Abstract
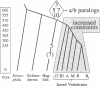
Diversification of the Wnt genes, a family of powerful developmental regulator molecules, is inferred by molecular evolutionary analyses. Fifty-five recently determined partial sequences from a variety of vertebrates and invertebrates, together with 17 published sequences, mostly from the mouse and Drosophila melanogaster, are analyzed. Wnt-1 through -7 originated before the last common ancestor of arthropods and deuterostomes lived. Another round of gene duplication, involving Wnt-3, -5, -7, and -10, occurred after the echinoderm lineage arose, on the ancestral lineage of jawed vertebrates. Increased constraints were imposed on the Wnt genes when jawed vertebrates originated, as indicated by an overall 4-fold lower rate of amino acid replacements in jawed vertebrates compared with invertebrates and jawless vertebrates. The Wnt genes are thus inferred to have undergone a disproportionately high amount of structural and functional evolution in the relatively short time (approximately 100 million years) between the origin of the echinoderm lineage and the first diversification of jawed vertebrates. A model is presented for the relationship of functional diversification of developmental regulators and their rates of amino acid replacement.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. E. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987 Jun;6(6):1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton M. J. Phylogeny of the major tetrapod groups: morphological data and divergence dates. J Mol Evol. 1990 May;30(5):409–424. doi: 10.1007/BF02101113. [DOI] [PubMed] [Google Scholar]
- Christian J. L., Gavin B. J., McMahon A. P., Moon R. T. Isolation of cDNAs partially encoding four Xenopus Wnt-1/int-1-related proteins and characterization of their transient expression during embryonic development. Dev Biol. 1991 Feb;143(2):230–234. doi: 10.1016/0012-1606(91)90073-c. [DOI] [PubMed] [Google Scholar]
- Duboule D., Dollé P. The structural and functional organization of the murine HOX gene family resembles that of Drosophila homeotic genes. EMBO J. 1989 May;8(5):1497–1505. doi: 10.1002/j.1460-2075.1989.tb03534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. J Mol Evol. 1981;17(6):368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Gavin B. J., McMahon J. A., McMahon A. P. Expression of multiple novel Wnt-1/int-1-related genes during fetal and adult mouse development. Genes Dev. 1990 Dec;4(12B):2319–2332. doi: 10.1101/gad.4.12b.2319. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Kishino H., Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22(2):160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Hein J. A new method that simultaneously aligns and reconstructs ancestral sequences for any number of homologous sequences, when the phylogeny is given. Mol Biol Evol. 1989 Nov;6(6):649–668. doi: 10.1093/oxfordjournals.molbev.a040577. [DOI] [PubMed] [Google Scholar]
- Kishino H., Hasegawa M. Evaluation of the maximum likelihood estimate of the evolutionary tree topologies from DNA sequence data, and the branching order in hominoidea. J Mol Evol. 1989 Aug;29(2):170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Moon R. T. Ectopic expression of the proto-oncogene int-1 in Xenopus embryos leads to duplication of the embryonic axis. Cell. 1989 Sep 22;58(6):1075–1084. doi: 10.1016/0092-8674(89)90506-0. [DOI] [PubMed] [Google Scholar]
- McMahon J. A., McMahon A. P. Nucleotide sequence, chromosomal localization and developmental expression of the mouse int-1-related gene. Development. 1989 Nov;107(3):643–650. doi: 10.1242/dev.107.3.643. [DOI] [PubMed] [Google Scholar]
- Molven A., Njølstad P. R., Fjose A. Genomic structure and restricted neural expression of the zebrafish wnt-1 (int-1) gene. EMBO J. 1991 Apr;10(4):799–807. doi: 10.1002/j.1460-2075.1991.tb08012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer J., Meijlink F., Verrijzer P., Rijsewijk F., Destrée O. Isolation of the Xenopus homolog of int-1/wingless and expression during neurula stages of early development. Nucleic Acids Res. 1989 Jan 11;17(1):11–18. doi: 10.1093/nar/17.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Brown A., Papkoff J., Scambler P., Shackleford G., McMahon A., Moon R., Varmus H. A new nomenclature for int-1 and related genes: the Wnt gene family. Cell. 1991 Jan 25;64(2):231–231. doi: 10.1016/0092-8674(91)90633-a. [DOI] [PubMed] [Google Scholar]
- Patel N. H., Schafer B., Goodman C. S., Holmgren R. The role of segment polarity genes during Drosophila neurogenesis. Genes Dev. 1989 Jun;3(6):890–904. doi: 10.1101/gad.3.6.890. [DOI] [PubMed] [Google Scholar]
- Pesole G., Bozzetti M. P., Lanave C., Preparata G., Saccone C. Glutamine synthetase gene evolution: a good molecular clock. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):522–526. doi: 10.1073/pnas.88.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987 Aug 14;50(4):649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Roelink H., Nusse R. Expression of two members of the Wnt family during mouse development--restricted temporal and spatial patterns in the developing neural tube. Genes Dev. 1991 Mar;5(3):381–388. doi: 10.1101/gad.5.3.381. [DOI] [PubMed] [Google Scholar]
- Roelink H., Wagenaar E., Lopes da Silva S., Nusse R. Wnt-3, a gene activated by proviral insertion in mouse mammary tumors, is homologous to int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4519–4523. doi: 10.1073/pnas.87.12.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Expression of the proto-oncogene int-1 is restricted to postmeiotic male germ cells and the neural tube of mid-gestational embryos. Cell. 1987 Jul 3;50(1):89–95. doi: 10.1016/0092-8674(87)90665-9. [DOI] [PubMed] [Google Scholar]
- Smith W. C., Harland R. M. Injected Xwnt-8 RNA acts early in Xenopus embryos to promote formation of a vegetal dorsalizing center. Cell. 1991 Nov 15;67(4):753–765. doi: 10.1016/0092-8674(91)90070-f. [DOI] [PubMed] [Google Scholar]
- Sokol S., Christian J. L., Moon R. T., Melton D. A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell. 1991 Nov 15;67(4):741–752. doi: 10.1016/0092-8674(91)90069-b. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990 Aug 30;346(6287):847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Wainwright B. J., Scambler P. J., Stanier P., Watson E. K., Bell G., Wicking C., Estivill X., Courtney M., Boue A., Pedersen P. S. Isolation of a human gene with protein sequence similarity to human and murine int-1 and the Drosophila segment polarity mutant wingless. EMBO J. 1988 Jun;7(6):1743–1748. doi: 10.1002/j.1460-2075.1988.tb03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- van Ooyen A., Nusse R. Structure and nucleotide sequence of the putative mammary oncogene int-1; proviral insertions leave the protein-encoding domain intact. Cell. 1984 Nov;39(1):233–240. doi: 10.1016/0092-8674(84)90209-5. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M., Nusse R., Johnston P., Lawrence P. A. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell. 1989 Nov 17;59(4):739–749. doi: 10.1016/0092-8674(89)90020-2. [DOI] [PubMed] [Google Scholar]