Abstract
Lonicerae japonicae flos is commonly used in traditional Chinese medicine for thousands of years with confirmed curative effects. Except for medicine, it is also used in healthy food, cosmetics, and soft beverages for its specific activities. Therefore, the chemical constituents, mainly including organic acids, flavonoids, iridoids, triterpenoids, and volatile oils, have been well studied by many scholars in recent years and a comprehensive and systematic review on chemical constituents of Lonicerae japonicae flos is indispensable. This paper aims at reviewing the chemical components of LJF in recent years through searching for the literatures both at home and abroad. Our results show that 212 components have been isolated from Lonicerae japonicae flos, including 27 flavonoids, 40 organic acids, 83 iridoids, 17 triterpenoids, and 45 other compounds, which could lay a foundation for the further application of Lonicerae japonicae flos.
1. Introduction
Lonicerae japonicae flos (LJF, also Jinyinhua in Chinese), coming from the dried buds of Lonicera japonica Thunb., has been widely used in traditional Chinese medicine (TCM) for several thousands of years due to its heat-clearing and detoxifying properties. In clinical practice, more than 500 prescriptions including LJF have been used to treat various diseases [1]. Pharmacological studies show that LJF possessed various actions, such as anti-inflammatory, antiviral, antidiabetic, antiallergic, and antioxidants [2–5], and could be used to treat many viral diseases, such as SARS and H7N9 virus and infections [6–9]. In addition, it is also used as healthy food, cosmetics, soft beverages, and ornamental groundcover, for its specific activities [10–13]. Therefore, many scholars inside and outside have drawn great attention on LJF in recent years and they have isolated a lot of chemical components from LJF, such as organic acids, flavones, iridoids, triterpenoids, and volatile oils [14], which have been reported as the active constituents with some potential pharmacological effects. Therefore, a comprehensive and systematic review on chemical constituents of LJF is indispensable.
Taking the abovementioned consideration, this paper comprehensively reviews chemical constituents of the dried flower buds of Lonicera japonica Thunb., in order to lay a foundation for the further study of LJF.
2. Constituents
More than 212 compounds have been isolated and identified from LJF so far, including organic acids, flavonoids, iridoids, triterpenoids, and volatile oils, which are the material basis of Lonicerae japonicae flos's pharmacological effects and constitute the primary effective substances. Beyond that, other groups of compounds were also reported.
2.1. Flavonoids
Up to now, 27 flavonoids have been isolated from LJF, mainly including quercetin (1), rutin (2), luteolin-7-O-β-D-glucopyranoside (3), kaempferol-3-O-β-D-glucopyranoside (4), apigenin-7-O-α-L-rhamnopyranoside (5), chrysoeriol-7-O-β-D-glucopyranosyl (6), luteolin-3′-L-rhamnoside (7), luteolin (8), flavoyadorinin-B (9), rhoifolin (10), quercetin-3-O-β-D-glucopyranoside (11), 3′-methoxy luteolin (12), 5,3′-dimethoxy luteolin (13), luteolin-5-O-β-D-glucopyranoside (14), apigenin (15), isorhamnetin-3-O-β-D-glucopyranoside (16), hyperoside (17), quercetin-7-O-β-D-glucopyranoside (18), kaempferol-3-O-β-D-rutinoside (19), isorhamnetin-3-O-β-D-rutinoside (20), 5-hydroxyl-3′,4′,7-trimethoxy flavone (21), 5-hydroxyl-6,7,8,4′-tetramethoxy flavone (22), corymbosin (23), 5-hydroxyl-7,4′-dimethoxy flavone (24), lonicerin (25), 5,7,3′,4′,5′-pentamethoxy flavone (26), and 5,4′-dihydroxy-3′,5′-dimethoxy-7-β-D-glucoxy-flavone (27). The structures of 27 flavonoids have been given in Figure 1 and Table 1.
Figure 1.
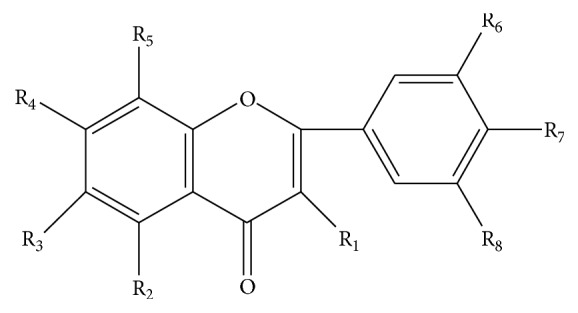
Skeleton of flavonoids.
Table 1.
The structures of compounds (1)–(27) isolated from LJF.
| Comp. number | Substitutional groups | References | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| (1) | OH | OH | H | OH | H | OH | OH | H | [25] |
| (2) | O-glc-rha | OH | H | OH | H | OH | OH | H | [25] |
| (3) | H | OH | H | O-glc | H | OH | OH | H | [25] |
| (4) | O-glc | OH | H | OH | H | H | OH | H | [19, 25] |
| (5) | H | OH | H | O-rha | H | H | OH | H | [19] |
| (6) | H | OH | H | O-glc | H | OCH3 | OH | H | [19] |
| (7) | H | OH | H | OH | H | O-rha | OH | H | [19] |
| (8) | H | OH | H | OH | H | OH | OH | H | [58] |
| (9) | H | H | H | OCH3 | H | O-glc | OCH3 | H | [26] |
| (10) | H | OH | H | O-rha-glc | H | H | OH | H | [26] |
| (11) | O-glc | OH | H | OH | H | OH | OH | H | [26] |
| (12) | H | OH | H | OH | H | OCH3 | OH | H | [20] |
| (13) | H | OCH3 | H | OH | H | OCH3 | OH | H | [20] |
| (14) | H | O-glc | H | OH | H | OH | OH | H | [20] |
| (15) | H | OH | H | OH | H | H | OH | H | [20] |
| (16) | O-glc | OH | H | OH | H | OCH3 | OH | H | [59] |
| (17) | O-gal | OH | H | OH | H | OH | OH | H | [59] |
| (18) | OH | OH | H | O-glc | H | OH | OH | H | [60] |
| (19) | O-glc-rha | OH | H | OH | H | H | OH | H | [28] |
| (20) | O-glc-rha | OH | H | OH | H | OCH3 | OH | H | [28] |
| (21) | H | OH | H | OCH3 | H | OCH3 | OCH3 | H | [15] |
| (22) | H | OH | OCH3 | OCH3 | OCH3 | H | OCH3 | H | [18] |
| (23) | H | OH | H | OCH3 | H | OCH3 | OCH3 | OCH3 | [61] |
| (24) | H | OH | H | OCH3 | H | H | OCH3 | H | [61] |
| (25) | H | OH | H | O-rha-glc | H | OH | OH | H | [61] |
| (26) | H | OCH3 | H | OCH3 | H | OCH3 | OCH3 | OCH3 | [62] |
| (27) | H | OH | H | O-glc | H | OCH3 | OH | OCH3 | [45] |
2.2. Organic Acids
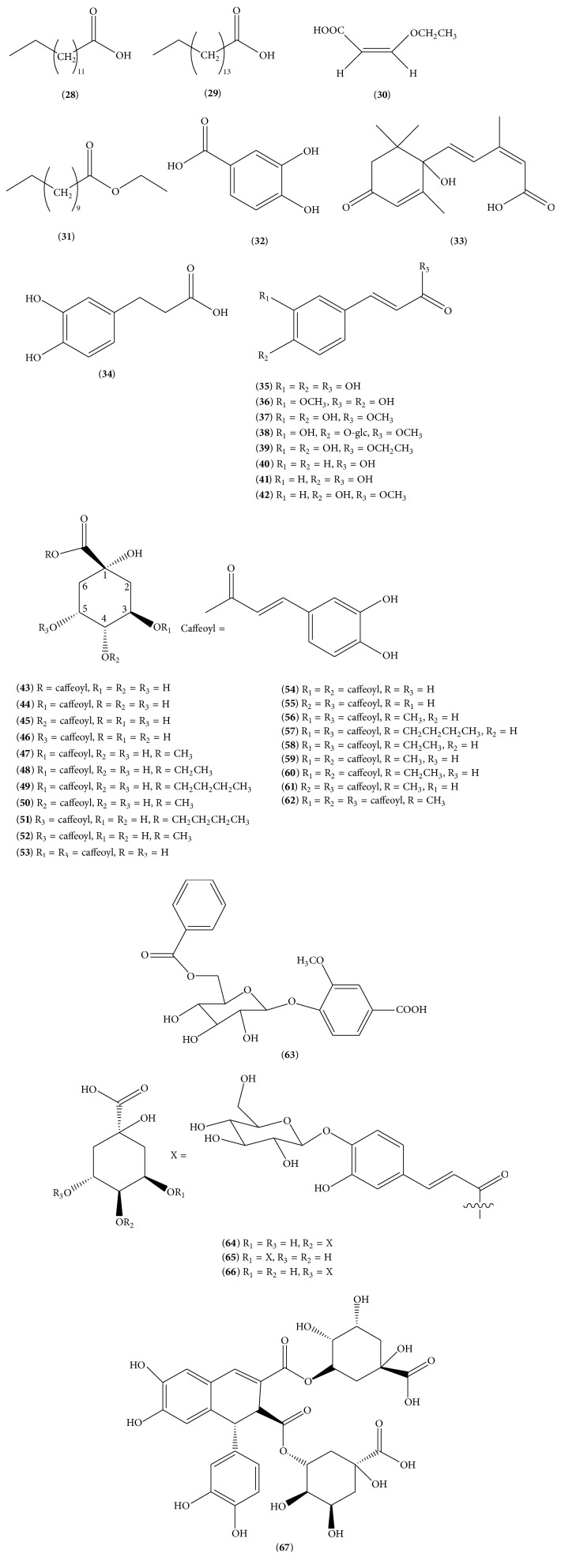
Just like many other herbs, LJF is also a rich source of organic acids, and until now, more than 40 organic acids (28–67) have been isolated from LJF, the structures of which are shown in Figure 2. The organic acids mainly include myristic acid (28), palmitic acid (29) [15], 2(E)-3-ethoxy acrylic acid (30) [16, 17], ethyl laurate (31), protocatechuic acid (32) [18], abscisic acid (33) [19], 3-(3, 4-dihydroxyphenyl) propionic acid (34) [20], caffeic acid (35), ferulic acid (36) [11], caffeic acid methyl ester (37) [20], methyl 4-O-β-D-glucopyranosyl caffeate (38) [21], caffeic acid ethyl ester (39) [18], cinnamic acid (40) [22], 4-hydroxycinnamic acid (41), and methyl 4-hydroxycinnamate (42) [20]. About 20 caffeic acid derivatives are isolated from LJF extract, including 1-O-caffeoylquinic acid (43) [23], 3-O-caffeoylquinic acid (44) [24], 4-O-caffeoylquinic acid (45), 5-O-caffeoylquinic acid (46) [25], 3-O-caffeoylquinic acid methyl ester (47), 3-O-caffeoylquinic acid ethyl ester (48) [26, 27], 3-O-caffeoylquinic acid butyl ester (49) [28], 4-O-caffeoylquinic acid methyl ester (50) [21], 5-O-caffeoylquinic acid butyl ester (51), 5-O-caffeoylquinic acid methyl ester (52), 3,5-O-dicaffeoylquinic acid (53), 3,4-O-dicaffeoylquinic acid (54), 4,5-O-dicaffeoylquinic acid (55), 3,5-O-dicaffeoylquinic acid methyl ester (56) [26, 27], 3,5-O-dicaffeoylquinic acid butyl ester (57) [29], 3,5-O-dicaffeoylquinic acid ethyl ester (58), 3,4-O-dicaffeoylquinic acid methyl ester (59), 3,4-O-dicaffeoylquinic acid ethyl ester (60), 4,5-O-dicaffeoylquinic acid methyl ester (61) [30], and 3,4,5-O-tricaffeoylquinic acid (62) [27]. In addition, some special organic acids, such as vanillic acid, 4-O-β-D-(6-O-benzoylglucopyranoside) (63) [26], (−)-4-O-(4-O-β-D-glucopyranosylcaffeoyl) quinic acid (64), (−)-3-O-(4-O-β-D-glucopyranosylcaffeoyl) quinic acid (65), (−)-5-O-(4-O-β-D-glucopyranosylcaffeoyl) quinic acid (66) [31], and dichlorogelignate (67) [21], have been obtained.
Figure 2.
The structures of 40 organic acids (28)–(67).
2.3. Iridoids
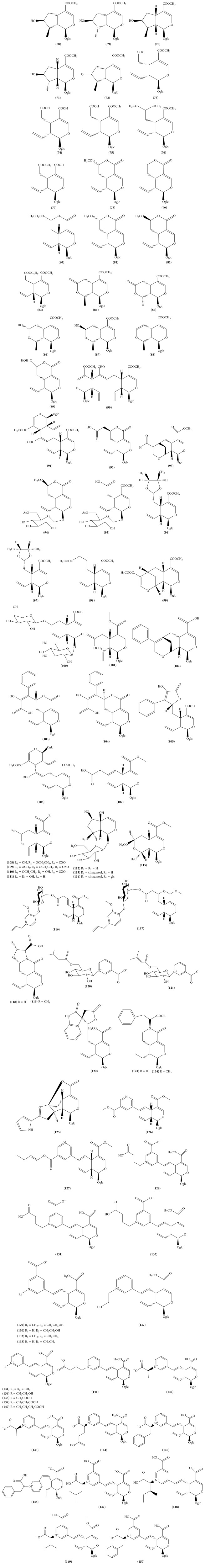
Iridoids are the most abundant compounds in LJF. Thus far, more than 83 iridoids have been isolated from LJF, which can be classified into three classes: iridoid glucosides, secoiridoid glycosides, and N-contained iridoid glycosides. Among them, six iridoid glucosides, such as loganin (68), 8-epiloganin (69), loganic acid (70), 8-epiloganic acid (71), and ketologanin (72) [32], have been isolated from LJF. Meanwhile, 47 secoiridoid glycosides (73–117) also are identified from LJF, including secologanin (73), secologanoside (74), secoxyloganin (75) [19], secologanin dimethyl acetal (76) [32], secologanoside-7-methyl ester (77) [33], secologanic acid (78), sweroside (79), 7-O-ethylsweroside (80), vogeloside (81), 7-epi-vogeloside (82) [25], secoxyloganin-7-butyl ester (83) [34], kingiside (84) [21], 8-epikingiside (85) [32], 7α-morroniside (86), 7β-morroniside (87) [21], dehydromorroniside (88) [35], 7-hydroxy-methyl-vogeloside (89) [17], (Z)-aldosecologanin (90), (E)-aldosecologanin (91) [32], loniaceticiridoside (92), lonimalondialiridoside (93) [36], 6′-O-acetylvogeloside (94), 6′-O-acetylsecoxyloganin (95) [22], loniceracetalide A (96), loniceracetalide B (97) [33], adinoside A (98), stryspinoside (99) [19], secologanoside A (100) [37], dimethyl secologanoside (101) [38], loniphenyruviridoside A~D (102–105) [39], centauroside (106) [23], loniceranan A (107), loniceranan B (108), loniceranan C (109), ethyl secologanoside (110), demethylsecologanol (111), harpagide (112), harpagoside (113), 6′′-O-β-glucopyranosylharpagoside (114), (7β)-7-O-methyl morroniside (115) [32], lonicerjaponin A (116), and lonicerjaponin B (117) [40]. 33 N-contained iridoid glycosides (118–150) have been isolated from LJF in recent years, including serinosecologanin (118), threoninosecologanin (119) [41], lonijaponinicotinosides A (120), lonijaponinicotinosides B (121) [42], lonijapospiroside A (122), L-phenylalaninosecologanin B (123), L-phenylalaninosecologanin C (124), and dehydroprolinoylloganin A (125) [30]. In 2013, Kashiwada et al. isolated two conjugates with a nicotinic acid derivative (126-127) [40]. Additionally, in 2008, Song et al. isolated three pyridinium alkaloid-coupled secoiridoids from an aqueous extract of the flower buds of Lonicera japonica, lonijaposides A–C (128–130) [43]. In 2011, Yu et al. isolated lonijaposides D–N (131–141) [39] and, in 2013, Yu et al. obtained lonijaposides O–W (142–150) from an aqueous extract of the flower buds of Lonicera japonica Thunb. [44]. The structures of 83 iridoids are listed in Figure 3.
Figure 3.
The structures of 83 iridoids (68)–(150).
2.4. Triterpenoids
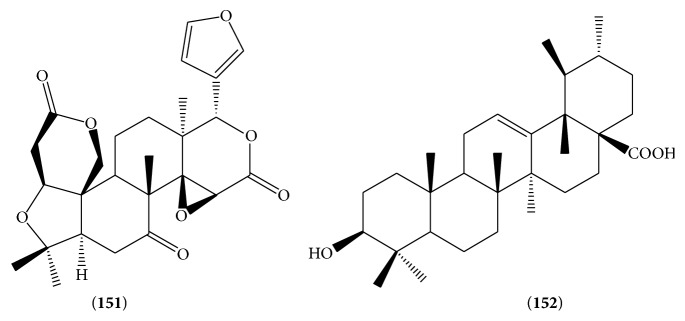
17 triterpenoids are found from LJF and their structures are listed in Figures 4 and 5 and Table 2, mainly including limonin (151) [45], ursolic acid (152) [22], and oleanolic acid triterpenoid saponins (153–156) and hederagenin triterpenoid saponins (157–167). Oleanolic acid triterpenoid saponins include oleanolic acid (153), 3-O-β-D-glucopyranosyl-(1→2)-α-L-arabinopyranosyl oleanolic acid-28-O-β-D-glucopyranosyl-(1→6)-β-D-glucopyranoside (154), oleanolic acid 28-O-α-L-rhamnopyranosyl-(1→2)-[β-D-xylopyranosyl(1→6)]-β-D-glucopyranosyl ester (155), loniceroside E (156), hederagenin 3-O-α-L-arabinopyranoside (157), loniceroside D (158), loniceroside A (159), loniceroside B (160), loniceroside C (161), 3-O-β-D-glucopyranosyl(1→4)-β-D-glucopyranosyl(1→3)-α-L-rhamnopyranosyl(1→2)-α-L-arabinopyranosyl-hederagenin-28-O-β-D-glucopyranosyl(1→6)-β-D-glucopyranosyl ester (162), hederagenin-3-O-α-L-rhamnopyranosyl(1→2)-α-L-arabinopyranoside (163), 3-O-α-L-rhamnopyranosyl(1→2)-α-L-arabinopyranosyl-hederagenin-28-O-β-D-xylopyranosyl(1→6)-β-D-glucopyranosyl ester (164), 3-O-α-L-rhamnopyranosyl(1→2)-α-L-arabinopyranosyl-hederagenin-28-O-β-D-glucopyranosyl(1→6)-β-D-glucopyranosyl ester (165), 3-O-α-L-rhamnopyranosyl(1→2)-α-L-arabinopyranosyl-hederagenin-28-O-β-D-rhamnopyranosyl(1→2)-[β-D-xylopyranosyl(1→6)]-β-D-glucopyranosyl ester (166), and 3-O-β-D-glucopyranosyl(1→3)-α-L-rhamnopyranosyl(1→2)-α-L-arabinopyranosyl-hederagenin-28-O-β-D-glucopyranosyl(1→6)-β-D-glucopyranosyl ester (167).
Figure 4.
The structures of compounds of (151)-(152).
Figure 5.
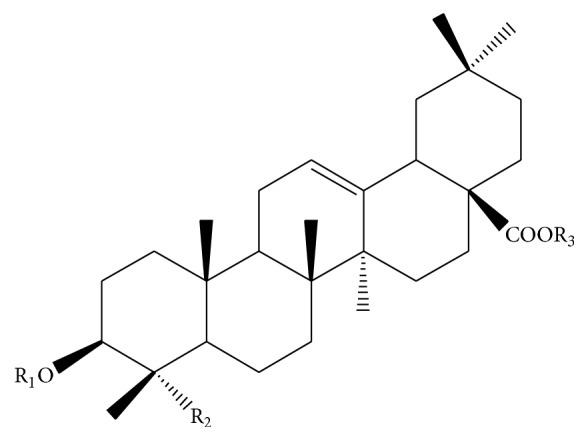
Skeleton of triterpenoids.
Table 2.
The structures of compounds (153)–(167) obtained from LJF.
| Comp. number | Substituent groups | References |
|---|---|---|
| (153) | R1 = R3 = H, R2 = CH3 | [28] |
| (154) | R1 = glc-(1→2)-ara, R2 = CH3
R3 = glc-(1→6)-glc |
[61] |
| (155) | R1 = H, R2 = CH3
R3 = rha-(1→2)-[xyl-(1→6)]-glc |
[55] |
| (156) | R1 = glc, R2 = CH3, R3 = rha(1→2)[xyl(1→6)]glc | [24, 63] |
| (157) | R1 = ara, R2 = CH2OH, R3 = H | [55] |
| (158) | R1 = glc, R2 = CH2OH, R3 = glc(1→2)[xyl(1→6)]glc | [24, 63] |
| (159) | R1 = ara, R2 = CH2OH, R3 = rha(1→2)[xyl(1→6)]glc | [24, 63] |
| (160) | R1 = rha, R2 = CH2OH, R3 = rha(1→2)[xyl(1→6)]glc | [24] |
| (161) | R1 = glc, R2 = CH2OH, R3 = rha(1→2)[xyl(1→6)]glc | [24, 63] |
| (162) | R1 = glc(1→4)glc(1→3)rha(1→2)ara, R2 = CH2OH, R3 = glc(1→6)glc | [64] |
| (163) | R1 = rha(1→2)ara, R2 = CH2OH, R3 = H | [64] |
| (164) | R1 = rha(1→2)ara, R2 = CH2OH, R3 = xyl(1→6)glc | [64] |
| (165) | R1 = rha(1→2)ara, R2 = CH2OH, R3 = glc(1→6)glc | [64] |
| (166) | R1 = rha(1→2)ara, R2 = CH2OH, R3 = rha(1→2)xyl(1→6)glc | [64] |
| (167) | R1 = glc(1→3)rha(1→2)ara, R2 = CH2OH, R3 = glc(1→6)glc | [64] |
2.5. Volatile Oils
Volatile oils, one of the important effective constituents of LJF, play a significant role on the pharmacological effects, which are also used in cosmetics, spices, and other industries. There are some differences of volatile oils components between different groups and different germplasms, mainly including alkylation, alcohol, alkene, and ketone. Du et al. [46] identified 35 volatile constituents in LJF from Guangxi Zhuang Autonomous Region, mainly including methyl linolenate, n-hexadecanoic acid, and ε-muurolene, and 18 volatile constituents in LJF from Hunan province, mainly including n-hexadecanoic acid, linoleic acid, and α-curcumene. Yang and Zhao [47] identified 49 volatile constituents in LJF from Ningxia province; three major components are linalool (13.59%), carvacrol (7.67%), and dibutyl phthalate (7.54%). Guan et al. [48] investigated the chemical constituents of essential oil in the fresh and dried buds of LJF “Jiu Feng 1,” and 44 volatile constituents were identified from the fresh buds, mainly including lower boiling point chemical compounds, such as linalool (5.21%), farnesol (2.60%), ascorbyl dipalmitate (9.49%), and nonacosane (17.38%), and 49 volatile constituents from the dried buds, mainly including higher boiling point chemical compounds, such as methyl hexadecanoate (13.99%) and methyl linolenate (9.20%). This may be chemical constituent changes from fresh buds and dried buds caused by different natural drying process. In addition, methods of extraction can also affect the class and content of the volatile oils. Du et al. [49] extracted volatile oils from LJF using steam distillation and fresh flowers homogenate extraction, respectively, and then extracted by diethyl ether and analyzed constituents by GC-MS. Results show that volatile oils extracted by fresh flowers homogenate extraction mainly include benzenepropanal (12.4%), ethylbenzene (8.58%), benzaldehyde (8.04%), linalool oxide trans (4.72%), and isophytol (2.94%), and volatile oils extracted by steam distillation mainly include cyclohexanol (8.06%), oxalic acid, cyclohexyl isobutyl ester (3.45%), cyclohexane-cyclopentylmethyl (18.35%), n-hexadecanoic acid (12.56%), and benzene cyclohexylmethyl (9.77%). The result shows that there are great differences between the compositions of volatile oils before and after heat treatment, which provides a new way of thinking for the use of fresh buds of LJF.
2.6. Others
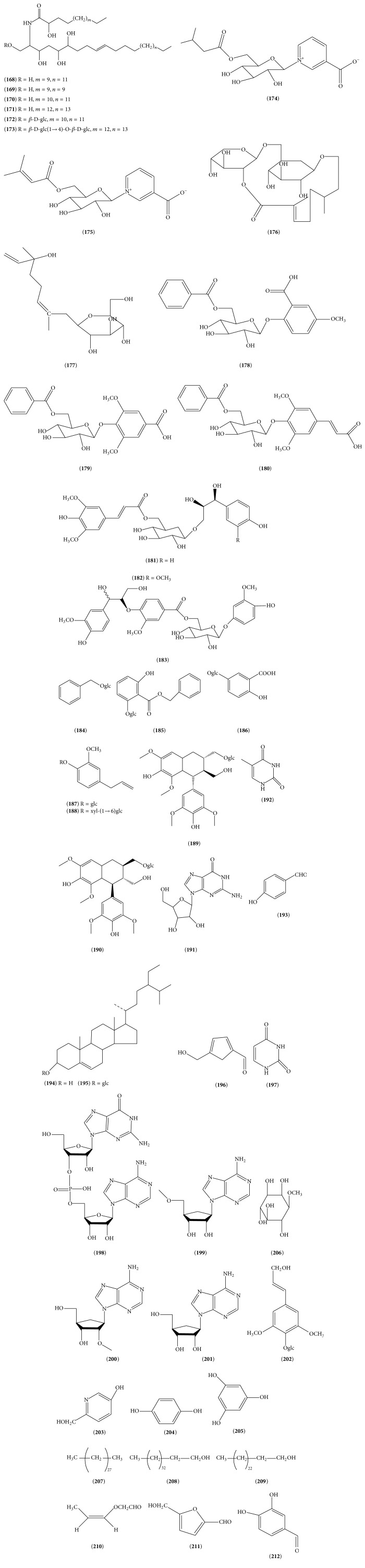
Other chemical constituents other than organic acids, flavonoids, iridoids, triterpenoids, and volatile oils were also found in LJF. In 2006, Kumar et al. [50] isolated six novel cerebrosides, lonijaposides A1–A4, B1, and B2 (168–173) from the flowers of Lonicera japonica Thunb. In 2008, Song [51] identified two nicotinic acids N-glycosides, (+)-N-(3-methybutyryl-β-D-glucopyranoyl)-nicotinate (174) and (+)-N-(3-methybut-2-enoyl-β-D-glucopyranoyl)-nicotinate (175). In 2008, Wang [52] isolated (2E)-(6S)-8-[α-L-arabinopyranosyl-(1′′ → 6′)-β-D-glucopyranosyloxy]-2,6-dimethyloct-2-eno-1,2′′-lactone (176) and 2,6-dimethyl-6-hydroxyl-2,7-diene-1-octyl alcoholglucopyranoside (177) from the flowers of Lonicera japonica. In 2013, Wang et al. [53] isolated six new glycosides from the flower buds of Lonicera japonica Thunb. These are (−)-2-hydroxy-5-methoxybenzoic acid 2-O-β-D-(6-O-benzoyl)-glucopyranoside (178), (−)-4-hydroxy-3,5-dimethoxybenzoic acid 4-O-β-D-(6-O-benzoyl)-glucopyranoside (179), (−)-(E)-3,5-dimethoxyphenylpropenoic acid 4-O-β-D-(6-O-benzoyl)-glucopyranoside (180), (−)-(7S,8R)-4-hydroxyphenylglycerol 9-O-β-D-[6-O-(E)-4-hydroxy-3,5-dimethoxyphenylpropenoyl]-glucopyranoside (181), (−)-(7S,8R)-4-hydroxy-3-methoxyphenylglycerol 9-O-β-D-[6-O-(E)-4-hydroxy-3,5-dimethoxyphenylpropenoyl]-glucopyranoside (182), and (−)-4-hydroxy-3-methoxyphenol β-D-{6-O-[4-O-(7S,8R)-(4-hydroxy-3-methoxyphenylglycerol-8-yl)-3-methoxybenzoyl]}-glucopyranoside (183). At the same year, Wang et al. [19] isolated benzyl alcohol β-D-glucoside (184), benzyl 2-O-β-D-glucopyranosyl-2,6-dihydroxybenzoate (185), gentisic acid 5-O-β-D-glucopyranoside (186), eugenyl β-D-glucopyranoside (187), eugenyl β-D-xylopyranosyl-(1→6)-β-D-glucopyranoside (188), (−)-lyoniresinol 9-O-β-D-glucopyranoside (189), (+)-lyoniresinol 9-O-β-D-glucopyranoside (190), guanosine (191), 5-methyluracil (192), p-hydroxybenzaldehyde (193), β-sitosterol (194), daucosterol (195), 5-hydroxymethyl-2-furancarboxaldehyde (196), and uracil (197) from LJF. In 2015, Yu et al. [21] identified guanosinyl-(3′ → 5′)-adenosine monophosphate (198), 5′-O-methyladenosine (199), 2′-O-methyladenosine (200), adenosine (201), syringing (202), and 6-hydroxymethyl-3-pyridinol (203). Additionally, P-hydroxy-phenol (204), 1,2,4-benzenetriol (205) [20], 1-O-methyl-myo-inositol (206), nonacontane (207) [28], pentatriaconta alcohol (208), pentacosa alcohol (209), 2-(2-propenyloxy)-ethanal (210) [54], 5-hydroxymethyl-2-furfural (211) [55], and 3,4-dihydroxybenzaldehyde (212) [15] were also isolated. The structures of compounds of (168)–(212) are shown in Figure 6.
Figure 6.
The structures of compounds of (168)–(212).
Certainly, some other constituents, such as proteins and amino acids, have been obtained from LJF, which were also found to be rich in metal elements, such as Ca, Mg, Mn, Cu, Fe, and Zn [56, 57].
3. Discussion and Conclusion
LJF is a widely used medicine which has been demonstrated to be useful for the treatment and prevention of severe acute respiratory syndromes, H1N1 influenza and hand-foot-and-mouth disease. The present review summarizes the chemical constituents of LJF found in recent years, and the results indicate that more than 212 components have been identified from extracts of LJF, which contain 27 flavonoids, 40 organic acids, 83 iridoids, 17 triterpenoids, and 45 other compounds. We can clearly see that LJF has complex chemical composition resulting in good clinical efficacy due to the interactions among the components. However, only chlorogenic acid and luteoloside are used as biomarkers in Chinese Pharmacopoeia in 2015 edition for evaluating the quality of LJF. At a certain stage, it cannot comprehensively inflect the quality of LJF and further studies of chemical constituents and pharmacological effects of LJF ought to be conducted, which could lay a foundation for the further application of LJF.
Acknowledgments
This work was financially supported by the National Science and Technology Pillar Program during the Twelfth Five-Year Plan Period (no. 2011BAI06B01) and the specialized fund for the dependent innovation of Shandong Province (2013CXC20401).
Competing Interests
The authors report no competing interests.
References
- 1.Shang X., Pan H., Li M., Miao X., Ding H. Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. Journal of Ethnopharmacology. 2011;138(1):1–21. doi: 10.1016/j.jep.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang O.-H., Choi J.-G., Lee J.-H., Kwon D.-Y. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-κB and MAPKs activation pathways in HMC-1 cells. Molecules. 2010;15(1):385–398. doi: 10.3390/molecules15010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y., Wang G. L. Effect of Flos Lonicerae extracts on herpes simplex keratitis. Herald of Medicine. 2011;30(11):1421–1424. doi: 10.3870/yydb.2011.11.006. [DOI] [Google Scholar]
- 4.Tian J., Che H., Ha D., Wei Y., Zheng S. Characterization and anti-allergic effect of a polysaccharide from the flower buds of Lonicera japonica . Carbohydrate Polymers. 2012;90(4):1642–1647. doi: 10.1016/j.carbpol.2012.07.044. [DOI] [PubMed] [Google Scholar]
- 5.Ning W., Peng X., Ma L., et al. Enhanced secondary metabolites production and antioxidant activity in postharvest Lonicera japonica Thunb. in response to UV radiation. Innovative Food Science and Emerging Technologies. 2012;13:231–243. doi: 10.1016/j.ifset.2011.10.005. [DOI] [Google Scholar]
- 6.Han J. M., Kim M. H., Choi Y. Y., Lee H., Hong J., Yang W. M. Effects of Lonicera japonica Thunb. on type 2 diabetes via PPAR-γ activation in rats. Phytotherapy Research. 2015;29(10):1616–1621. doi: 10.1002/ptr.5413. [DOI] [PubMed] [Google Scholar]
- 7.National Health and Family Planning Commission ofthe People's Republic of China. Diagnostic and Treatment Protocol for Human Infections with Avian Influenza A (H7N9), 2014, http://www.moh.gov.cn/yzygj/s3593g/201401/3f69 fe196ecb4cfc8a2d6d96182f8b22.shtml.
- 8.Liu N., Lin X. F., Zuo J. L., Ye Z. Z., Huang X. P. Treatment of 70 cases of SARS clinical observation by clear away heat and toxic material. Journal of Sichuan Traditional Chinese Medicine. 2005;23(3):54–57. [Google Scholar]
- 9.Pan Z. Z., Wang X. F., Yan L. J., Nan C. H., Yue Z. J. Inhibitory effect of extracts from Lonicerae on influena A virus FM1 strain in vitro. Chinese Journal of Information on TCM. 2007;14(6):37–38. [Google Scholar]
- 10.Wang X. Y. Study on health beverage of honeysuckle and mungbean skin fiber. Food Research and Development. 2013;34(10):65–68. [Google Scholar]
- 11.Jeong Y. T., Jeong S. C., Hwang J. S., Kim J. H. Modulation effects of sweroside isolated from the Lonicera japonica on melanin synthesis. Chemico-Biological Interactions. 2015;238:33–39. doi: 10.1016/j.cbi.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Shen T. C., Cheng Q. Q., Shan W. G., Tang L. Study on preparation of Lonicerae japonicae flos effervescent tables for beverage. Science and Technology of Food Industry. 2012;33(12):255–258. [Google Scholar]
- 13.Chu X. B., Yan B. X. Application and prospect of medicinal ornamental plant Lonicera in the landscape field. Journal of Anhui Agricultural Sciences. 2014;13:3854–3862. doi: 10.3969/j.issn.0517-6611.2014.13.030. [DOI] [Google Scholar]
- 14.Li Y., Cai W., Weng X., et al. Lonicerae japonicae flos and Lonicerae flos: a systematic pharmacology review. Evidence-Based Complementary and Alternative Medicine. 2015;2015:16. doi: 10.1155/2015/905063.905063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang L. Y., Lv Z. Z., Li J. B., Zhou B. N. Studies on the chemical constituents of Japanese honeysuekle. Chinese Traditional and Herbal Drugs. 1996;27(11):645–647. [Google Scholar]
- 16.Bi Y. F., Tian Y., Pei S. S., Zhang C. The chemical constituents of Lonicera japonica Thunb. Journal of Zhengzhou University (Natural Science Edition) 2007;39(2):184–186. [Google Scholar]
- 17.Tian Y. Study on chemical constituents of Lonicera japonica thunb [M.S. dissertation] Zhengzhou, China: Zhengzhou University; 2007. [Google Scholar]
- 18.Jiang N. H. Study on chemical constituents of Lonicera japonica bud. Journal of Chinese Medicinal Materials. 2015;38(2):315–317. [PubMed] [Google Scholar]
- 19.Wang F., Jiang Y. P., Wang X. L., Lin S., Pu P. B., Zhu C. G. Chemical constituents from flower buds of Lonicera japonica . China Journal of Chinese Materia Medica. 2013;38(9):1378–1384. [PubMed] [Google Scholar]
- 20.Feng W.-S., Chen X., Zheng X.-K., Zhang C.-L., Li D.-D. Study on chemical constituents of Lonicerae japonicae flos. Chinese Pharmaceutical Journal. 2011;46(5):338–340. [Google Scholar]
- 21.Yu Y., Song W. X., Guo Q. L., et al. Studies on chemical constituents of aqueous extract of Lonicera japonica flower buds. The Journal of Chinese Medicine & Traditional Chinese Medicine. 2015;40(17):3496–3504. [PubMed] [Google Scholar]
- 22.Xu D. D., Jiang X. Z., Gao Y., Zheng W., He M., Gao Q. P. Studies on chemical constituents of effective fraction of Honeysuckle on inhibition of Escherichia coli biofilms. Chinese Journal of Experimental Traditional Medical Formulae. 2012;18(20):122–125. [Google Scholar]
- 23.Bai X., Huang H. F., Wu X. H., Chi L. L. Analysis on chemical constituents in Lonicerae japonicae flos by HPLC-DAD-MS/MS. Food Drug. 2015;17(1):5–8. [Google Scholar]
- 24.Lin L.-M., Zhang X.-G., Zhu J.-J., Gao H.-M., Wang Z.-M., Wang W.-H. Two new triterpenoid saponins from the flowers and buds of Lonicera japonica . Journal of Asian Natural Products Research. 2008;10(10):925–929. doi: 10.1080/10286020802217366. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L. Y., Li Y. B., Li L. X., Wang Y. M., Jin J. Chemical constituent of Lonicera japonica Thunb. by RRLC-O-TOF/MS. Central South Pharmacy. 2012;10(3):204–208. doi: 10.3969/j.issn.1672-2981.2012.03.014. [DOI] [Google Scholar]
- 26.Lee E. J., Kim J. S., Kim H. P., Lee J.-H., Kang S. S. Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities. Food Chemistry. 2010;120(1):134–139. doi: 10.1016/j.foodchem.2009.09.088. [DOI] [Google Scholar]
- 27.Ma S. C., Bi P. X., Huang R. C., Lee S. H., Lee S. F. Determination of the antiviral caffeoyl quinic acids isolaetd from Lonicera japonica Thunb. Chinese Journal of Pharmaceutical Analysis. 2005;25(7):751–755. [Google Scholar]
- 28.Wang Q. Study on chemical constituents of Lonicare japonica flos [M.S. thesis] Shenyang, China: Shenyang Pharmaceutical University; 2008. [Google Scholar]
- 29.Peng L.-Y., Mei S.-X., Jiang B., Zhou H., Sun H.-D. Constituents from Lonicera japonica . Fitoterapia. 2000;71(6):713–715. doi: 10.1016/s0367-326x(00)00212-4. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Z.-F., Zhang Q.-J., Chen R.-Y., Yu D.-Q. Four new N-contained iridoid glycosides from flower buds of Lonicera japonica . Journal of Asian Natural Products Research. 2012;14(8):729–737. doi: 10.1080/10286020.2012.688038. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y., Jiang Z. B., Song W. X., et al. Glucosylated caffeoylquinic acid derivatives from the flower buds of Lonicera japonica . Acta Pharmaceutica Sinica B. 2015;5(3):210–214. doi: 10.1016/j.apsb.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z.-X., Liu C.-T., Liu Q.-B., et al. Iridoid glycosides from the flower buds of Lonicera japonica and their nitric oxide production and α-glucosidase inhibitory activities. Journal of Functional Foods. 2015;18, article 1255:512–519. doi: 10.1016/j.jff.2015.08.017. [DOI] [Google Scholar]
- 33.Kakuda R., Imai M., Yaoita Y., Machida K., Kikuchi M. Secoiridoid glycosides from the flower buds of Lonicera japonica . Phytochemistry. 2000;55(8):879–881. doi: 10.1016/s0031-9422(00)00279-x. [DOI] [PubMed] [Google Scholar]
- 34.Song Y., Li S.-L., Wu M.-H., Li H.-J., Li P. Qualitative and quantitative analysis of iridoid glycosides in the flower buds of Lonicera species by capillary high performance liquid chromatography coupled with mass spectrometric detector. Analytica Chimica Acta. 2006;564(2):211–218. doi: 10.1016/j.aca.2006.01.068. [DOI] [Google Scholar]
- 35.Li H. J., Li P., Wang M. C., Ye W. C. A new secoiridoid glucoside from Lonicera japonica . Chinese Journal of Natural Medicines. 2003;1(3):132–133. [Google Scholar]
- 36.Song W.-X., Guo Q.-L., Yang Y.-C., Shi J.-G. Two homosecoiridoids from the flower buds of Lonicera japonica . Chinese Chemical Letters. 2015;26(5):517–521. doi: 10.1016/j.cclet.2014.11.035. [DOI] [Google Scholar]
- 37.Li C., Dai Y., Zhang J.-B., Liu M.-L., Yao X.-S. A new iridoid glycoside from Lonicerae flos. Chinese Traditional and Herbal Drugs. 2013;44(21):2951–2954. doi: 10.7501/j.issn.0253-2670.2013.21.001. [DOI] [Google Scholar]
- 38.Ma S. C., Paul B. X., Huang R. C., Lee S. H., Lee S. F. Determination of the antiviral Caeffoyl quinie acids isolated from Lonicera japonica Thunb. Chinese Journal of Pharmaceutical Analysis. 2005;25(7):751–755. [Google Scholar]
- 39.Yu Y., Song W., Zhu C., et al. Homosecoiridoids from the flower buds of Lonicera japonica . Journal of Natural Products. 2011;74(10):2151–2160. doi: 10.1021/np2004566. [DOI] [PubMed] [Google Scholar]
- 40.Kashiwada Y., Omichi Y., Kurimoto S.-I., et al. Conjugates of a secoiridoid glucoside with a phenolic glucoside from the flower buds of Lonicera japonica Thunb. Phytochemistry. 2013;96:423–429. doi: 10.1016/j.phytochem.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Song W.-X., Yang Y.-C., Shi J.-G. Two new β-hydroxy amino acid-coupled secoiridoids from the flower buds of Lonicera japonica: isolation, structure elucidation, semisynthesis, and biological activities. Chinese Chemical Letters. 2014;25(9):1215–1219. doi: 10.1016/j.cclet.2014.05.037. [DOI] [Google Scholar]
- 42.Jiang Z.-B., Song W.-X., Shi J.-G. Two 1-(6′-O-acyl-β-d-glucopyranosyl)pyridinium-3-carboxylates from the flower buds of Lonicera japonica . Chinese Chemical Letters. 2015;26(1):69–72. doi: 10.1016/j.cclet.2014.10.011. [DOI] [Google Scholar]
- 43.Song W. X., Li S., Wang S. J., et al. Pyridinium alkaloid-coupled secoiridoids from the flower buds of Lonicera japonica . Journal of Natural Products. 2008;71(5):922–925. doi: 10.1021/np800040k. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y., Zhu C. G., Wang S. J., Song W. X., Yang Y. C., Shi J. G. Homosecoiridoid alkaloids with amino acid units from the flower buds of Lonicera japonica . Journal of Natural Products. 2013;76(12):2226–2233. doi: 10.1021/np4005773. [DOI] [PubMed] [Google Scholar]
- 45.Zheng Z. F. Studies on chemical constituents and bioactivities of Lonicera japonica and Morus australia [Ph.D. thesis] Beijing, China: Chinese Academy of Medical Science & Peking Union Medical College; 2010. [Google Scholar]
- 46.Du C. Z., Feng X., Wang H. Analysis of volatile constituents in Lonicera japonica Thunb. from different originals by GC-MS. Agricultural Science & Technology. 2015;5:1081–1083. [Google Scholar]
- 47.Yang M. L., Zhao Y. G. Analysis of volatile components of Honeysuckle in Ningxia by GC-MS. Journal of Zhengzhou University. (Natural Science Edition) 2006;38(1):95–97. doi: 10.3969/j.issn.1671-6841.2006.01.021. [DOI] [Google Scholar]
- 48.Guan R. W., Wang L., Qu Y. S., et al. Volatile constituents in Lonicarae japonicae flos ‘Jiu feng yi hao’ by GC-MS. Chinese Traditional Patent Medicine. 2014;36(11):2367–2371. doi: 10.3969/j.issn.1001-1528.2014.11.032. [DOI] [Google Scholar]
- 49.Du H. F., Zhang Y., Weng D. Q., Yang D. J. GC-MS analysis of volatile oils extracted from fresh Lonicarae japonicae flos by different methods. Chongqing Journal of Research on Chinese Drugs and Herbs. 2009;2:13–17. [Google Scholar]
- 50.Kumar N., Singh B., Gupta A. P., Kaul V. K. Lonijaposides, novel cerebrosides from Lonicera japonica . Tetrahedron. 2006;62(18):4317–4322. doi: 10.1016/j.tet.2006.02.070. [DOI] [Google Scholar]
- 51.Song W. X. Study the water-soluble chemical constituents of the flower buds of Lonicera japonica [Ph.D. thesis] Beijing, China: Chinese Academy of Medical Science & Peking Union Medical College; 2008. [Google Scholar]
- 52.Wang Y. Study the water-soluble chemical constituents of the flower buds of Lonicera japonica [M.S. thesis] Changsha, China: Human University of Traditional Chinese Medicine; 2008. [Google Scholar]
- 53.Wang F., Jiang Y.-P., Wang X.-L., et al. Aromatic glycosides from the flower buds of Lonicera japonica . Journal of Asian Natural Products Research. 2013;15(5):492–501. doi: 10.1080/10286020.2013.785531. [DOI] [PubMed] [Google Scholar]
- 54.Bi Y.-F., Tian Y., Pei S.-S., Liu H.-M. Secoiridoid glycosides from Flos Lonicerae . Chinese Traditional and Herbal Drugs. 2008;39(1):18–21. [Google Scholar]
- 55.Choi C.-W., Jung H. A., Kang S. S., Choi J. S. Antioxidant constituents and a new triterpenoid glycoside from flos Lonicerae . Archives of Pharmacal Research. 2007;30(1):1–7. doi: 10.1007/bf02977770. [DOI] [PubMed] [Google Scholar]
- 56.Han J.-T., Liu Y.-M., Wang H. Determination of eleven trace elements in chinese traditional and herbal drugs for relieving heat and toxic by FAAS. Spectroscopy and Spectral Analysis. 2006;26(10):1931–1934. [PubMed] [Google Scholar]
- 57.Lai H. Y., Wu Y. Y. Analysis of primary chemical speciation of nine trace elements in Lonicera japonica . Journal of Anhui Agricultural Sciences. 2013;41(28):11326–11353. [Google Scholar]
- 58.Song Y.-L., Wang H.-M., Ni F.-Y., et al. Study on anti-inflammatory activities of phenolic acids from Lonicerae Japonicae Flos . Chinese Traditional and Herbal Drugs. 2015;46(4):490–495. doi: 10.7501/j.issn.0253-2670.2015.04.006. [DOI] [Google Scholar]
- 59.Li X.-Q., Sun X.-H., Cai S., Ying X.-X., Li F.-M. Investigation on the chemical constituents and variation of the flower buds of Lonicera species by UPLC-ESI-MS/MS and principle component analysis. Acta Pharmaceutica Sinica. 2009;44(8):895–904. [PubMed] [Google Scholar]
- 60.Chen Q. Z., Lin R. C., Wang G. L., Li F. M. Studies on chemical constituents of the extract of Lonicera japonica . Journal of Chinese Medicinal Materials. 2010;33(6):920–922. [PubMed] [Google Scholar]
- 61.Xing J. B., Li H. J., Li P., Liu Y. Studies on chemical constituents in dried buds of Lonicera japonica Thunb. Chinese Journal of New Drugs. 2002;11(11):856–859. [Google Scholar]
- 62.Cui C. Y., Liu Z. P., Zhou M., Su D. H., Tang X. Z. Chemical constituents of Lonicera japonica . Journal of Guangxi University: Natural Science Edition. 2012;37:530–533. [Google Scholar]
- 63.Zhang X. G. Chemical studies on the water-soluble fraction extracted from flos Lonicerae japonicae [M.S. thesis] Xianyang, China: Shannxi University of Chinese Medicine; 2007. [Google Scholar]
- 64.Chen C. X., Wang W. W., Ni W., Chen N. Y., Zhou J. Triterpenoid glycosides from the Lonicera japonica . Acta Botanica Yunnanica. 2000;22:201–208. [Google Scholar]