.
FIG. 3.
Effect of initial YF207/pGA11/pUFLA cell density on noncooking fermentation from raw corn starch, which correspond to 200 g of total sugar, as the sole carbon source, per liter. Symbols represent initial cell densities of 10 (squares), 25 (triangles), 50 (inverted triangles), 75 (circles), and 100 (diamonds) g [wet weight] of cells per liter. Open and closed symbols show ethanol and total sugar concentrations, respectively. Data are averages from two or three independent experiments.
Conclusions.
We succeeded in producing ethanol directly from raw corn starch using yeast strain YF207/pGA11/pUFLA, which codisplays R. oryzae glucoamylase and S. bovis α-amylase, using α-agglutinin and Flo1p with no time lag in the decrease in corn starch. In sequential reactions of α-amylase and glucoamylase codisplayed on the cell surface, raw corn starch was hydrolyzed to glucose. The noncooking fermentation system using a cell surface-engineered yeast strain thus promises to be very effective in reducing the production costs of ethanol.
Acknowledgments
This work was supported by a Grant-in-Aid for the Creation of Innovations through Business-Academic-Public Sector Cooperation from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
REFERENCES
- 1.Ashikari, T., S. Kunisaki, N. Matsumoto, T. Amachi, and H. Yoshizumi. 1989. Direct fermentation of raw corn to ethanol by yeast transformants containing a modified Rhizopus glucoamylase gene. Appl. Microbiol. Biotechnol. 32:129-133. [Google Scholar]
- 2.Birol, G., Z. I. Önsan, B. Kirdar, and S. G. Oliver. 1998. Ethanol production and fermentation characteristics of recombinant Saccharomyces cerevisiae strains grown on starch. Enzyme Microb. Technol. 22:672-677. [Google Scholar]
- 3.Cole, G. E., P. C. McCabe, D. Inlow, D. H. Gelfand, A. Ben-Basaat, and M. A. Innis. 1988. Stable expression of Aspergillus awamori glucoamylase in distiller's yeast. Bio/Technology 6:417-421. [Google Scholar]
- 4.De Moraes, L. M., S. Astolfi-Filho, and S. G. Oliver. 1995. Development of yeast strains for the efficient utilization of starch: evaluation of constructs that express alpha-amylase and glucoamylase separately or as bifunctional fusion proteins. Appl. Microbiol. Biotechnol. 43:1067-1076. [DOI] [PubMed] [Google Scholar]
- 5.Dubois, M., K. A. Gilles, L. K. Hamilton, P. A. Reberse, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 6.Eksteen, J. M., P. van Rensburg, R. R. C. Otero, and I. S. Pretorius. 2003. Starch fermentation by recombinant Saccharomyces cerevisiae strains expressing the α-amylase and glucoamylase genes from Lipomyces kononenkoae and Saccharomycopsis fibuligera. Biotechnol. Bioeng. 84:639-646. [DOI] [PubMed] [Google Scholar]
- 7.Inlow, D., J. McRae, and A. Ben-Bassat. 1988. Fermentation of corn starch to ethanol with genetically engineered yeast. Biotechnol. Bioeng. 32:227-234. [DOI] [PubMed] [Google Scholar]
- 8.Jespersen, H. M., E. A. MacGregor, M. R. Sierks, and B. Svensson. 1991. Comparison of the domain-level organization of starch hydrolases and related enzymes. Biochem. J. 280:51-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo, A., H. Shigechi, M. Abe, K. Uyama, T. Matsumoto, S. Takahashi, M. Ueda, A. Tanaka, M. Kishimoto, and H. Fukuda. 2002. High-level ethanol production from starch by a flocculent Saccharomyces cerevisiae strain displaying cell-surface glucoamylase. Appl. Microbiol. Biotechnol. 58:291-296. [DOI] [PubMed] [Google Scholar]
- 10.Lo, H. F., L. L. Lin, W. Y. Chiang, M. C. Chie, W. H. Hsu, and C. T. Chang. 2002. Deletion analysis of the C-terminal region of the α-amylase of Bacillus sp. strain TS-23. Arch. Microbiol. 178:115-123. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto, N., O. Fukushi, M. Miyanaga, K. Kakihara, E. Nakajima, and H. Yoshizumi. 1982. Industrialization of a noncooking system for alcoholic fermentation from grains. Agric. Biol. Chem. 46:1549-1558. [Google Scholar]
- 12.Matsumoto, N., H. Yoshizumi, S. Miyata, and S. Inoue. 1985. Development of the noncooking and low temperature cooking systems for alcoholic fermentation of grains. Nippon Nogeikagaku Kaishi 59:291-299. [Google Scholar]
- 13.Matsumoto, T., H. Fukuda, M. Ueda, A. Tanaka, and A. Kondo. 2002. Construction of yeast strains with high cell surface lipase activity by using novel display systems based on the Flo1p flocculation functional domain. Appl. Environ. Microbiol. 68:4517-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murai, T., M. Ueda, M. Yamamura, H. Atomi, Y. Shibasaki, N. Kamasawa, M. Osumi, T. Amachi, and A. Tanaka. 1997. Construction of a starch-utilizing yeast by cell surface engineering. Appl. Environ. Microbiol. 63:1362-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murai, T., M. Ueda, Y. Shibasaki, N. Kamasawa, M. Osumi, T. Imanaka, and A. Tanaka. 1999. Development of an arming yeast strain for efficient utilization of starch by co-display of sequential amylolytic enzymes on the cell surface. Appl. Microbiol. Biotechnol. 51:65-70. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura, Y., F. Kobayashi, M. Ohnaga, and T. Sawada. 1997. Alcohol fermentation of starch by a genetic recombinant yeast having glucoamylase activity. Biotechnol. Bioeng. 53:21-25. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez, S. R., J. Morlon-Guyot, J. Jore, J. Pintado, N. Juge, and J. P. Guyot. 2000. Comparative characterization of complete and truncated forms of Lactobacillus amylovorus α-amylase and role of the C-terminal direct repeats in raw-starch binding. Appl. Environ. Microbiol. 66:3350-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satoh, E., Y. Niimura, T. Uchimura, M. Kozaki, and K. Komagata. 1993. Molecular cloning and expression of two α-amylase genes from Streptococcus bovis 148 in Escherichia coli. Appl. Environ. Microbiol. 59:3669-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satoh, E., T. Uchimura, T. Kudo, and K. Komagata. 1997. Purification, characterization, and nucleotide sequence of an intracellular maltotriose-producing α-amylase from Streptococcus bovis 148. Appl. Environ. Microbiol. 63:4941-4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shibasaki, S., M. Ueda, T. Iizuka, M. Hirayama, Y. Ikeda, N. Kamasawa, M. Osumi, and A. Tanaka. 2001. Quantitative evaluation of the enhanced green fluorescent protein displayed on the cell surface of Saccharomyces cerevisiae by fluorometric and confocal laser scanning microscopic analyses. Appl. Microbiol. Biotechnol. 55:471-475. [DOI] [PubMed] [Google Scholar]
- 21.Shigechi, H., K. Uyama, Y. Fujita, T. Matsumoto, M. Ueda, A. Tanaka, H. Fukuda, and A. Kondo. 2002. Efficient ethanol production from starch through development of novel flocculent yeast strains displaying glucoamylase and co-displaying or secreting α-amylase. J. Mol. Cat. B. 17:179-187. [Google Scholar]
- 22.Shigechi, H., Y. Fujita, J. Koh, M. Ueda, H. Fukuda, and A. Kondo. 2000. Energy-saving direct ethanol production from low-temperature-cooked corn starch using a cell-surface engineered yeast strain co-displaying glucoamylase and α-amylase. Biochem. Eng. J. 18:149-153. [Google Scholar]
- 23.Sumitani, J., T. Tottori, T. Kawaguchi, and M. Arai. 2000. New type of starch-binding domain: the direct repeat motif in the C-terminal region of Bacillus sp. no. 195 α-amylase contributes to starch binding and raw starch degrading. Biochem. J. 350:477-484. [PMC free article] [PubMed] [Google Scholar]
- 24.Takahashi, S., M. Ueda, and A. Tanaka. 2001. Function of the prosequence for in vivo folding and secretion of active Rhizopus oryzae lipase in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 55:454-462. [DOI] [PubMed] [Google Scholar]
- 25.Tibbot, B. K., D. W. Wong, and G. H. Robertson. 2000. A function raw starch-binding domain of barley α-amylase expressed in Escherichia coli. J. Protein Chem. 19:663-669. [DOI] [PubMed] [Google Scholar]
- 26.Watari, J., Y. Takata, M. Ogawa, H. Sahara, S. Koshino, M.-L. Onnela, U. Airaksinen, R. Jaatinen, M. Penttilä, and S. Keränen. 1994. Molecular cloning and analysis of the yeast flocculation gene FLO1. Yeast 10:211-225. [DOI] [PubMed] [Google Scholar]