Abstract
Escherichia coli O157:H7 causes hemorrhagic colitis and life-threatening complications. Because healthy cattle are reservoirs for the bacterium, ruminant infection models have applications in analyzing the relationship between cattle and this human pathogen and in testing interventions to reduce or prevent bovine colonization with this bacterium. Current approaches often do not reliably mimic natural, long-term bovine colonization with E. coli O157:H7 in older calves and adult animals (ages that enter our food chain). Based on the recent identification of the bovine rectoanal junction mucosa as a site of E. coli O157:H7 colonization, we developed a novel rectal swab administration colonization model. We compared this method with oral dosing and direct contact transmission (Trojan) methods. E. coli O157:H7 carriage status was determined by fecal or rectoanal mucosa swab culture. High (∼1010 CFU) and low (∼107 CFU) oral doses of E. coli O157:H7 in sheep and cattle resulted in variable infection with the bacterium. Some animals became colonized with the bacteria and remained culture positive for several weeks, and some animals did not become colonized and rapidly cleared the bacteria in a few days. Pen mates of E. coli O157:H7 culture-positive Trojan cattle had a low infection rate and variable colonization status. However, rectal swab administration of E. coli O157:H7 to cattle resulted in consistent long-term colonization in all animals. The surprising ease with which long-term infections resulted from a single application of bacteria to the rectoanal mucosa also strongly supported this location as a site of E. coli O157:H7 colonization in cattle.
Escherichia coli O157:H7 is an enteric pathogen of humans that causes a spectrum of illnesses, including hemorrhagic colitis and renal failure, and can be fatal (14, 24, 29). Domestic cattle are an important reservoir of this pathogen and are the source for most food-borne infections (8). Experimental and field studies of E. coli O157:H7 in beef and dairy cattle have found within-group variation between individual animals with respect to occurrence and duration of fecal culture-positive status (2, 3, 6, 10, 15, 17, 20, 21, 27, 28). This variation suggests that host factors play an important role in E. coli O157:H7 colonization of cattle. A thorough understanding of the host-bacterium interaction could lead to the development of novel interventions, and in order to investigate interactions between the ruminant host and E. coli O157:H7 researchers must recreate infections in an experimental setting. Moreover, to evaluate the efficacy of traditional types of interventions, such as vaccinations (25), probiotics (4, 5), and antimicrobials, it is necessary to be able to reproduce the state of colonization.
The earliest publications of ruminant E. coli O157:H7 inoculation studies reported on the use of very young calves, either preweaned or early postweaning, receiving doses of 1010 CFU administered orally (6, 10) or through rumen cannulae (16, 30). An oral dose of 108 CFU given to four calves in one study resulted in all four being fecal culture positive for 20 to 45 days, but the calves were 1 week old at the start of the experiment (27) and lacked a developed ruminant gastrointestinal tract. When older fully ruminant animals are orally dosed, some animals have brief infections that likely reflect passage of the inocula rather than colonization. Four of eight 4-month-old calves given an oral dose of 1010 CFU became fecal culture negative after 7 days (23), and in another study, 9 of 18 yearling steers given 1010 CFU were fecal culture negative by day 9 of the study (7). In order to experimentally mimic natural, long-term colonization with E. coli O157:H7 in older calves and adults, a new method is needed.
A site of E. coli O157:H7 colonization has recently been identified as the mucosal lining of the rectoanal junction (22). This finding was supported by earlier observations that E. coli O157:H7 persists in the lower gastrointestinal tract of ruminants but is not detected in the upper gastrointestinal tract a few days after oral inoculation (7, 12) and the detection of the bacterium only in the distal rectal tissue in experimentally inoculated sheep (12). As a logical follow-up to these findings, our laboratory recently demonstrated that rectoanal mucosal swab (RAMS) culture is a more sensitive detection method than traditional fecal culture (26). We further reasoned that to establish reliably colonized animals, we would have greater success using a rectoanal inoculation route than an oral route.
In this study, we developed a novel rectal swab administration of E. coli O157:H7 to establish experimental colonization of ruminants with this bacterium. We compared this methodology with two other challenge strategies and various inoculation doses. The methods included: (i) oral dosing, (ii) direct contact transmission by copenning with culture-positive animals (Trojan model), and (iii) a novel rectal swab inoculation. Both sheep and cattle were used, and E. coli O157:H7 colonization status was determined by standard fecal or RAMS culture (26).
MATERIALS AND METHODS
Animals.
Cattle and sheep were healthy, had normal weight gains throughout the study, and had fully developed ruminant gastrointestinal tracts at the start of the experiments. Animals were housed in quarantined facilities in which feed, water, bedding, manure, and other waste were not in contact with any nonexperimental animals. All personnel followed strict biosafety procedures, and all procedures were approved by the Institutional Animal Care and Use and Biosafety Committees.
(i) Sheep.
Twenty-four 5-month-old cross-bred white-faced ram lambs were housed in groups of eight animals per pen. Lambs were fed pelleted alfalfa twice daily, with free access to drinking water.
(ii) Cattle.
Eighty-seven 5- to 8-month-old Holstein steers were housed in groups of three to five animals per pen except for cattle exposed to Trojans (see below), in which animals were in one large pen. Steers were fed alfalfa hay twice daily, with free access to drinking water. For procedures requiring animal restraint, a squeeze chute was used.
E. coli O157:H7 strains.
Strains used for the study were ATCC 43894 (American Type Culture Collection, Manassas, Va.) and Washington State University (WSU) isolates designated WSU 180, WSU 400, and WSU 588 (kindly provided by D. D. Hancock, WSU). E. coli O157:H7 ATCC 43894 is a human isolate, and WSU 180, WSU 400, and WSU 588 are naturally occurring bovine isolates. All strains possess the genes for Shiga toxin type 1 and type 2. E. coli O157:H7 cultures were grown in Luria-Bertani broth (Difco, Detroit, Mich.) and incubated with aeration at 37°C for 24 h. Adjustment to the desired inoculum number was made by dilution in phosphate-buffered saline and confirmed by viable plate count.
Challenge methods.
Three methods of introducing experimental infections with E. coli O157:H7 in ruminants were used.
(i) Oral inoculation.
The indicated number of E. coli O157:H7 cells were suspended in 10 to 60 ml of phosphate-buffered saline and placed directly into the caudal oral cavity of the experimental animals by using a disposable syringe. The single oral dose was immediately followed by 60 ml of water.
(ii) Inoculation by exposure to culture-positive Trojan animals.
Two or three steers were inoculated with a single oral dose of 1010 E. coli O157:H7 (the Trojan animals) and copenned with culture-negative steers. A similar direct contact transmission (Trojan) model was previously described by Besser et al. (3).
(iii) Rectal inoculation.
Feces were manually removed from the terminal rectum. A 10-cm by 3.5-cm-diameter cylindrical sponge (Rubbermaid, St. Francis, Wis.) with a wooden handle was saturated with 10 ml of the E. coli O157:H7 culture. The anal sphincter was relaxed by digital stimulation, and the sponge was inserted into the terminal rectum and gently and rapidly rubbed against the rectoanal junction mucosa. After swabbing, defecation was prevented for 10 min by holding the steer's tail firmly against the anus. This presumably ensured that the inoculum was retained in the rectal lumen for a sufficient period of time for bacteria to associate with the mucosa.
E. coli O157:H7 culture and analysis.
Fecal samples were aseptically acquired by rectal palpation or as an animal defecated (referred to as free catch) and held on ice during transport to the laboratory. RAMS samples were acquired as previously described, placed directly into Trypticase soy broth (TSB), and held on ice for transport to the laboratory (26). All samples were cultured within 3 h of collection. Samples were collected prior to inoculation (on day zero) and thereafter as indicated in Results. In addition, to investigate if E. coli O157:H7 differentially colonized dorsal or ventral sites, RAMS samples were obtained separately from the dorsal and ventral rectoanal junction mucosa without cross-contamination. The anal sphincter was digitally stimulated to open, and either the dorsal or ventral rectoanal junction mucosa was swabbed.
Fecal and RAMS samples were analyzed for E. coli O157:H7 by direct and enrichment culture as previously described (18, 26). Briefly, 10 g of feces was suspended in TSB (50 ml; BBL-Becton Dickinson) supplemented with cefixime (50 ng/ml; Wyeth-Ayerst, Pearl River, N.Y.; generously provided by D. D. Hancock, WSU), potassium tellurite (2.5 mg/liter; Sigma Chemical Co., St. Louis, Mo.), and vancomycin (40 mg/liter; Sigma) (referred to hereafter as TSB-CTV). Direct cultures were prepared from serial dilutions in sterile saline (0.15 M NaCl) and plated onto sorbitol MacConkey agar supplemented with 4-methylumbelliferyl-β-d-glucuronide (Biosynth AG, Staad, Switzerland), cefixime (50 μg/liter; Lederle Laboratories), potassium tellurite (2.5 mg/liter; Sigma Chemical Co.), and vancomycin (40 mg/liter; Sigma Chemical Co.) (SMAC-CTVM). Plates were incubated overnight at 37°C and observed for colonies not fermenting sorbitol (colorless colonies) and not hydrolyzing 4-methylumbelliferyl-β-d-glucuronide (no fluorescence at 363 nm). Presumptive E. coli O157:H7 colonies were confirmed by latex agglutination (Pro-Lab Diagnostics, Toronto, Canada). Positive cultures from this technique resulted in a quantitative measure of E. coli O157:H7 bacteria as CFU per gram of feces. Samples negative by direct culture were further analyzed by an enrichment procedure that entailed incubating the TSB-CTV flasks with aeration at 37°C for 18 h prior to plating on SMAC-CTM. RAMS samples were vortexed for 60 s, serially diluted, plated, and processed as described for fecal samples (26). RAMS samples that were negative by direct culture were enriched by incubation in TSB with aeration at 37°C for 18 h prior to plating. These enrichment procedures are similar to or more sensitive than standard immuno-magnetic bead separation culture techniques (13, 26). Animals were considered culture positive if a fecal or RAMS sample was culture positive by either direct or enrichment techniques.
Necropsy.
Animals inoculated by rectal application of bacteria and that remained colonized for 60 days were necropsied. After euthanasia, three to six samples of tissue, digesta, and swabs of the mucosal surface were taken aseptically from the rumen, ascending colon, descending colon, anterior and posterior to the spiral colon, anterior and posterior rectum, and the rectoanal junction. Samples were collected aseptically and placed in sterile whirl-pak bags (NASCO, New Haven, Conn.) and kept on ice until processing. Five grams of digesta samples was mixed with 45 ml of TSB and cultured for E. coli O157:H7 as described for fecal samples (see above). Mucosal surface samples were collected using foam swabs vigorously rubbed over the mucosal surface of the tissue and placed into 3 ml of TSB. Ten-gram tissue samples were placed into 90 ml of TSB. Both mucosal swab samples and tissue samples were cultured for E. coli O157:H7 as described for the RAMS samples (see above).
Statistical methods.
To compare the duration of E. coli O157:H7 infections induced by oral and rectal inoculation of cattle, a survival analysis was conducted on the data from the cattle experiments (see Tables 3, 4, 7, and 8, below). The Kaplan-Meier estimate of the cumulative probability of survival (here, survival denotes duration of infection) was computed using the PEPI (Programs for Epidemiologists) program (1). Survival time was defined as the number of days from the first positive sample until the first consistently negative sample. The log-rank test was used to evaluate the statistical significance of the difference between durations of infection following oral and rectal inoculations (1). The two-tailed Fisher's exact test was used to compare the difference between the dorsal and ventral rectoanal mucosa with respect to frequency of positive culture results.
TABLE 3.
Duration and number of E. coli O157:H7 in cattle after oral dosing: low-dose experimenta
| Animal |
E. coli O157:H7 CFU/g of feces on postdose day:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 6 | 9 | 13 | 16 | 20 | 23 | 27 | 30 | |
| LC1 | 0 | 3.0 × 103 | 5.5 × 103 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LC2 | 0 | E+b | 6.5 × 103 | 1.7 × 104 | E+ | 0 | E+ | 0 | 0 | 0 |
| LC3 | 0 | E+ | 6.0 × 103 | E+ | 0 | 0 | E+ | 0 | 0 | 0 |
| LC4 | 0 | 1.5 × 102 | 8.5 × 103 | 5.0 × 103 | 0 | E+ | E+ | 0 | E+ | E+ |
| LC5 | 0 | 1.0 × 102 | 8.5 × 103 | 6.5 × 102 | E+ | 0 | 0 | 0 | 0 | 0 |
| LC6 | 0 | 1.5 × 102 | 1.1 × 104 | 1.7 × 103 | 1.0 × 102 | 0 | 0 | 0 | 0 | 0 |
| LC7 | 0 | 3.5 × 102 | 1.7 × 104 | 5.0 × 103 | E+ | E+ | 0 | 0 | 0 | 0 |
| LC8 | 0 | 2.5 × 102 | E+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LC9 | 0 | 2.0 × 103 | 1.8 × 104 | 2.4 × 103 | 0 | E+ | E+ | E+ | E+ | E+ |
| LC10 | 0 | 7.0 × 102 | 1.1 × 103 | 4.4 × 103 | 0 | 0 | 0 | 0 | 0 | 0 |
| LC11 | 0 | 5.0 × 101 | 7.1 × 104 | 2.0 × 104 | 5.0 × 101 | 9.0 × 101 | E+ | 0 | 0 | 0 |
| LC12 | 0 | 8.5 × 102 | 0 | E+ | 0 | 0 | 0 | 0 | 0 | 0 |
| LC13 | 0 | 1.0 × 103 | 1.1 × 103 | 7.4 × 103 | 5.0 × 101 | 0 | 0 | 0 | 0 | 0 |
| LC14 | 0 | 5.0 × 101 | 5.5 × 103 | 1.7 × 105 | E+ | E+ | 0 | 0 | 0 | 0 |
| LC15 | 0 | 1.0 × 103 | 1.0 × 102 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LC16 | 0 | 5.0 × 102 | 5.7 × 104 | 5.1 × 105 | E+ | 0 | 0 | 0 | 0 | 0 |
| LC17 | 0 | 1.00 × 102 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LC18 | 0 | 7.50 × 102 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Five-month-old Holstein steers received a single oral dose of 1.0×107 E. coli O157:H7 ATCC 43894.
E+, sample was positive by enrichment culture technique but not by direct culture technique; this indicates that the number of CFU/g was ≤50.
TABLE 4.
Duration and number of E. coli O157:H7 in cattle after oral dosing: high-dose experimenta
| Animal |
E. coli O157:H7 CFU/g of feces on postdose day:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 9 | 12 | 17 | 22 | 25 | 36 | 44 | 54 | |
| HC1 | 0 | 4.1 × 104 | 5.4 × 104 | E+b | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HC2 | 0 | 2.5 × 104 | 2.9 × 104 | E+ | E+ | E+ | E+ | 0 | 0 | 0 | 0 |
| HC3 | 0 | 3.4 × 104 | 1.4 × 104 | 3.5 × 102 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HC4 | 0 | 3.1 × 104 | 1.1 × 104 | 1.5 × 102 | E+ | 1.5 × 102 | E+ | 0 | 0 | E+ | E+ |
| HC5 | 0 | 9.3 × 103 | 3.2 × 103 | 5.0 × 101 | E+ | E+ | E+ | E+ | E+ | E+ | E+ |
| HC6 | 0 | 4.6 × 104 | 1.7 × 104 | 1.5 × 102 | E+ | E+ | E+ | 0 | E+ | 0 | 0 |
| HC7 | 0 | 5.4 × 103 | 3.2 × 104 | 1.7 × 104 | E+ | 0 | E+ | E+ | E+ | 0 | 0 |
| HC8 | 0 | 4.3 × 104 | 3.3 × 104 | 9.9 × 104 | 0 | 1.5 × 102 | 0 | 0 | 0 | 0 | 0 |
| HC9 | 0 | 6.3 × 103 | 5.0 × 101 | 5.0 × 101 | 0 | 0 | 2.5 × 104 | 5.0 × 102 | 0 | 0 | 0 |
| HC10 | 0 | 5.3 × 103 | 1.8 × 103 | 3.0 × 103 | 5.0 × 101 | 6.5 × 103 | 1.1 × 104 | 5.0 × 101 | 0 | 5.0 × 101 | E+ |
Seven-month-old Holstein steers received a single oral dose of 5.1×1010 E. coli O157:H7; the inoculum included equal amounts of strains ATCC 43894, WSU 180, WSU 400, and WSU 588.
E+, sample was positive by enrichment culture technique but not by direct culture technique; this indicates that the number of CFU/g was ≤50.
TABLE 7.
Duration and number of E. coli O157:H7 in cattle after rectal swab inoculation: high-dose experimenta
| Animal |
E. coli O157:H7 CFU/swab or CFU/g of feces on postdose day:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 22 | 29 | 36 | 44 | 58 | 66 | |
| HR1 | 0 | 6.0 × 103 | 2.2 × 103 | 1.2 × 102 | E+b | E+ | E+ | 0 | 0 |
| HR2 | 0 | 1.2 × 102 | 5.4 × 102 | 9.0 × 102 | 3.3 × 102 | E+ | E+ | E+ | E+ |
| HR3 | 0 | 5.7 × 102 | 3.0 × 102 | 4.5 × 102 | 3.0 × 101 | E+ | E+ | E+ | E+ |
| HR4 | 0 | 1.1 × 103 | 9.0 × 101 | 3.0 × 101 | E+ | E+ | E+ | E+ | 0 |
| HR5 | 0 | 3.1 × 103 | 1.1 × 102 | 3.3 × 102 | E+ | 6.0 × 101 | E+ | E+ | 0 |
| HR6 | 0 | 2.0 × 105 | 2.4 × 102 | 9.0 × 101 | 2.4 × 101 | E+ | E+ | 0 | 0 |
| HR7 | 0 | 6.9 × 102 | 1.5 × 102 | 6.6 × 102 | E+ | 2.4 × 101 | E+ | E+ | E+ |
| HR8 | 0 | 1.8 × 104 | 3.6 × 102 | 1.2 × 102 | 1.5 × 102 | 6.0 × 101 | E+ | E+ | 0 |
Seven-month-old Holstein steers received a single rectal dose of 109 CFU of a four-strain mixture of E. coli O157:H7; the mixture contained equal amounts of strains ATCC 43894, WSU 180, WSU 400, and WSU 588.
E+, sample was positive by enrichment culture technique but not by direct culture technique; this indicates that the number of CFU/g was ≤50.
RESULTS AND DISCUSSION
The purpose of this work was to develop reliable methodology for experimental colonization of ruminants with E. coli O157:H7. Colonization models have broad applications in analyzing the relationship between cattle and this human pathogen and in testing interventions to reduce or prevent colonization with this bacterium. In this study, all animals were fecal culture negative prior to exposure to E. coli O157:H7, and after exposure to or infection with the bacteria all animals were in good health for the duration of the experiments. Animals were not considered colonized with E. coli O157:H7 if they were culture positive for only 1 week or less, because although the inoculum may have replicated in the intestinal lumen, this short duration does not indicate that a stable association between the mucosa and the bacterium has been established (12, 26). There was variation in the duration of culture-positive status among the inoculated animals, depending on the route of exposure and/or the number of bacteria in the inoculum.
An oral dose of E. coli O157:H7 in sheep and cattle resulted in variable colonization with the bacteria.
Sheep are an established ruminant model for E. coli O157:H7 colonization and have advantages compared to cattle that include ease of handling and economy (9, 11, 18, 19, 31). Five-month-old ram lambs were inoculated orally with either a low dose (1.0 × 107 CFU) or a high dose (6.0 × 109 CFU) of E. coli O157:H7, and fecal samples were cultured for 15 or 40 days, respectively (Tables 1 and 2). Although 100% of the sheep in the low-dose group were fecal culture positive 24 h postinoculation, the number of culture-positive lambs declined rapidly so that by day 5 postinoculation only 33.3% (8 of 24) of the animals remained positive. These culture-positive lambs were likely colonized by the bacterium, because they remained culture positive for 12 to 15 days or longer (Table 1). Lambs inoculated with the high dose had higher numbers of fecal E. coli O157:H7, and most of these animals remained culture positive longer than animals in the low-dose group (Table 2). Sixty percent of the animals that received the high oral dose were culture positive 25 days postinoculation, and 20% remained culture positive for the 40-day duration of the experiment. However, some animals in the high-dose group rapidly cleared the bacterium in 1 week or less (HS10, HS14, HS3, and HS4) and were likely not colonized by E. coli O157:H7.
TABLE 1.
Duration and number of fecal E. coli O157:H7 in sheep after oral dosing: low-dose experimenta
| Animal |
E. coli O157:H7 CFU/g of feces on postdose day:
|
|||||
|---|---|---|---|---|---|---|
| 0 | 1 | 5 | 8 | 12 | 15 | |
| LS1 | 0 | E+b | 0 | 0 | 0 | 0 |
| LS2 | 0 | E+ | 0 | 0 | 0 | 0 |
| LS3 | 0 | 5.0 × 102 | 0 | 0 | 0 | 0 |
| LS4 | 0 | 1.0 × 102 | 0 | 0 | 0 | 0 |
| LS5 | 0 | 5.0 × 102 | 0 | 0 | 0 | 0 |
| LS6 | 0 | 5.0 × 102 | 4.2 × 104 | 4.2 × 104 | 3.0 × 102 | 1.0 × 102 |
| LS7 | 0 | 7.0 × 102 | 1.8 × 104 | 5.5 × 104 | E+ | E+ |
| LS8 | 0 | 1.4 × 104 | 0 | 0 | 0 | 0 |
| LS9 | 0 | E+ | 4.2 × 104 | 5.0 × 102 | E+ | E+ |
| LS10 | 0 | 2.5 × 103 | 0 | 0 | 0 | 0 |
| LS11 | 0 | 5.7 × 104 | 1.0 × 103 | 6.0 × 103 | 3.7 × 104 | 2.8 × 104 |
| LS12 | 0 | 8.2 × 103 | 0 | 0 | 0 | 0 |
| LS13 | 0 | E+ | 0 | 0 | 0 | 0 |
| LS14 | 0 | 3.0 × 103 | 7.5 × 102 | E+ | 6.5 × 104 | 2.0 × 103 |
| LS15 | 0 | 5.0 × 102 | 0 | 0 | 0 | 0 |
| LS16 | 0 | 2.0 × 102 | 5.0 × 102 | E+ | 2.3 × 104 | 4.8 × 103 |
| LS17 | 0 | 2.0 × 102 | 0 | 0 | 0 | 0 |
| LS18 | 0 | 3.5 × 103 | 0 | 0 | 0 | 0 |
| LS19 | 0 | 1.0 × 103 | 7.5 × 103 | 6.5 × 103 | 5.0 × 103 | E+ |
| LS20 | 0 | E+ | 0 | 0 | 0 | 0 |
| LS21 | 0 | 2.0 × 103 | 0 | 0 | 0 | 0 |
| LS22 | 0 | 1.5 × 103 | 5.0 × 103 | 6.0 × 103 | E+ | 0 |
| LS23 | 0 | E+ | 0 | 0 | 0 | 0 |
| LS24 | 0 | 2.2 × 103 | 0 | 0 | 0 | 0 |
Animals (5-month-old white-faced ram lambs) received a single oral dose of 1.0×107 E. coli O157:H7 ATCC 43894.
E+, fecal sample was positive by enrichment culture technique but not by direct culture technique; this indicates that the number of CFU/g was ≤50.
TABLE 2.
Duration and number of fecal E. coli O157:H7 in sheep after oral dosing: high-dose experimenta
| Animal |
E. coli O157:H7 CFU/g of feces on postdose day:
|
||||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 7 | 11 | 25 | 32 | 40 | |
| HS1 | 0 | 2.5 × 105 | 5.0 × 103 | 0 | 0 | E+b | 0 |
| HS2 | 0 | 4.0 × 104 | E+ | E+ | E+ | E+ | E+ |
| HS3 | 0 | 7.0 × 104 | E+ | 0 | 0 | 0 | 0 |
| HS4 | 0 | 4.7 × 104 | 2.5 × 103 | 0 | 0 | 0 | 0 |
| HS5 | 0 | 5.4 × 104 | 3.8 × 103 | 1.5 × 103 | E+ | 0 | 0 |
| HS6 | 0 | 9.5 × 104 | 1.0 × 104 | 3.0 × 104 | E+ | 0 | 0 |
| HS7 | 0 | 1.7 × 105 | 4.0 × 104 | E+ | E+ | 0 | 0 |
| HS8 | 0 | 2.0 × 104 | E+ | E+ | E+ | 0 | 0 |
| HS9 | 0 | 5.0 × 104 | 2.0 × 103 | 0 | 1.5 × 104 | E+ | E+ |
| HS10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HS11 | 0 | 1.5 × 103 | 4.5 × 102 | 2.5 × 102 | 1.0 × 103 | 0 | 0 |
| HS12 | 0 | 5.0 × 104 | 5.0 × 103 | E+ | E+ | E+ | E+ |
| HS13 | 0 | 6.0 × 105 | 5.0 × 103 | E+ | E+ | 0 | 0 |
| HS14 | 0 | E+ | 0 | E+ | 0 | 0 | 0 |
| HS15 | 0 | E+ | 1.0 × 103 | 0 | 0 | 0 | 0 |
Five-month-old white-faced ram lambs received a single oral dose of 6.×109 E. coli O157:H7 ATCC 43894.
E+, fecal sample was positive by enrichment culture technique but not by direct culture technique; this indicates that the number of CFU/g was ≤50.
Similar results were obtained in orally inoculated cattle. Five- to 7-month-old Holstein steers were inoculated orally with either a low dose (1.0 × 107 CFU) or a high dose (5.1 × 1010 CFU) of E. coli O157:H7, and fecal and RAMS samples were cultured for the bacterium for 30 or 54 days, respectively (Tables 3 and 4). All 18 steers that received a low oral dose of bacteria were culture positive for E. coli O157:H7 1 day postinoculation, but the proportion of culture-positive steers declined rapidly from day 6 postinoculation, when 15 of 18 (83.3%) steers were culture positive, to day 23, when only 1 steer remained culture positive (5.6%) (Table 3). Among the 10 steers that received a high oral dose of E. coli O157:H7, the proportion of animals that were culture positive remained higher for a longer period of time than in the low-dose group. On day 22 postinoculation 7 of 10 (70%) were culture positive, and during days 36 through 54 postinoculation 3 of 10 (30%) remained culture positive for E. coli O157:H7 (Table 4). Two animals that were culture positive through the end of the study had one or more samples test negative. This could have been due to culture detection limits or reinfection.
The infections that resulted from a single oral dose of E. coli O157:H7 were similar in both sheep and cattle. Some animals became colonized with the bacterium and remained culture positive for more than 1 week, and some animals did not become colonized and rapidly cleared the bacterium in 1 week or less. The number of animals that were colonized increased as the dose increased, so that a higher proportion of animals were colonized when exposed to a higher dose. Low-dose inoculations in sheep and cattle resulted in fewer culture-positive animals (29.2 and 27.8%, respectively) 2 weeks postinoculation, while high-dose inoculations resulted in 60% culture-positive animals 3 weeks postinoculation. No oral inoculation tested resulted in more than 70% of the animals being culture positive 3 weeks postinoculation.
Exposure to E. coli O157:H7-positive Trojan cattle resulted in low infection rates and variable colonization status.
Exposure to the unnaturally high bolus of E. coli O157:H7 (1010 CFU) required to establish colonization for 3 weeks in more than 60% of the test animals is unlikely to happen on the farm, so to more closely mimic the presumed farm conditions that lead to infections we tested a direct contact transmission (Trojan) model, previously described by Besser et al. (3). Two experiments using Trojan cattle as the challenge source were done, one in the cool fall season and one in the warm summer season. The duration and number of fecal E. coli O157:H7 in the Trojan steers and their pen mates are shown in Tables 5 and 6 and Fig. 1. This method of infection was not efficient, and many animals did not acquire E. coli O157:H7 or were culture positive for a brief time. The low infection rates and sporadic culture status occurred even though the Trojan animals remained culture positive for most of the study, and feces from the Trojan animals contained as much as 104 CFU of E. coli O157:H7 per gram for several days. In the fall experiment, 5 of 15 pen mates were culture positive within 2 days of exposure to three Trojan animals, but none of these animals remained culture positive for E. coli O157:H7 on day 9 or 14 postexposure (Fig. 1). In the summer experiment, 7 out of the 15 pen mates were culture positive for E. coli O157:H7 within 5 days of copenning with two Trojan animals (Fig. 1). Thereafter, the number of positive animals varied between 4 and 10, and the number of fecal E. coli O157:H7 recovered fluctuated between 4.0 × 104 CFU/g and zero. Seven of the 15 animals (47%) were culture positive for E. coli O157:H7 after 54 days. These infection rates were similar, although lower, than the infection rates reported by Besser et al. (3). We followed the Besser et al. protocol, except that the Trojan animals received 1010 CFU of E. coli O157:H7 and our animals were housed in a quarantined open-air barn rather than in biosafety level 3 containment; therefore, both air humidity and animal-to-animal contact were less in our setting. Although we did not measure environmental contamination, it is likely that the confined housing of the biosafety level 3 building resulted in an environment more heavily contaminated with E. coli O157:H7 than the open-air barn. The level of environmental contamination probably contributed to the culture status of the animals. The improved infection rates in the warm-season experiment may have been due to several factors: the weather and its impact on bacterial survival in the environment, the extended duration of the experiment, and/or the stagnant water bin that became contaminated and contained 103 CFU of E. coli O157:H7/ml. The presence of this water bin was in keeping with the original protocol (3) and may have provided a continuous source of freshly ingested E. coli O157:H7 to any animals that took a drink or played in this water, so that the increase in the percentage of culture-positive animals may have been due to transient passage of bacteria through the gastrointestinal tract rather than an increase in the number of colonized animals. The stagnant water bin was not the main water source, and an automatic watering system was present from which animals drank fresh water ad libitum.
TABLE 5.
Duration and number of fecal E. coli O157:H7 in cattle after exposure to Trojan animals: fall experimenta
| Animal |
E. coli O157:H7 CFU/g of feces on postexposure day:
|
||||
|---|---|---|---|---|---|
| 0 | 2 | 7 | 9 | 14 | |
| TF1 | 0 | 0 | E+b | 0 | 0 |
| TF2 | 0 | E+ | 0 | 0 | 0 |
| TF3 | 0 | 0 | 0 | 0 | 0 |
| TF4 | 0 | E+ | E+ | 0 | 0 |
| TF5 | 0 | E+ | 0 | 0 | 0 |
| TF6 | 0 | 0 | 0 | 0 | 0 |
| TF7 | 0 | 0 | E+ | 0 | 0 |
| TF8 | 0 | 0 | 0 | 0 | 0 |
| TF9 | 0 | 5.0 × 102c | E+ | 0 | 0 |
| TF10 | 0 | 0 | 0 | 0 | 0 |
| TF11 | 0 | 0 | 0 | 0 | 0 |
| TF12 | 0 | 0 | 0 | 0 | 0 |
| TF13 | 0 | 0 | 0 | 0 | 0 |
| TF14 | 0 | 0 | 0 | 0 | 0 |
| TF15 | 0 | E+ | E+ | 0 | 0 |
| Trojan A | 0 | 1.5 × 103 | E+ | E+ | E+ |
| Trojan B | 0 | 6.5 × 104 | E+ | 5.0 × 101 | 0 |
| Trojan C | 0 | 3.6 × 104 | E+ | 5.0 × 101 | E+ |
Fifteen 6-month-old Holstein steers were penned together in contact with three Trojan cattle inoculated orally with 3.5×1010 CFU of E. coli O157:H7 ATCC 43894 per animal on day zero in November (fall).
E+, sample was positive by enrichment culture technique but not by direct culture technique; this indicates that the number of CFU/g was ≤50.
TABLE 6.
Duration and number of fecal E. coli O157:H7 in cattle after exposure to Trojan animals: Summer experimenta
| Animal |
E. coli O157:H7 CFU/g of feces on postexposure day:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 9 | 15 | 22 | 29 | 36 | 47 | 54 | |
| TS1 | 0 | 0 | 0 | 1.5 × 102 | 2.0 × 104 | 2.2 × 104 | 1.0 × 103 | E+b | 0 |
| TS2 | 0 | 0 | E+ | 0 | 0 | 0 | 0 | 0 | 0 |
| TS3 | 0 | 0 | 0 | 0 | 0 | 1.0 × 102 | 1.5 × 102 | 0 | 0 |
| TS4 | 0 | 5.0 × 101 | 0 | 0 | 0 | 0 | 2.0 × 103 | 0 | 0 |
| TS5 | 0 | 0 | 0 | 0 | 0 | 5.0 × 102 | 0 | 0 | 0 |
| TS6 | 0 | 1.0 × 102 | E+ | 5.0 × 102 | 2.0 × 102 | 1.5 × 102 | 5.0 × 103 | 4.1 × 104 | 0 |
| TS7 | 0 | E+ | 0 | 0 | 4.0 × 104 | 8.5 × 103 | 1.0 × 102 | E+ | E+ |
| TS8 | 0 | 0 | 5.0 × 101 | 0 | E+ | 2.0 × 104 | 5.0 × 101 | 0 | E+ |
| TS9 | 0 | 0 | E+ | 0 | 0 | 3.0 × 102 | 0 | 0 | 0 |
| TS10 | 0 | 1.5 × 102 | 1.0 × 102 | 1.0 × 103 | 5.0 × 103 | 5.0 × 101 | 0 | E+ | E+ |
| TS11 | 0 | E+ | 0 | 0 | 0 | E+ | 0 | E+ | E+ |
| TS12 | 0 | 0 | 0 | 0 | 5.0 × 103 | 2.5 × 102 | 0 | 0 | 0 |
| TS13 | 0 | 5.0 × 102 | E+ | 5.0 × 102 | E+ | 0 | 0 | E+ | E+ |
| TS14 | 0 | E+ | 0 | 0 | 0 | 0 | E+ | 0 | E+ |
| TS15 | 0 | 0 | 0 | 0 | 0 | E+ | E+ | 0 | E+ |
| Trojan D | 0 | 5.0 × 101 | E+ | 0 | 2.5 × 104 | 2.0 × 102 | 0 | 0 | 0 |
| Trojan E | 0 | 1.8 × 103 | 3.0 × 103 | 1.5 × 105 | 1.1 × 104 | 7.5 × 102 | 0 | E+ | E+ |
Fifteen 6-month-old Holstein steers were penned together in contact with two Trojan cattle inoculated orally with a single dose of 3.5×1010 CFU of a four-strain mixture of E. coli O157:H7, including equal amounts of ATCC 43894, WSU 180, WSU 400, and WSU 588 at day zero in June (summer).
E+, sample was positive by enrichment culture technique but not by direct culture technique; this indicates that the number of CFU/g was ≤50.
FIG. 1.
Comparison of the proportion of cattle culture positive for E. coli O157:H7 following exposure to Trojan cattle in two trials. In both the cool weather fall trial (Δ) and the warm weather summer trial (▴), 15 6-month-old Holstein steers were penned together with three or two Trojan steers, respectively. Data points do not include the Trojan steers that were introduced into the groups immediately after receiving a single oral dose of 3.5 × 1010 CFU of an E. coli O157:H7 mixture containing equal amounts of ATCC 43894, WSU 180, and WSU 400. A stagnant water bucket became contaminated with approximately 10 g of cattle feces, as indicated by the arrow on day 21, and contained approximately 103 CFU of E. coli O157:H7/ml through the duration of the trial.
Rectal swab administration of E. coli O157:H7 to cattle resulted in a consistent colonization status in all animals.
The recent demonstration by Naylor et al. (22) that the rectoanal junction mucosa is a site of E. coli O157:H7 colonization in cattle and work in our laboratory that supports this finding (12, 26) led us to hypothesize that cattle could be efficiently colonized with this bacterium by direct application to the distal rectal mucosa. Although natural colonization begins by ingestion of the bacterium, an important advantage to applying the inoculum at a site of colonization in experimental animals is that it avoids the confounding situation of inocula replicating in the upper gastrointestinal tract and being passively shed in the feces. We presumed that rectally applied E. coli O157:H7 organisms that did not associate with the mucosa would be rapidly eliminated. Five-month-old Holstein steers were inoculated with a rectal swab containing either a low dose (2.0 × 107 CFU) or high dose (1.0 × 1010 CFU) and tested for E. coli O157:H7 for 55 or 66 days, respectively. The number of bacteria delivered onto the mucosa was not measured but was less than the starting inoculum, because some bacteria remained on the swab and some were extruded immediately. The eight steers that received a high rectal dose were uniformly culture positive through day 44 postinoculation. On day 66, the last sampling day, three of eight (38%) were culture positive (Table 7). The 16 steers that received the lower dose were all culture positive on day 6 postinoculation, and the proportion of culture-positive animals decreased until day 55, the last sampling day, when there were 4 of 16 (25%) culture-positive animals (Table 8). In addition, we tested the ability of lower bacteria numbers, 103, 105, or 107 CFU of E. coli O157:H7, to result in infections by rectal swab application. Similar to the results in Tables 7 and 8, the steers that received 107 E. coli O157:H7 CFU remained culture positive for 35 days (data not shown). However, animals that received 105 bacteria were culture positive for only 1 week, and animals that received 103 bacteria were not culture positive on any day (data not shown). Surprisingly, inoculum concentrations of 105 or less even applied to the relatively small area of the rectoanal junction mucosa were not sufficient to colonize animals. This may have been due to the rapid and ongoing removal of the inocula as the animals defecated.
TABLE 8.
Duration and number of E. coli O157:H7 in cattle after rectal swab inoculation: low-dose experimenta
| Animal |
E. coli O157:H7 CFU/swab or CFU/g of feces on postdose day:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 13 | 20 | 27 | 34 | 41 | 48 | 55 | |
| LR1 | 0 | 6.0 × 102 | 9.0 × 101 | 0 | 0 | 0 | 0 | 0 | 0 |
| LR2 | 0 | 1.5 × 103 | E+b | 1.2 × 102 | E+ | 0 | E+ | 0 | E+ |
| LR3 | 0 | 2.0 × 104 | 0 | 0 | 3.0 × 101 | E+ | E+ | 0 | 0 |
| LR4 | 0 | E+ | 1.2 × 102 | E+ | 0 | 0 | 0 | 0 | 0 |
| LR5 | 0 | 4.6 × 103 | 3.0 × 101 | E+ | E+ | 0 | 0 | E+ | 0 |
| LR6 | 0 | E+ | E+ | E+ | E+ | E+ | E+ | E+ | 0 |
| LR7 | 0 | 9.0 × 101 | 1.2 × 103 | E+ | 0 | 0 | 0 | E+ | 0 |
| LR8 | 0 | 2.1 × 102 | 6.0 × 101 | 0 | E+ | E+ | E+ | E+ | E+ |
| LR9 | 0 | 6.0 × 101 | E+ | E+ | 0 | 0 | 0 | 0 | E+ |
| LR10 | 0 | 4.2 × 103 | 6.0 × 101 | 0 | E+ | E+ | 0 | 0 | 0 |
| LR11 | 0 | E+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| LR12 | 0 | 5.1 × 104 | E+ | E+ | 6.0 × 101 | E+ | E+ | E+ | 0 |
| LR13 | 0 | 9.0 × 101 | 6.0 × 101 | E+ | 0 | 0 | |||
| LR14 | 0 | 1.3 × 104 | 2.1 × 102 | 9.0 × 102 | 3.0 × 102 | 1.5 × 102 | 1.5 × 102 | E+ | 0 |
| LR15 | 0 | 6.5 × 104 | 6.0 × 101 | E+ | 0 | 0 | 0 | 0 | 0 |
| LR16 | 0 | 2.0 × 104 | 7.2 × 102 | E+ | 0 | E+ | E+ | E+ | E+ |
Five-month-old Holstein steers received a single rectal dose of 2.0×107 CFU of E. coli O157:H7 ATCC 43894.
E+, sample was positive by enrichment culture technique but not by direct culture technique; this indicates that the number of CFU/g was ≤50.
E. coli O157:H7 was restricted to the rectoanal junction mucosa in animals that were inoculated by rectal swab.
To determine if E. coli O157:H7 was restricted to the rectoanal junction mucosa in animals inoculated only at this site, necropsy was performed on animals that remained colonized for 60 days. Tissue, digesta, and mucosal swab samples from numerous sites, including the rumen, colon, and rectum (refer to Materials and Methods) were taken and cultured for the bacterium. All samples from the rectoanal junction were culture positive, and all samples from other gastrointestinal sites were culture negative (data not shown). To determine if E. coli O157:H7 was distributed evenly around the circumference of the rectoanal junction mucosa, we sampled seven animals by separate RAMS samples obtained from the dorsal and ventral mucosa after colonization was established. RAMS samples were not cross-contaminated and were obtained on 18 separate days over a 3-week period. All 18 ventral samples were culture positive, while 12 of 18 dorsal samples were culture positive for E. coli O157:H7. The difference between dorsal and ventral culture results was significant (P < 0.05, Fisher's exact two-tailed test). The propensity for E. coli O157:H7 to colonize the ventral rather than dorsal mucosa may be an artifact of the inoculation method or may be related to some anatomical, physiological, and/or immunological difference between the dorsal and ventral mucosa at that site.
Repeated rectal swabbing or lack thereof did not affect colonization with E. coli O157:H7.
We tested the effects of rectal palpation (to aseptically acquire fecal samples) and swabbing the rectoanal mucosa (for the RAMS culture) on inducing or prolonging E. coli O157:H7 colonization of the mucosa. Four steers were inoculated by rectal swab with a high dose of E. coli O157:H7 ATCC 43894 (1010 CFU). Two animals were not rectally palpated or rectally swabbed for the first 3 weeks postinoculation; the other two animals had RAMS samples done weekly. Fecal samples from all four steers were collected by free catch weekly and cultured for E. coli O157:H7. Twenty-one days postinoculation, all animals were culture positive for E. coli O157:H7, including the animals that were not repeatedly swabbed at the rectum (Table 9). Also, both groups had one animal with a positive fecal culture on day 50 postinoculation (Table 9). On some occasions, fecal samples from an animal were culture negative while RAMS samples were culture positive, reflecting the superior sensitivity of the RAMS culture technique (26). The results suggested that repeated rectal swabbing to obtain the RAMS sample did not influence the duration of E. coli O157:H7 infection in cattle.
TABLE 9.
Duration and number of E. coli O157:H7 in cattle with and without repeated swabbing at the rectoanal junction mucosaa
| Animal | No. of E. coli O157:H7 on postinoculation dayb:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 7 | 14 | 21 | 29 | 35 | 43 | 50 | |
| T801 | 0 | NDc | ND | ND | 7.5 × 102 | 9.0 × 103 | ND | ND | E+ |
| (0) | (E+d) | (4.0 × 103) | (0) | (0) | (0) | (5.4 × 102) | (E+) | (0) | |
| T802 | 0 | ND | ND | ND | 5.4 × 102 | E+ | ND | ND | 0 |
| (0) | (E+) | (2.0 × 103) | (0) | (0) | (0) | (0) | (0) | (0) | |
| S803 | 0 | 6.0 × 101 | 6.0 × 101 | E+ | E+ | E+ | 3.3 × 102 | E+ | 0 |
| (0) | (E+) | (E+) | (0) | (0) | (0) | (0) | (0) | (0) | |
| S804 | 0 | 6.9 × 103 | 5.6 × 102 | 1.2 × 103 | 7.8 × 102 | 9.0 × 103 | 9.0 × 104 | 6.0 × 101 | E+ |
| (0) | 8.5 × 103 | 6.3 × 103 | (0) | (E+) | (E+) | 3.3 × 103 | (E+) | (0) | |
Four 5-month-old Holstein steers were given a single rectal dose of 1010 CFU of E. coli O157:H7 (ATCC 43894) after sampling at day zero. Two animals (T801 and T802) were not RAMS sampled until 21 days postinoculation and then less frequently until day 50. Two animals (S803 and S804) were RAMS sampled every week from day 3 postinoculation until the end of experiment.
Number of E. coli O157:H7 CFU/swab of RAMS or number of E. coli O157:H7 CFU/g of free-catch feces (in parentheses).
ND, not done, because RAMS was not collected.
E+, sample was positive by enrichment culture but not by direct culture; this indicates the number of E. coli O157:H7 was ≤30 CFU/swab or in feces was ≤50 CFU/g.
Inoculation by rectal swab resulted in more colonized animals with longer durations of infection than traditional oral inoculation.
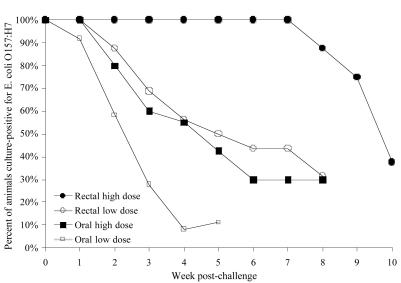
We compared the proportion of animals culture positive for E. coli O157:H7 each week following a low- or high-dose inoculation by oral or rectal swab methods (Fig. 2). To evaluate the statistical significance of the difference between durations of experimental infections induced by these routes, we analyzed the culture data using the log-rank test for comparison of the Kaplan-Meier survival curves (1). This analysis compared duration of infection induced by rectal and oral inoculation within strata of high and low doses. The resulting summary P value was 3.4 × 10−6 (Table 10). Within-strata comparisons were also significant. The comparison between durations of infection induced by the high oral dose and the low rectal dose indicated no significant difference (Table 10). Although we did not test the rectal swab application method in sheep, we expect that, similar to cattle, this method would be superior to oral dosing in this species as well.
FIG. 2.
Comparison of the oral dose and rectal swab bovine E. coli O157:H7 infection models. Data points represent the proportion of animals positive for E. coli O157:H7 each week following a single dose at day zero. All animals were culture negative for the bacterium prior to inoculation with E. coli O157:H7. Eight 5-month-old steers received a rectal swab inoculation containing 1010 CFU (high dose; •), and 16 5- to 7-month-old steers received a rectal swab inoculation containing 2.0 × 107 CFU (low dose; ○). Ten 7-month-old Holstein steers received an oral dose of 5.1 × 1010 CFU (high dose; ▪), and 18 5-month-old Holstein steers received an oral dose of 1.0 × 107 CFU (low dose; □ ).
TABLE 10.
Results of Kaplan-Meier survival analysis comparing duration of infection induced by oral versus rectal inoculation of cattle with E. coli O157:H7
| Group | Route | Median duration (days) | Stratum-specific P valuea | Summary P valueb | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High dosec | Rectal | 63.3 | 0.012 | |||||||||||
| Oral | 36.0 | 0.974 | 3.4 × 10−6 | |||||||||||
| Low dosed | Rectal | 30.5 | 2.5 × 10−4 | |||||||||||
| Oral | 14.5 |
P value for the comparison between rectal and oral routes of administration, within strata of dose and for the comparison between high-dose oral and low-dose rectal inoculations.
P value for the comparison between rectal and oral routes of administration, controlling for dose level.
High dose ranged from 1×109 to 5×1010 CFU of E. coli O157:H7.
Low dose ranged from 1×107 to 2×107 CFU of E. coli O157:H7.
Conclusion.
Rectal swab administration of E. coli O157:H7 resulted in a consistent colonization of cattle and was superior to the traditional oral dosing and to the Trojan calf methods of creating experimental infections. The surprising ease with which a long-term infection resulted from a single application of bacteria to the rectoanal junction mucosa also strongly supported this location as a site of E. coli O157:H7 colonization in cattle. Rectal swab application of bacteria is a powerful tool to investigate host-bacterium interactions in vivo. Ongoing studies in our laboratory include live animal testing of various E. coli O157:H7 virulence genes for their role in bovine colonization and adherence to the rectoanal junction mucosa.
Acknowledgments
This work was supported, in part, by the Idaho Agriculture Experiment Station, U.S. Department of Agriculture NRICGP grant 99-35201-8539, and Public Health Service grants NO1-HD-0-3309, P20 RR15587, P20 RR16454, and 1U54-AI 5714 from the National Institutes of Health.
We thank D. D. Hancock for providing the cefixime, L. Austin for animal handling, and L. J. Grauke and D. H. Rice for technical assistance.
REFERENCES
- 1.Abramson, J. H., and P. M. Gahlinger. 1999. Computer programs for epidemiologists: PEPI version 3. Brixton Books, Llanidloes, Powys, Wales.
- 2.Besser, T. E., D. D. Hancock, L. C. Pritchett, E. M. McRae, D. H. Rice, and P. I. Tarr. 1997. Duration of detection of fecal excretion of Escherichia coli O157:H7 in cattle. J. Infect. Dis. 175:726-729. [DOI] [PubMed] [Google Scholar]
- 3.Besser, T. E., B. L. Richards, D. H. Rice, and D. D. Hancock. 2001. Escherichia coli O157:H7 infection of calves: infectious dose and direct contact transmission. Epidemiol. Infect. 127:555-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brashears, M. M., M. L. Galyean, G. H. Loneragan, J. E. Mann, and K. Killinger-Mann. 2003. Prevalence of Escherichia coli O157:H7 and performance by beef feedlot cattle given Lactobacillus direct-fed microbials. J. Food Prot. 66:748-754. [DOI] [PubMed] [Google Scholar]
- 5.Brashears, M. M., D. Jaroni, and J. Trimble. 2003. Isolation, selection, and characterization of lactic acid bacteria for a competitive exclusion product to reduce shedding of Escherichia coli O157:H7 in cattle. J. Food Prot. 66:355-363. [DOI] [PubMed] [Google Scholar]
- 6.Brown, C. A., B. G. Harmon, T. Zhao, and M. P. Doyle. 1997. Experimental Escherichia coli O157:H7 carriage in calves. Appl. Environ. Microbiol. 63:27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchko, S. J., R. A. Holley, W. O. Olson, V. P. Gannon, and D. M. Veira. 2000. The effect of different grain diets on fecal shedding of Escherichia coli O157:H7 by steers. J. Food Prot. 63:1467-1474. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2000. Summary of outbreaks of Escherichia coli O157 and other Shiga toxin-producing E. coli reported to the CDC in 1999 (updated June, 2000). [Online.] http://www.cdc.gov/ncidod/dbmd/diseaseinfo/files/ecoli_99summary.pdf.
- 9.Cookson, A. L., A. D. Wales, J. M. Roe, C. M. Hayes, G. R. Pearson, and M. J. Woodward. 2002. Variation in the persistence of Escherichia coli O157:H7 in experimentally inoculated 6-week-old conventional lambs. J. Med. Microbiol. 51:1032-1040. [DOI] [PubMed] [Google Scholar]
- 10.Cray, W. C., Jr., and H. W. Moon. 1995. Experimental infection of calves and adult cattle with Escherichia coli O157:H7. Appl. Environ. Microbiol. 61:1586-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edrington, T. S., T. R. Callaway, K. M. Bischoff, K. J. Genovese, R. O. Elder, R. C. Anderson, and D. J. Nisbet. 2003. Effect of feeding the ionophores monensin and laidlomycin propionate and the antimicrobial bambermycin to sheep experimentally infected with E. coli O157:H7 and Salmonella typhimurium. J. Anim. Sci. 81:553-560. [DOI] [PubMed] [Google Scholar]
- 12.Grauke, L. J., I. T. Kudva, J. W. Yoon, C. W. Hunt, C. J. Williams, and C. J. Hovde. 2002. Gastrointestinal tract location of Escherichia coli O157:H7 in ruminants. Appl. Environ. Microbiol. 68:2269-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grauke, L. J., S. A. Wynia, H. Q. Sheng, J. W. Yoon, C. J. Williams, C. W. Hunt, and C. J. Hovde. 2003. Acid resistance of Escherichia coli O157:H7 from the gastrointestinal tract of cattle fed hay or grain. Vet. Microbiol. 95:211-225. [DOI] [PubMed] [Google Scholar]
- 14.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 15.Hancock, D. D., T. E. Besser, D. H. Rice, D. E. Herriott, and P. I. Tarr. 1997. A longitudinal study of Escherichia coli O157 in fourteen cattle herds. Epidemiol. Infect. 118:193-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmon, B. G., C. A. Brown, S. Tkalcic, P. O. Mueller, A. Parks, A. V. Jain, T. Zhao, and M. P. Doyle. 1999. Fecal shedding and rumen growth of Escherichia coli O157:H7 in fasted calves. J. Food Prot. 62:574-579. [DOI] [PubMed] [Google Scholar]
- 17.Jonsson, M. E., A. Aspan, E. Eriksson, and I. Vagsholm. 2001. Persistence of verocytotoxin-producing Escherichia coli O157:H7 in calves kept on pasture and in calves kept indoors during the summer months in a Swedish dairy herd. Int. J. Food Microbiol. 66:55-61. [DOI] [PubMed] [Google Scholar]
- 18.Kudva, I. T., P. G. Hatfield, and C. J. Hovde. 1995. Effect of diet on the shedding of Escherichia coli O157:H7 in a sheep model. Appl. Environ. Microbiol. 61:1363-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudva, I. T., C. W. Hunt, C. J. Williams, U. M. Nance, and C. J. Hovde. 1997. Evaluation of dietary influences on Escherichia coli O157:H7 shedding by sheep. Appl. Environ. Microbiol. 63:3878-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magnuson, B. A., M. Davis, S. Hubele, P. R. Austin, I. T. Kudva, C. J. Williams, C. W. Hunt, and C. J. Hovde. 2000. Ruminant gastrointestinal cell proliferation and clearance of Escherichia coli O157:H7. Infect. Immun. 68:3808-3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mechie, S. C., P. A. Chapman, and C. A. Siddons. 1997. A fifteen month study of Escherichia coli 0157:H7 in a dairy herd. Epidemiol. Infect. 118:17-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naylor, S. W., J. C. Low, T. E. Besser, A. Mahajan, G. J. Gunn, M. C. Pearce, I. J. McKendrick, D. G. Smith, and D. L. Gally. 2003. Lymphoid follicle-dense mucosa at the terminal rectum is the principal site of colonization of enterohemorrhagic Escherichia coli O157:H7 in the bovine host. Infect. Immun. 71:1505-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohya, T., T. Marubashi, and H. Ito. 2000. Significance of fecal volatile fatty acids in shedding of Escherichia coli O157 from calves: experimental infection and preliminary use of a probiotic product. J. Vet. Med. Sci. 62:1151-1155. [DOI] [PubMed] [Google Scholar]
- 24.Park, S., R. Worobo, and R. Durst. 2001. Escherichia coli O157:H7 as an emerging foodborne pathogen: a literature review. Crit. Rev. Biotechnol. 21:27-48. [DOI] [PubMed] [Google Scholar]
- 25.Potter, A. A., S. Klashinsky, Y. Li, E. Frey, H. Townsend, D. Rogan, G. Erickson, S. Hinkley, T. Klopfenstein, R. A. Moxley, D. R. Smith, and B. B. Finlay. 2004. Decreased shedding of Escherichia coli O157:H7 by cattle following vaccination with type III secreted proteins. Vaccine 22:362-369. [DOI] [PubMed] [Google Scholar]
- 26.Rice, D. H., H. Q. Sheng, S. A. Wynia, and C. J. Hovde. 2003. Rectoanal mucosal swab culture is more sensitive than fecal culture and distinguished Escherichia coli O157:H7-colonized cattle and those transiently shedding the same organism. J. Clin. Microbiol. 41:4924-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanderson, M. W., T. E. Besser, J. M. Gay, C. C. Gay, and D. D. Hancock. 1999. Fecal Escherichia coli O157:H7 shedding patterns of orally inoculated calves. Vet. Microbiol. 69:199-205. [DOI] [PubMed] [Google Scholar]
- 28.Shere, J. A., C. W. Kaspar, K. J. Bartlett, S. E. Linden, B. Norell, S. Francey, and D. M. Schaefer. 2002. Shedding of Escherichia coli O157:H7 in dairy cattle housed in a confined environment following waterborne inoculation. Appl. Environ. Microbiol. 68:1947-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tarr, P. I. 1995. Escherichia coli O157:H7: clinical, diagnostic, and epidemiological aspects of human infection. Clin. Infect. Dis. 20:1-8. [DOI] [PubMed] [Google Scholar]
- 30.Tkalcic, S., T. Zhao, B. G. Harmon, M. P. Doyle, C. A. Brown, and P. Zhao. 2003. Fecal shedding of enterohemorrhagic Escherichia coli in weaned calves following treatment with probiotic Escherichia coli. J. Food Prot. 66:1184-1189. [DOI] [PubMed] [Google Scholar]
- 31.Woodward, M. J., A. Best, K. A. Sprigings, G. R. Pearson, A. M. Skuse, A. Wales, C. M. Hayes, J. M. Roe, J. C. Low, and R. M. La Ragione. 2003. Nontoxigenic Escherichia coli O157:H7 strain NCTC12900 causes attaching-effacing lesions and eae-dependent persistence in weaned sheep. Int. J. Med. Microbiol. 293:299-308. [DOI] [PubMed] [Google Scholar]