Abstract
Chromosomal abnormalities lead to the development of hematologic malignancies such as Myelodysplastic Syndrome (MDS). Known chromosomal changes causing MDS include deletion of the long arm of chromosome 5, runt-related transcription factor 1 (RUNX1) also known as acute myeloid leukemia 1 protein (AML1), and very rarely fusion genes involving RUNX1 at t(5;21)(q15;q22). We present a case of a 71-year-old female with MDS, refractory anemia with excess blasts, type 1, with a combination of two cytogenetic abnormalities, specifically a concomitant translocation between chromosomes 5q15 and 21q22 and deletion of chromosome 5q13q33. Fluorescence in-situ hybridization (FISH) using a probe for RUNX1 (AML1), localized to 21q22, showed three FISH signals for RUNX1, consistent with rearrangement of RUNX1. Therapy was started with Lenalidomide leading to normal blood counts. Most significantly, repeat cytogenetics revealed normal karyotype and resolution of deletion on the long arm of chromosome 5 and a t(5;21). FISH negative for deletion 5q. The results altogether meet criteria for a complete cytogenetic remission (CR). We report a new case of t(5;21)(q15;q22) involving the RUNX1 gene and del(5)(q13q33) in a MDS patient, a combination of chromosomal abnormalities heretofore not reported in the literature. RUNX1 rearrangement is usually associated with an adverse prognosis in AML and MDS. Deletions of 5q are typically associated with poor prognosis in AML, however it is usually associated with a favorable prognosis in MDS. Our patient responded very well to Lenalidomide therapy with achievement of CR. Lenalidomide is approved for treatment of anemia in low and intermediate risk MDS with del (5q), however based on a search of literature it seems that RUNX1 mutations are also more prominent in patients who have responded to Lenalidomide therapy. MDS is a genomically unstable disease. Hence, it is conceivable that our patient started with a 5q minus syndrome and then acquired the second hit RUNX1 translocation leading to an accelerated phase of myeloid neoplasm or refractory anemia with excess blasts, type 1. Hence, the temporal relationship between acquisition of del 5q and RUNX1 rearrangement may have influenced the clinical outcome and possibly response to therapy.
Keywords: Myelodysplastic Syndrome (MDS), Deletion 5q, runt-related transcription factor 1 (RUNX1), lenalidomide
Introduction
Chromosomal abnormalities (deletions) and gene fusion lead to development of cancer, including hematologic malignancies like Myelodysplastic Syndrome (MDS) (1). Deletion of all or part of the long arm of chromosome 5 (5q) is the most common chromosomal abnormality encountered in MDS, constituting 12–15% of cases (2). Runt-related transcription factor 1 (RUNX1), also known as acute myeloid leukemia 1 protein (AML1) is found to be mutated in >10% of MDS patients (3). The gene RUNX1 at chromosome 21q22 encodes the alpha subunit of core binding factor (CBF), a heterodimeric transcription factor involved in the development of normal hematopoiesis. Fusion genes by translocations of RUNX1 have been reported in acute myeloid leukemia (AML), the most frequent being t(8;21)(q22;q22). In contrast, t(5;21)(q15;q22) resulting in rearrangement of RUNX1 have been very rarely reported (4,5). To date, there has not been a reported case of a combination of deletion 5q [del(5q)] along with translocation (5;21). We report such a case, in which there was a concomitant translocation between chromosomes 5q15 and 21q22, and deletion of chromosome 5q13q33.
Case presentation
The patient referenced is a 71-year-old Caucasian female who was admitted to the hospital for lower extremity edema, fatigue, and dyspnea with exertion. She was found to have macrocytic anemia and was in atrial fibrillation, thought to be anemia-associated. She had no personal or family history of hematologic disease; however, the patient’s mother did have breast and colon cancer. She had no history of radiation or industrial exposures. The patient was treated symptomatically and discharged from the hospital. However, in the coming weeks, the patient required multiple transfusions of packed red blood cells for symptomatic anemia.
On physical examination the patient did not have any petechiae or ecchymoses, lymphadenopathy, or hepatosplenomegaly. She did have inflamed but not pale conjunctivae, 2/6 systolic murmur at the upper sternal borders, and bilateral 2+ lower extremity edema. Laboratory findings included the following: CBC Hgb 7.9 mg/dL, MCV 127 fL, RDW 14.0%, platelets 387,000/µL, and WBC 4,700/µL with an eosinophilia of 8%. Chemistry, LFTs, and UA were within normal limits. There was a negative antibody screen on type and cross. Thyroid dysfunction, vitamin B12, and folic acid deficiencies were ruled out. Erythropoietin (Epo) levels were found to be elevated at 1,029 mU/mL. Epo score was calculated at −1 which usually predicts a poor response to erythropoietin therapy.
The blood smear showed a macrocytic anemia, thrombocytosis with monolobulated megakaryocytes, slightly increased basophils (4%), and 5% blasts. Flow cytometry showed a mild increase in atypical myeloblasts (6%), which were positive for CD4, CD13, CD33, CD34, and HLA-DR. BCR/ABL mRNA transcripts were not detected.
The bone marrow was hypercellular (50–60%) with increased megakaryopoiesis, severely decreased erythropoiesis (2%), and 7% blasts. There was greater than 10% granulocytic dyspoiesis, indicating multi-lineage dysplasia.
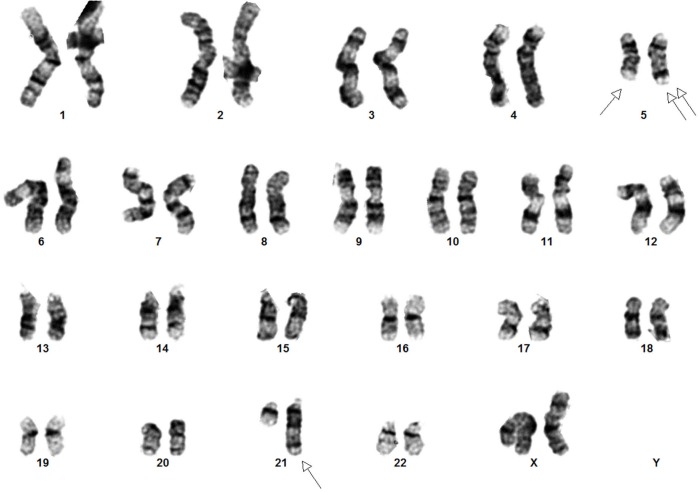
Cytogenetics showed an interstitial deletion on the long arm of chromosome 5 and a t(5;21) chromosome translocation with break points at 5q15 and 21q22 at each metaphase (Figure 1). Fluorescence in-situ hybridization (FISH) using a probe for RUNX1 (AML1), localized to 21q22, showed three FISH signals for RUNX1, consistent with rearrangement of RUNX1 (Figure 2).
Figure 1.
Representative G-banded karyogram showing t(5; 21)(q15;q22) and del(5)(q13q33). Single arrows indicate t(5;12)(q15;q22) and double arrows indicate del(5)(q13q33).
Figure 2.
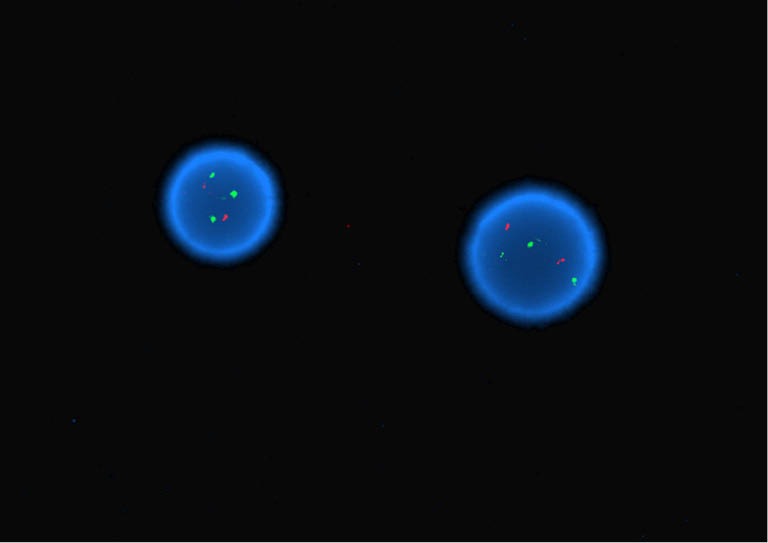
Interphase FISH with two orange signals for 8q21.3 (RUNX1T1) and three green signals for 21q22 (RUNX1) consistent with rearrangement of RUNX1 (Dual color, dual fusion RUNX1/RUNX1T1 probes, Abbott Molecular, Des Plaines, Illinois, 66018).
The patient was diagnosed with MDS with IPSS is intermediate-1. R-IPSS was 5.5 (high). She was started on lenalidomide 10 mg daily, along with aspirin for prophylaxis of deep vein thrombosis. The patient became pancytopenic on this regimen approximately one month after starting, requiring a transfusion of two units of packed red blood cells. The lenalidomide was held for one week, after which she had improvement in her blood counts, and was restarted on lenalidomide at a lower dose of 5 mg 21/28 days. Ten months after diagnosis, the patient is still taking lower dose lenalidomide. Her blood counts have normalized with rise in hemoglobin to 12.9 with stable WBC and platelet counts at 3,100 and 204,000/µL, respectively. Repeat bone marrow evaluation revealed normocellular bone marrow for age (30–35%) with increased lymphocytes and 1% blasts, with only rare dyserythropoiesis and rare atypical megakaryocytes. Cytogenetics revealed normal karyotype and resolution of deletion on the long arm of chromosome 5 and a t(5;21). FISH negative for deletion 5q. Thus indicating a complete cytogenetic remission (CR). The patient is maintaining a good functional status with Lenalidomide.
Discussion
In this study, we report a new case of t(5;21)(q15;q22) involving the RUNX1 gene and del(5)(q13q33) in a MDS patient. The deletion of all or part of long arm of chromosome 5 [del(5q)] is a recurrent abnormality in AML and MDS patients (6). While the association between del(5q) and AML are still unclear, a deletion at 5q31.1 has been reported to be related with AML (7). Moreover, del(5q) is frequently a part of the complex karyotype in several AML cases. Hence it is difficult to ascertain the significant relevance of del(5q) though some researchers suggest that it portends a poor prognosis. On the other hand del 5q is associated with a favorable clinical course and prognosis in MDS.
Ribosomal protein S14 (RPS14) is one of the genes located on chromosome 5q, which is essential for the maintenance of normal erythroid progenitor cells (8). Loss of chromosome 5q leads to haploinsufficiency of RPS14 (9), which in turn results in failure of erythropoiesis (10). In our case, the patient had a history of anemia, and the karyotype showed del(5q). We hypothesize that the anemia in this patient is related with haploinsufficiency of RPS14, however, this hypothesis would need to be confirmed by further molecular testing.
There have been rare but similar reports of t(5;21) (q13;q22) with RUNX1 rearrangement in cases of AML and MDS (specifically CMML) (4,5,11,12). An adverse prognosis was associated with the t(5;21) in the majority of cases. However, the combination of del(5q) along with t(5;21) has never been reported in the literature.
This patient’s clinical and pathological features of female sex, macrocytic anemia with preserved platelet counts, monolobulated megakaryocytes, with elevated erythropoietin levels and lack of myeloproliferative disorder-associated fibrosis in the bone marrow does raise a suspicion of an underlying 5q minus syndrome. MDS is a genomically unstable disease. Hence, it is conceivable that our patient started with a 5q minus syndrome and then acquired the second hit RUNX1 translocation leading to an accelerated phase of myeloid neoplasm or refractory anemia with excess blasts, type 1. Hence, the temporal relationship between acquisition of del 5q and RUNX1 rearrangement may have influenced the clinical outcome and possibly response to therapy in our patient with MDS. We will elucidate this further in our discussion.
Lenalidomide is approved for treatment of transfusion-dependent anemia in IPSS low- and intermediate-1-risk MDS patients with the del(5q). It has been shown to reduce transfusion requirements and improvement in cytologic and cytogenetic abnormalities (13). In addition, there is also evidence indicating that the immunomodulatory effects of lenalidomide show efficacy in MDS both with and without the del(5q) (14,15). However, at present time, apart from del(5q) we have not identified any other routine molecular biomarkers to predict response to Lenalidomide therapy. In order to answer this question, investigators from Cleveland clinic carried out next-generation sequencing study in MDS patients treated with Lenalidomide, where it was revealed that mutations in RUNX1 (17%) were more prevalent in Lenalidomide responders (16,17).
A different chromosomal abnormality, trisomy 13 has been associated with RUNX1 mutation (18). Of note, two patients with AML due to trisomy 13 as the sole cytogenetic abnormality achieved sustained morphologic and cytogenetic complete remission with high-dose, single-agent lenalidomide. Hence, suggesting RUNX1 as a potential target to the lenalidomide activity (19).
As the patient has a 5q minus syndrome, and was ineligible for immunosuppressive therapy with ATG and cyclosporine in the setting of cardiac comorbidities, lenalidomide therapy was initiated with a positive hematologic response and cytogenetic CR. Based on above discussion in addition to targeting del(5q), there is evidence that RUNX1 inhibition mediated by Lenalidomide therapy may have resulted in positive clinical outcome as indicated by improvement of bone marrow dyspoiesis and peripheral blood counts.
In our best knowledge, this is the first case report of combination of t(5;21)(q15;q22) involving the RUNX1 gene and del(5)(q13q33) in MDS, that responded favorably to Lenalidomide with resultant CR. In addition, this case highlights the importance of not only focusing on the mutation that gives rise to MDS or other malignancies, but also considering the sequential acquisition of such mutations, as clinical outcome was affected depending on the temporal sequence of genetic abnormalities. In conclusion, a comprehensive understanding and the clinical significance of such mutations and their sequential acquisition could further guide physicians in utilizing targeted therapeutics.
Acknowledgements
Deborah Peterson, RN for helping with obtaining consent from patient. Carol Webb for administrative assistance.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- 1.Mitelman F, Johansson B, Mertens F. The impact of translocations and gene fusions on cancer causation. Nat Rev Cancer 2007;7:233-45. [DOI] [PubMed] [Google Scholar]
- 2.Jerez A, Gondek LP, Jankowska AM, et al. Topography, clinical, and genomic correlates of 5q myeloid malignancies revisited. J Clin Oncol 2012;30:1343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haferlach T, Nagata Y, Grossmann V, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2014;28:241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gogineni SK, da Costa M, Verma RS. A new translocation, t(5;21)(q13;q22) in acute myelogenous leukemia. Cancer Genet Cytogenet 1996;88:167-9. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Li C, Bo L, et al. AML1/RUNX1 fusion gene and t(5;21)(q13;q22) in a case of chronic myelomonocytic leukemia with progressive thrombocytopenia and monocytosis. Cancer Genet Cytogenet 2004;152:172-4. [DOI] [PubMed] [Google Scholar]
- 6.Van den Berghe H, Cassiman JJ, David G, et al. Distinct haematological disorder with deletion of long arm of no. 5 chromosome. Nature 1974;251:437-8. [DOI] [PubMed] [Google Scholar]
- 7.Giagounidis AA, Germing U, Aul C. Biological and prognostic significance of chromosome 5q deletions in myeloid malignancies. Clin Cancer Res 2006;12:5-10. [DOI] [PubMed] [Google Scholar]
- 8.Jeandidier E, Gervais C, Radford-Weiss I, et al. A cytogenetic study of 397 consecutive acute myeloid leukemia cases identified three with a t(7;21) associated with 5q abnormalities and exhibiting similar clinical and biological features, suggesting a new, rare acute myeloid leukemia entity. Cancer Genet 2012;205:365-72. [DOI] [PubMed] [Google Scholar]
- 9.Ebert BL, Pretz J, Bosco J, et al. Identification of RPS14 as a 5q- syndrome gene by RNA interference screen. Nature 2008;451:335-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boultwood J, Pellagatti A, McKenzie AN, et al. Advances in the 5q- syndrome. Blood 2010;116:5803-11. [DOI] [PubMed] [Google Scholar]
- 11.Mitelman F, Nilsson PG, Brandt L, et al. Chromosome pattern, occupation, and clinical features in patients with acute nonlymphocytic leukemia. Cancer Genet Cytogenet 1981;4:197-214. [DOI] [PubMed] [Google Scholar]
- 12.Roulston D, Espinosa R, 3rd, Nucifora G, et al. CBFA2(AML1) translocations with novel partner chromosomes in myeloid leukemias: association with prior therapy. Blood 1998;92:2879-85. [PubMed] [Google Scholar]
- 13.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med 2006;355:1456-65. [DOI] [PubMed] [Google Scholar]
- 14.Sugimoto Y, Sekeres MA, Makishima H, et al. Cytogenetic and molecular predictors of response in patients with myeloid malignancies without del[5q] treated with lenalidomide. J Hematol Oncol 2012;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leitch HA, Buckstein R, Shamy A, et al. The immunomodulatory agents lenalidomide and thalidomide for treatment of the myelodysplastic syndromes: a clinical practice guideline. Crit Rev Oncol Hematol 2013;85:162-92. [DOI] [PubMed] [Google Scholar]
- 16.2807 Molecular predictors of response to lenalidomide in myeloid malignancies. Available online: https://ash.confex.com/ash/2013/webprogram/Paper64103.html
- 17.4665 Molecular predictors of response in patients with myeloid neoplasms treated with lenalidomide. Available online: https://ash.confex.com/ash/2014/webprogram/Paper75038.html [DOI] [PMC free article] [PubMed]
- 18.Silva FP, Lind A, Brouwer-Mandema G, et al. Trisomy 13 correlates with RUNX1 mutation and increased FLT3 expression in AML-M0 patients. Haematologica 2007;92:1123-6. [DOI] [PubMed] [Google Scholar]
- 19.Fehniger TA, Byrd JC, Marcucci G, et al. Single-agent lenalidomide induces complete remission of acute myeloid leukemia in patients with isolated trisomy 13. Blood 2009;113:1002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]