Abstract
Cyanobacteria synthesize several types of bioactive secondary metabolites. Anabaena strain 90 produces three types of bioactive peptides, microcystins (inhibitors of protein phosphatases 1 and 2A), anabaenopeptilides, and anabaenopeptins (serine protease inhibitors). To investigate the role of the anabaenopeptilides in Anabaena, wild-type strain 90 (WT) and its anabaenopeptilide deficient mutant (MU) were cultured with various light and phosphate levels to evaluate the effects and coeffects of these growth factors on the concentrations of the three classes of peptides and the growth characteristics. WT and MU grew in comparable ways under the different growth conditions. The total peptide concentration in WT was significantly higher than that in MU (2.5 and 1.4 μg/mg [dry weight], respectively). Interestingly, the average concentration of anabaenopeptins was significantly higher in MU than in WT (0.59 and 0.24 μg/mg [dry weight], respectively). The concentration of microcystins was slightly but not statistically significantly higher in MU than in WT (1.0 and 0.86 μg/mg [dry weight], respectively). In WT, the highest peptide concentrations were usually found after 13 days in cultures grown at medium light intensities (23 μmol m−2 s−1) and with the highest phosphate concentrations (2,600 μg liter−1). In MU, the highest peptide concentrations were found in 13-day-old cultures grown at medium light intensities (23 μmol m−2 s−1) and with phosphate concentrations greater than 100 μg liter−1. The higher concentrations of anabaenopeptins in MU may compensate for the absence of anabaenopeptilides. These findings clearly indicate that these compounds may have some linked function in the producer organism, the nature of which remains to be discovered.
Cyanobacteria produce a large number of secondary metabolites, which are compounds that are not essential for cell metabolism. Many of these compounds are peptides (20, 23) and have been reported to possess a number of bioactivities, such as toxicity for eukaryotic organisms and antimicrobial, antifungal, antiviral, and enzyme-inhibiting activities (2, 11, 22, 23, 31).
Anabaena strain 90 is a filamentous heterocystous cyanobacterium that was isolated from a toxic bloom. It produces four nonribosomal heptapeptides (microcystins MCYST-LR, MCYST-RR, d-Asp3-MCYST-LR, and d-Asp3-MCYST-RR) (30, 31), two seven-residue depsipeptides (anabaenopeptilides 90A and 90B), and three six-residue anabaenopeptins (anabaenopeptins A, B, and C) (7, 8). Microcystins have been reported to inhibit protein phosphatases 1 and 2A (17, 41), and anabaenopeptilides and anabaenopeptins have been reported to have protease-inhibiting activity (20, 39). Although bioactivities have been assigned to these compounds, their functions in the producing organisms have remained largely unresolved. The roles of secondary metabolites in nature are not completely understood (4, 18), but it is generally agreed that these compounds somehow increase the competitive ability of the producer in the environment. Generally, bioactive peptides are considered secondary metabolites, but even the roles of the best-studied compounds, microcystins, as secondary metabolites are disputed (14, 21). It has been proposed that microcystins may function in cell metabolism, perhaps in chelating iron (35), and that nontoxic clones could survive by producing other peptides (21). Microbial secondary metabolites are usually produced in the stationary phase (1), but cyanobacteria contain bioactive peptides in all growth phases.
Studying the effects of external growth stimuli on the production of bioactive peptides should provide insight into their functions in the producers and help optimize the growth conditions for particular pharmacologically interesting compounds. The effects of environmental factors on microcystin concentrations have been extensively studied, and microcystin production is usually highest under optimal growth conditions (31). Because the cyanobacterial products that are toxic to vertebrates, such as microcystins and nodularins, cause problems for human and animal health, scientific and public interest has been concentrated on them. However, from the point of view of the cyanobacteria these compounds probably do not have a special position among the vast array of bioactive compounds produced.
A comprehensive understanding of the possible functions of bioactive peptides and their ecological benefits requires studying bioactive peptides as a group rather than focusing on microcystins. There have been few studies on the effects of growth factors on cyanobacterial bioactive peptides other than microcystins and nodularins. Recently, the biosynthesis of certain cyanobacterial peptides has been resolved (27, 28, 34). This has enabled studies of gene expression of the biosynthetic genes as a function of growth and environmental changes. It has been observed that transcription of the microcystin synthethase gene is activated under high light, especially red light, conditions (13). It is now possible to inactivate the genes responsible for biosynthesis of these peptides (5, 27). Such mutants are still scarce due to the lack of effective transformation methods for planktonic cyanobacteria.
We succeeded in inactivating the genes responsible for production of anabaenopeptilides in Anabaena strain 90 and in producing a unique mutant (27). The aim of this study was to compare wild-type Anabaena strain 90 (WT) and an anabaenopeptilide-deficient mutant (MU) grown at different light intensities and with different phosphate concentrations. We wanted to discover differences in growth and the concentrations of bioactive peptides in order to reveal the possible role of anabaenopeptilides in the producer. In this study we investigated for the first time the roles of all the known small peptides that the organism produces. Light was chosen as a growth factor because it was shown previously that light influences microcystin synthesis (13). Phosphate was studied because it is the most important limiting nutrient for nitrogen-fixing cyanobacteria (9). Our experimental design made it possible to investigate the coeffects of these growth factors.
MATERIALS AND METHODS
Cyanobacterial strains and cultivation.
Anabaena strain 90 was isolated in 1986 from Lake Vesijärvi in Finland (30). The mutant used in this study (MU), which is unable to produce anabaenopeptilides, was generated by insertion of the chloramphenicol resistance gene into the apdA gene in the anabaenopeptilide operon (27). WT and MU were maintained in Z8 mineral growth medium without nitrogen (16, 29), and the mutant was usually kept on plates in medium containing 2 μg of chloramphenicol ml−1. For the comparative experiments in this study, chloramphenicol was omitted from the medium to exclude the influence of the antibiotic on physiological processes and morphological characteristics. The persistence of the mutation, as indicated by the lack of anabaenopeptilides, was shown by high-performance liquid chromatography (HPLC) analysis in this study (Fig. 1).
FIG. 1.
HPLC chromatograms of the identified peptides present in Anabaena strain 90 (A) and its anabaenopeptilide-deficient mutant (B). Mcyst, microcystin; Peptin, anabaenopeptin; Peptilide, anabaenopeptilide.
Experiments.
Two batch culture experiments were performed at 22°C in temperature-controlled water baths by using 250-ml Erlenmeyer flasks containing 100 ml of liquid medium. The experiments were conducted under continuous cool white fluorescent light (Daylight DeLuxe tubes; AIRAM, Helsinki, Finland). The growth medium was Z8 medium without nitrogen (16), in which the concentration of PO4 phosphorus was reduced by replacing K2HPO4 with KCl, so that the concentration of potassium remained unchanged. The light and phosphate levels used (Fig. 2) were determined by a central composite design (19) to minimize the number of replicates and to allow estimation of quadratic effects and interactions between factors.
FIG. 2.
Design of the batch culture experiments with wild-type Anabaena strain 90 (WT) and its anabaenopeptilide mutant (MU). Nine combinations of phosphate concentrations (P) (in micrograms of P per liter) and light intensities (L) (in micromoles per square meter per second) are indicated by dots. There were five replicates for the middle point (P100L23). The phosphate concentrations and light levels were determined by a central composite design (see Materials and Methods). Samples of the underlined treatments were taken on days 6 and 20. On day 13, four replicates of P100L23 and the treatments not underlined were sampled. On day 25, one replicate of P100L23 was sampled.
Before the experiments were started, the cultures were grown in phosphate-free media for 7 days to deplete the phosphorus stored in the cells. The cultures were not axenic, and the bacterial flora in the cultures was equalized by first removing the cyanobacteria by filtering with GF52 filters (Schleicher & Schuell), combining the filtrates, and adding 5 ml of the combined filtrate to both WT and MU cultures. During the experiments, the positions of the culture vials were randomized and rearranged, and the vials were manually shaken three times a week.
Growth was monitored on days 1, 2, 3, 4, 5, 8, 10, and 16 by determining the optical density at 750 nm (Perkin-Elmer) and dry weight. Complete sampling was performed on days 0, 6, 13, 21, and 25 (Fig. 2). On sampling days, the following parameters were determined: optical density, dry weight, chlorophyll a (Chl a) concentration, cell number, cell length, nitrogenase activity, peptide concentrations, and phosphatase enzyme activity.
Analytical methods.
To determine the dry weight, approximately 50 ml of culture was filtered on tared G52 filters (Scheichler & Schuell). Subsequently, the filters were dried in an oven (105°C) for over 2 h and weighed again. The Chl a concentration was measured as described by Tandeau de Marsac and Houmard (33). Cell densities and cell sizes were determined from samples preserved with Lugol's iodine solution. The cells were counted with a hemocytometer (Bürker, Marienfeld, Germany) and a light microscope (Olympus BH-2) at a magnification of ×400. A minimum of 400 cells or 12 replicate samples were counted. The cell sizes were measured at a magnification of ×400. Nitrogenase activity (N2 fixation) was determined by the acetylene reduction technique (3). In short, three replicate 5-ml aliquots of samples were transferred to 10-ml tubes. The cells in one sample were killed with 0.2 ml of 37% HCl to quantify the trace amounts of ethylene in acetylene. Subsequently, 1 ml of acetylene was injected into the sealed tubes. After 2 h of incubation the other two cultures were killed. The acetylene and ethylene from a 1-ml sample were analyzed within 2 days by using a Varian 3700 gas chromatograph equipped with a flame ionization detector and a Porapak T column (1 m by 0.5 in.). The temperature settings for the column, injector, and detector were 50, 150, and 150°C, respectively. Ethylene was used as a standard. Phosphatase exoenzyme activities were measured by using 2 mmol of the fluorescent substrate 4-methylumbelliferyl phosphate per liter as described by Wittmann et al. (40).
The rest of the culture was centrifuged at 6,000 × g for 20 min and subsequently freeze-dried. Peptides were extracted from approximately 20 mg of freeze-dried material by adding 10 ml of 75% methanol and sonicating the preparation for 10 min (Labsonic-U; Braun, Melsungen, Germany). After sonication, the mixture was stirred with a magnetic stirrer for 1 h and the supernatant was collected. Methanol was then added, and the stirring procedure was repeated twice. After this, the methanol was evaporated, water was added, and the peptides were concentrated on C18 cartridges according to the manufacturer's instructions (Oasis; Waters, Milford, Mass.). The supernatants that eluted from the cartridges with 2 ml of methanol were subsequently dried in an air stream and dissolved in 0.5 ml of 50% methanol. Prior to analyses, the samples were stored at −20°C. The concentrations of peptides were determined with a Hewlett-Packard HP1090 liquid chromatograph equipped with a Hewlett-Packard UV/VIS diode array detector and a Hewlett-Packard Hypersil C18 column (125 by 4.0 mm) as described by Spoof et al. (32). The mobile phase was a 74:26 (vol/vol) mixture of 10 mM ammonium acetate and acetonitrile. The flow rate was 1 ml min−1, the injection volume 25 μl, and detection was at 238 nm. Nodularin was used as an external quantitative standard. Qualitative standards of MCYST-LR, MCYST-RR, d-Asp3-MCYST-LR, d-Asp3-MCYST-RR, anabaenopeptins A and B, and anabaenopeptilides 90A and 90B isolated in previous studies (7, 30) were used to identify these compounds on the basis of their retention times and UV spectra. The identities of the peptides were verified by determining their molecular masses by HPLC-electron spray ionization mass spectrometry, using an Esquire mass spectrometer (Bruker Daltonics, Billerica, Mass.) equipped with an ion trap interface coupled to an Agilent Technologies 1100 series HPLC system.
Concentrations equivalent to 5 μg of microcystin ml−1of the microcystins MCYST-LR, MCYST-RR, d-Asp3-MCYST-LR, and d-Asp3-MCYST-RR, anabaenopeptins A and B, and anabaenopeptilides 90A and 90B were tested for trypsin (0.5 μg ml−1) inhibition activity with a QuantiCleave fluorescent protease assay kit (Pierce, Rockford, Ill.) and fluorometer (Fluoroskan; Ascent) used according to the manufacturers' instructions.
Statistical analyses.
To test the significance of the differences between WT and MU for biomass parameters and concentrations of bioactive peptides, 95% confidence intervals were calculated by performing a t test.
For each strain, the effects of culture age, light, and phosphate were tested after linear transformation of the design matrices with multivariate regression analyses. This analysis was performed with MATLAB statistical software for Windows (MathWorks, Inc., Natick, Mass.). Composite design matrices were created with prior transformation of the original variables into coded variables as described by Vezie et al. (37). The matrices were expanded to include the coeffects and quadratic (second power) effects of experimental factors. Multivariate regression models describing the variation of the measured parameters were obtained by omitting insignificant (P > 0.05) factors.
RESULTS
Growth.
The biomass estimates obtained by using optical density, dry weight, and cell numbers correlated closely with each other (Spearman correlations [r], >0.90). The correlations for Chl a concentrations with other biomass parameters were weaker (r, <0.78). The growth results for WT and MU at the different light and phosphate levels did not differ when either dry weight, the Chl a concentration, or cell numbers were measured (Table 1 and Fig. 3 and 4).
TABLE 1.
Means and 95% confidence intervals for growth parameters and peptide concentrations of WT and Mu for different growth conditions and culture agesa
| Strain | Dry wt (mg liter−1) | Chl a concn (μg liter−1) | Cell concn (106 cells ml−1) | Chl a concn/dry wt (μg of Chl a mg−1 [dry wt]) | Cell length (μm)b | Cell mass (pg [dry wt] cell−1) | Nitrogenase activity (nmol liter−1) | Total peptides (μg mg−1 [dry wt])b | Total peptides (fg cell−1) | Anabaenopeptin concn (μg mg−1 [dry wt])b | Anabaenopeptin concn (fg cell−1) | Microcystin concn (μg mg−1 [dry wt]) | Microcystin concn (fg cell−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT | 35.7 (29.4-42) | 476 (302-650) | 1.09 (0.54-1.64) | 21 (16-26) | 4.35 (4.09-4.61) | 65.6 (43.3-87.9) | 653 (177-830) | 2.54 (1.79-3.3) | 121 (81-161) | 0.24 (0.17-0.31) | 22.9 (15.3-30.5) | 0.86 (0.76-0.96) | 49.1 (31.2-67.0) |
| Mu | 37.6 (30.8-44.4) | 605 (423-787) | 1.35 (0.73-1.96) | 29 (13-45) | 4.94 (4.66-5.22) | 45.4 (34.0-56.8) | 607 (463-751) | 1.44 (1.08-1.80) | 71.1 (49.9-92.3) | 0.59 (0.51-0.67) | 30.1 (20.7-39.5) | 1.0 (0.8-1.2) | 41.0 (27.6-54.4) |
The values are means (95% confidence intervals).
The confidence intervals do not overlap, and the means are statistically significantly different.
FIG. 3.
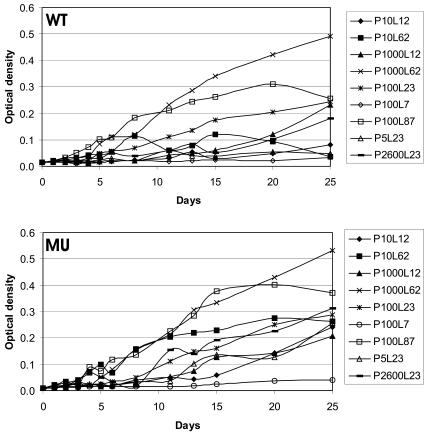
Growth based on optical density of wild-type Anabaena strain 90 (WT) and its anabaenopeptilide-deficient mutant (MU) at various light intensities (L) (in micromoles per square meter per second) and phosphate concentrations (P) (in micrograms of P per liter) in batch cultures.
FIG. 4.
Means and 95% confidence intervals of dry weight of wild-type Anabaena strain 90 (WT) and its anabaenopeptilide mutant (MU) at a phosphate concentration of 100 μg liter−1 and a light intensity of 23 μmol m−2 s−1 in batch cultures (P100L23 in Fig. 2).
At the two lowest light intensities (7 μmol m−2 s−1) the growth was poor (Fig. 3). With the other treatments, both strains were at the exponential growth phase from day 1 onward. The stationary phase was seen with all treatments, but the onset of the stationary phase depended on the treatment. The growth pattern in the medium treatment (100 μg of P liter−1 and 23 μmol of light m−2 s−1) (Fig. 3) shows that around day 20, the exponential growth phase ended, and there was very little production of new biomass between day 20 and day 25 (Fig. 4). By this time, the two strains had produced very similar yields (Fig. 4).
The amount of Chl a per unit of dry weight (i.e., the Chl a content) did not differ in the strains (Table 1). The cells of MU were significantly (Table 1) longer than those of WT. The cells of WT were, however, heavier than those of MU, but the difference was not statistically significant (Table 1). The mean nitrogenase activities of WT and MU were 653 ± 476 and 607 ± 144 nmol liter−1. The confidence intervals were, however, large and overlapped, and thus there was not a statistically significant difference in the nitrogenase activity.
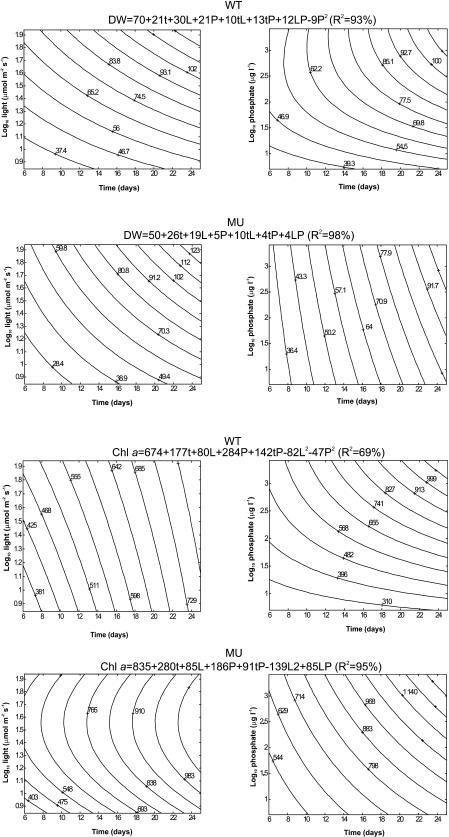
For both strains, the highest levels of dry weight were observed in the oldest cultures at the highest light intensities and phosphate levels (Table 2 and Fig. 5). The highest Chl a concentrations were found in the oldest cultures at the highest phosphate concentrations and the medium or highest light levels (Table 2 and Fig. 5). Light and phosphate had positive interactions with dry weight and Chl a concentration (Table 2). Neither strain grew with the low phosphate concentration (10 μg liter−1) and the high light intensity (62 μmol m−2 s−1) from day 20 onward (Fig. 3), indicating that there was phosphate limitation. Phosphatase enzyme activity was observed more often with MU than with WT. In both strains on day 25, phosphatase enzyme activity was detected (Table 3).
TABLE 2.
Results of multiple regression analysis for WT and MU grown in batch culturesa
| Parameter | Regression coefficients (b values)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Culture age | Light intensity | Phosphate concn | Culture age2 | Culture age- light intensity | Culture age- phosphate concn | Light intensity2 | Light intensity- phosphate concn | Phosphate concn2 | |
| Dry wt (WT) | 20.8b | 29.9b | 20.9b | −0.8 | 9.5c | 13.2b | 0.2 | 12.0c | −9.1c |
| Dry wt (MU) | 31.1d | 26.5d | 6.5b | −0.1 | 12.1b | 4.0c | −0.8 | 4.9c | −1.7 |
| Chl a concn (WT) | 170b | 70c | 285d | −24 | −21 | 142b | −80b | 52c | −42c |
| Chl a concn (MU) | 286b | 75c | 184b | 17 | −23 | 91b | −141b | 85c | −26 |
| Microcystin concn (WT) | −0.10 | 0.10 | 0.29b | −0.24b | −0.30b | 0.0005 | −0.39b | −0.018 | −0.22b |
| Microcystin concn (MU) | −0.074 | 0.062 | 0.12c | −0.22b | −0.26b | 0.005 | −0.32 | −0.01 | −0.06 |
| Anabaenopeptin concn (WT) | 0.12c | −0.0007 | 0.14c | −0.08 | −0.10 | 0.05 | −0.17c | −0.05 | −0.06 |
| Anabaenopeptin concn (MU) | 0.084 | −0.042 | −0.0007 | −0.091 | −0.10 | 0.022 | −0.17c | −0.030 | −0.12c |
| Anabaenopeptilide concn (WT) | 0.05 | 0.41b | 0.25b | −0.16c | −0.09 | 0.03 | −0.24b | −0.003 | −0.18c |
The independent variables were culture age, light intensity, and phosphate concentration. In addition, their coeffects (culture age-light intensity, culture age-phosphate concentration, light intensity-phosphate concentration) and quadratic effects (culture age2, light intensity2, phosphate concentration2) were included in the model.
P ≤ 0.01.
P ≤ 0.05.
P ≤ 0.0001.
FIG. 5.
Contour plots describing dry weight (DW) (in milligrams per liter) and Chl a concentrations (in micrograms per liter) of wild-type Anabaena strain 90 (WT) and its anabaenopeptilide mutant (MU) in relation to light intensity (L), phosphate concentration (P), and culture age (t).
TABLE 3.
Phosphatase enzyme activities in batch cultures of WT and MU cultured with different phosphate concentrations and light intensitiesa
| Day | Phosphatase activity (nmol liter−1 h−1) under the following conditions:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 10 μg of P liter−1, 62 μmol of light m−2 s−1
|
1,000 μg of P liter−1, 62 μmol of light m−2 s−1
|
100 μg of P liter−1, 23 μmol of light m−2 s−1
|
100 μg of P liter−1, 62 μmol of light m−2 s−1
|
|||||
| WT | MU | WT | MU | WT | MU | WT | MU | |
| 13 | NM | NM | NM | NM | 0 | 0 | 0 | 79 |
| 20 | 0 | 220 | 0 | 100 | NM | NM | NM | NM |
| 25 | NM | NM | NM | NM | 83 | 150 | NM | NM |
Data for only the treatments and sampling days on which phosphatase enzyme activity was detected are shown. All treatments and sampling days are shown in Fig. 2.
Bioactive peptides.
As expected, MU did not produce anabaenopeptilides (Fig. 1). WT produced two types of anabaenopeptilides, anabaenopeptilide 90A and anabaenopeptilide 90B. MCYST-LR, MCYST-RR, d-Asp3-MCYST-LR, and d-Asp3-MCYST-RR were produced by both WT and MU. Anabaenopeptins A, B, and C and two other unidentified anabaenopeptins were found in both strains (Fig. 1).
In gravimetric units, the total amount of peptides in WT was significantly higher than the amount in MU (Table 1). However, the difference in the cell quotas of total peptides (41% higher concentration of peptides in WT) was not statistically significant. In MU, the gravimetric anabaenopeptin concentration was approximately twice as high as that in WT. The anabenopeptin cell quota of MU was also higher than that of WT, but the difference was not statistically significant (Table 1). When the individual anabaenopeptins were examined, the largest difference between the strains was in the concentration of anabaenopeptin B (7.9 and 5.7 fg cell−1 in MU and WT, respectively). The gravimetric microcystin concentration and microcystin cell quota were slightly higher in MU than in WT, but the differences were not statistically significant (Table 1).
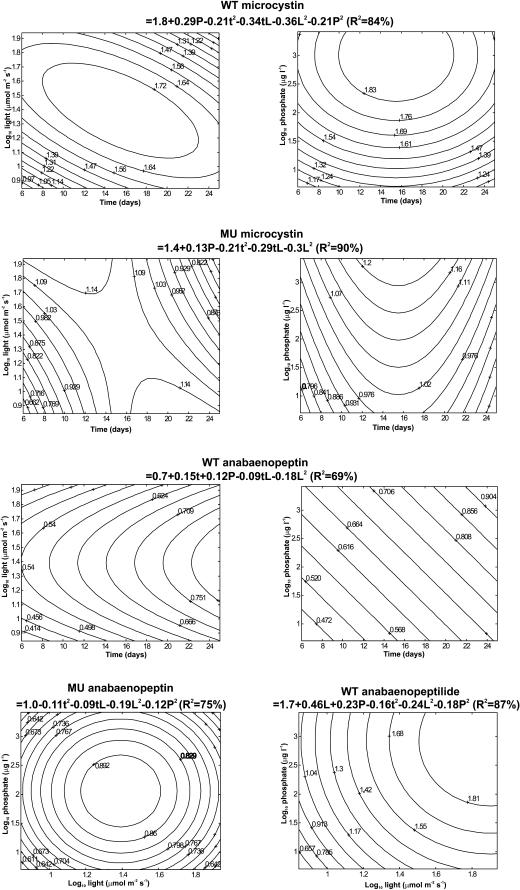
The anabaenopeptin concentration in WT was highest in the oldest cultures grown at the medium light levels and the highest phosphate concentrations (Table 2 and Fig. 6). In MU, the anabaenopeptin concentration was not influenced by the culture age but was highest at the medium light and phosphate levels (Table 2 and Fig. 6). In WT, the total microcystin concentration was highest at the medium light intensities, whereas in MU the microcystin concentration was not related to light. In both strains, the total microcystin concentration was highest at the middle of the culture period at the highest phosphate concentrations (Table 2 and Fig. 6). The anabaenopeptilide concentration of WT was not influenced by the culture age but was highest at the highest light and phosphate levels (Table 2 and Fig. 6).
FIG. 6.
Contour plots describing peptide concentrations (in micrograms per milligram [dry weight]) of wild-type Anabaena strain 90 (WT) and its anabaenopeptilide mutant (MU) in relation to light intensity (L), phosphate concentration (P), and culture age (t).
In addition to the peptides identified with standards or based on mass numbers, two previously unidentified anabaenopeptins were found based on their spectra. The four microcystin variants, anabaenopeptilide 90B, and anabaenopeptin A did not inhibit trypsin activity. Anabaenopeptilide 90A and anabaenopeptin B inhibited trypsin activity 5.2 and 9.4%, respectively.
DISCUSSION
The two strains grew under variable growth conditions, including light limitation and phosphate limitation, in comparable ways. However, the MU cells were longer and slightly lighter than the WT cells. Nitrogen fixation was higher in WT, and phosphatase activity was observed more often with MU. Although anabaenopeptilides are clearly not essential for growth, they may have some effects on the cells. The Chl a contents of WT and MU did not differ. A microcystin-deficient Microcystis mutant contained less Chl a and other pigments under light limitation conditions than the wild type contained (10). It was speculated that microcystin may influence light adaptation processes, but the same does not seem to be true for anabaenopeptilides.
The total concentrations of peptides expressed in gravimetric units were significantly different in the two strains, whereas the cell quotas, regardless of considerable differences in the means, did not differ significantly statistically. Counting cells microscopically can be regarded as a rather inaccurate means of estimating biomass. In addition, due to the design of our experiments, cells were exposed to variable growth conditions, additionally increasing the error variation in the cell numbers and peptide concentrations and further decreasing the power of the statistical test.
The highest peptide concentrations were produced under the medium or high light conditions and with the medium phosphate levels, conditions which promoted optimal or slightly suboptimal growth. The same has been true for microcystins (21, 31). The highest microcystin concentrations were observed at the middle of the growth period, whereas the highest anabaenopeptin and anabaenopeptilide concentrations were usually observed at the end of the culture period. Because we measured intracellular peptide concentrations, it may be that some peptides leaked or were transported out of the cells in the older cultures. High extracellular microcystin concentrations in old cultures, especially under high light levels, have been observed previously (25). The existence of putative ABC transporters linked to microcystins (34) and anabaenopeptilides (27) supports the proposal that there are active efflux mechanisms for these peptides (13, 14).
The highest microcystin concentrations were observed in WT at medium light intensities (23 μmol m−2 s−1). In previous studies, an increase in the light intensity between 2 and 40 μmol m−2 s−1 has been shown to increase the microcystin concentration, but at intensities greater than 40 μmol m−2 s−1 there was no further increase (25, 35, 36, 38). In MU, however, light had no effect on the microcystin concentration. Since the metabolic roles of these peptides are still unknown, it is difficult to speculate how this apparent insensitivity of microcystin production to light in MU could be linked to the absence of anabaenopeptilides. It has been proposed that microcystins may act as an intercellular signal in Microcystis and that shading (i.e., light intensity and quality) is tightly coupled to the abundance of cells (6).
It is difficult to make predictions about the metabolic costs related to the production of bioactive peptides. Such costs have been assumed to be high since both large enzyme complexes and nitrogen-rich peptides are produced. The concentrations of peptides in the cell can be substantial; in this study peptides accounted for up to 0.25% of the dry weight. Because peptides are nitrogen-rich compounds, it could be supposed that WT needs more nitrogen than MU because the total amount of peptides is larger in WT (the number of peptide synthetase complexes is larger). In a laboratory experiment, the biomass concentrations of nontoxic Microcystis strains were higher than those of toxic strains at low phosphate and nitrate concentrations (37). In this study, both Anabaena strains were grown without nitrogen, and they had to acquire all of their nitrogen by nitrogen fixation. However, there was no clear difference in nitrogen fixation between the strains. Probably the increase in nitrogen demand was so low that acetylene reduction was too insensitive to reveal a potential difference. It is also possible that the difference is compensated for because MU produces more anabenopeptins. Although MU does not produce anabaenopeptilides, a chloramphenicol resistance gene has been inserted. Chloramphenicol resistance is based on the production of O-acetyltransferase, which has some costs for the cells as well. Because the growth of WT and the growth of MU were comparable, the costs of producing anabaenopeptilides were outweighed by the increased production of other cyclic peptides and the production of O-acetyltransferase. A Microcystis aeruginosa mutant in which the microcystin synthethase has been disrupted also grows in a manner comparable to the manner of growth of the wild type (5).
MU contained, as expressed in gravimetric units, approximately twice as much anabaenopeptins as WT. MU also produced more microcystins, but the difference was not statistically significant. This raises the question of the significance of anabaenopeptilides to the producer and the question of possibilities to compensate for its absence by increasing the production of other cyclic peptides. Both anabaenopeptilides and anabaenopeptins are considered to be protease inhibitors (12, 20; unpublished observation) and could thus potentially replace each other in this respect.
It has recently been shown that microviridin, another protease inhibitor, from Microcystis disrupts molting in a grazer, Daphnia, due to inhibition of hardening of the chitin-protein complexes (15, 26). It is possible that the same is true for anabaenopeptilides and anabaenopeptins. The morphology of Anabaena in itself is a good defense mechanism against Daphnia. The microcystin synthetase complex has been shown to be ancient and probably predates zooplankton (24). In this respect, it seems logical to look for some other functions for these compounds.
We concluded that the absence of anabaenopeptilides had only minor measurable effects on the cells of MU. However, the higher concentrations of anabaenopeptins in MU point toward compensation with a closely related peptide and thus the importance of these molecules for the producer. More information is needed to determine the roles of these peptides in the producers. In the future, successful production of mutants lacking the other peptides may be crucial for this research.
Acknowledgments
This work was supported by grants from the Academy of Finland (grants 46812, 201576, and 48860), from TEKES (grant 40008), and from the European Union PEPCY (grant QLK4-CT-2002-02634) to K.S.
We thank David Fewer for critically reading the manuscript.
REFERENCES
- 1.Brock, T. D., M. T. Madigan, J. M. Martinko, and J. Parker. 2000. Biology of microorganisms, p. 363-366, Prentice-Hall Inc., Upper Saddle River, N.J.
- 2.Burja, A. M., B. Banaigs, E. Abou-Mansour, J. G. Burgess, and P. C. Wright. 2001. Marine cyanobacteria—a prolific source of natural products. Tetrahedron 57:9347-9377. [Google Scholar]
- 3.Burris, R. H. 1972. Measurement of biological N2 fixation with 15N2 and acetylene, p. 3-14. In Y. I. Sorokin and H. Kadota (ed.), Techniques for the assessment of microbial production and decomposition in fresh waters. IBP handbook 23. Blackwell Scientific International Biological Program, Oxford, United Kingdom.
- 4.Dakshini, I., and K. M. M. Dakshini. 1994. Algal allelopathy. Bot. Rev. 60:182-196. [Google Scholar]
- 5.Dittmann, E., B. Neilan, M. Erhard, H. Von Döhren, and T. Börner. 1997. Insertional mutagenesis of peptide synthetase gene that is responsible for hepatotoxin production in the cyanobacterium Microcystis aeruginosa PCC 7806. Mol. Microbiol. 26:779-787. [DOI] [PubMed] [Google Scholar]
- 6.Dittmann, E., M. Erhard, M. Kaebernick, C. Scheler, B. A. Neilan, H. von Döhren, and T. Börner. 2001. Altered expression of two light-dependent genes in a microcystin-lacking mutant of Microcystis aeruginosa PCC 7806. Microbiology 147:3113-3119. [DOI] [PubMed] [Google Scholar]
- 7.Fujii, K., K.-I. Harada, M. Suzuki, F. Kondo, Y. Ikai, H. Oka, W. W. Carmichael, and K. Sivonen. 1996. Occurrence of novel cyclic peptides together with microcystins from toxic cyanobacteria, Anabaena species, p. 559-562. In T. Yasumoto, Y. Oshima, and Y. Fukuyo (ed.), Harmful and toxic algal blooms. Intergovernmental Oceanographic Commission of UNESCO, Paris, France.
- 8.Fujii, K., K. Sivonen, T. Nakano, and K.-I. Harada. 2002. Structural elucidation of cyanobacterial peptides encoded by peptide synthetase gene in Anabaena species. Tetrahedron 58:6863-6871. [Google Scholar]
- 9.Hecky, R. E., and P. Kilham. 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment. Limnol. Oceanogr. 33:796-822. [Google Scholar]
- 10.Hesse, K., E. Dittmann, and T. Börner. 2001. Consequences of impaired microcystin production for light-dependent growth and pigmentation of Microcystis aeruginosa PCC 7806. FEMS Microbiol. Ecol. 37:39-43. [Google Scholar]
- 11.Honkanen, R. E., F. R. Caplan, K. K. Baker, C. L. Baldwin, S. C. Bobzin, C. M. Bolis, G. M. Cabrera, L. A. Johnson, J. H. Jung, L. K. Larsen, I. A. Levine, R. E. Moore, C. S. Nelson, G. M. L. Patterson, K. L. Tschappat, G. D. Tuang, A. L. Boynton, A. R. Arment, J. An, W. W. Carmichael, K. D. Rodland, B. E. Magun, and R. A. Lewin. 1995. Protein phosphatase inhibitory activity in extracts of cultured blue-green algae (Cyanophyta). J. Phycol. 31:478-486. [Google Scholar]
- 12.Itou, Y. S., S. Suzuki, K. Ishida, and M. Murakami. 1999. Anabaenopeptins G and H, potent carboxypeptidase A inhibitors from the cyanobacterium Oscillatoria agardhii (NIES-595). Bioorg. Med. Chem. Lett. 9:1243-1246. [DOI] [PubMed] [Google Scholar]
- 13.Kaebernick, M., B. A. Neilan, T. Börner, and E. Dittmann. 2000. Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl. Environ. Microbiol. 66:3387-3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaebernick, M., and B. A. Neilan. 2001. Ecological and molecular investigations of cyanotoxin production. FEMS Microbiol. Ecol. 35:1-9. [DOI] [PubMed] [Google Scholar]
- 15.Kaebernick, M., T. Rohrlack, K., Christofferssen, and B. A. Neilan. 2001. A spontaneous mutant of microcystin biosynthesis: genetic characterization and effect on Daphnia. Environ. Microbiol. 3:669-679. [DOI] [PubMed] [Google Scholar]
- 16.Kotai J. 1972. Instructions for preparation of modified nutrient solution Z8 for algae. Publication B-11/69. Norwegian Institute for Water Research, Blindern, Oslo, Norway.
- 17.MacKintosh, C., K. A. Beattie, S. Klumpp, P. Cohen, and G. A. Codd. 1990. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatase 1 and 2A from both mammals and higher plants. FEBS Lett. 264:187-192. [DOI] [PubMed] [Google Scholar]
- 18.Metting, B., and J. W. Pyne. 1986. Biologically active compounds from microalgae. Enzyme Microb. Technol. 8:386-394. [Google Scholar]
- 19.Montgomery D. C. 1997. Response surface methods and other approaches to process optimization, p. 575-604. In Design and analysis of experiments. John Wiley & Sons, New York, N.Y.
- 20.Namikoshi, M., and K. L. Rinehart. 1996. Bioactive compounds produced by cyanobacteria. J. Ind. Microbiol. 17:373-384. [Google Scholar]
- 21.Orr, P. T., and G. J. Jones. 1998. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr. 43:1604-1614. [Google Scholar]
- 22.Patterson, G. M. L., K. K. Baker, C. L. Baldwin, C. M. Bolis, F. R. Caplan, L. K. Larsen, I. A. Levine, R. E. Moore, C. S. Nelson, K. D. Tschappat, G. D. Tuang, M. R. Boyd, J. H. Cardellina II, R. P. Collins, K. R. Gustafson, K. M. Snader, O. S. Weislow, and R. A. Lewin. 1993. Antiviral activity of cultured blue-green algae (Cyanophyta). J. Phycol. 29:125-130. [Google Scholar]
- 23.Patterson, G. M. L., L. K. Larsen, and R. E. Moore. 1994. Bioactive natural products from blue-green algae. J. Appl. Phycol. 6:151-157. [Google Scholar]
- 24.Rantala, A., D. P. Fewer, M. Hisbergues, L. Rouhiainen, J. Vaitomaa, T. Börner, and K. Sivonen. 2004. Phylogenetic evidence for the early evolution of microcystin synthesis. Proc. Natl. Acad. Sci. USA. 101:568-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapala, J., K. Sivonen, C. Lyra, and S. I. Niemelä. 1997. Variation of microcystins, cyanobacterial hepatotoxins, in Anabaena spp. as a function of growth stimuli. Appl. Environ. Microbiol. 63:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohrlack, T., K. Christoffersen, P. E. Hansen, W. Zhang, O. Czarnecki, M. Henning, J. Fastner, M. Erhard, B. A. Neilan, and M. Kaebernick. 2003. Isolation, characterization, and quantitative analysis of microviridin J, a new Microcystis metabolite toxic to Daphnia. J. Chem. Ecol. 29:1757-1770. [DOI] [PubMed] [Google Scholar]
- 27.Rouhiainen, L., L. Paulin, S. Suomalainen, H. Hyytiäinen, W. Buikema, R. Haselkorn, and K. Sivonen. 2000. Genes encoding synthetases of cyclic depsipeptides, anabaenopeptilides, in Anabaena strain 90. Mol. Microbiol. 37:156-167. [DOI] [PubMed] [Google Scholar]
- 28.Rouhiainen, L., T. Vakkilainen, B. L. Siemer, W. Buikema, R. Haselkorn, and K. Sivonen. 2004. Genes coding for hepatotoxic heptapeptides (microcystins) in the cyanobacterium Anabaena strain 90. Appl. Environ. Microbiol. 70: 686-692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sivonen, K., K. Kononen, A.-L. Esala, and S. I. Niemelä. 1989. Toxicity and isolation of the cyanobacterium Nodularia spumigena from the southern Baltic Sea. Hydrobiologia 185:3-8. [Google Scholar]
- 30.Sivonen, K., M. Namikoshi, W. R. Evans, W. W. Carmichael, F. Sun, L. Rouhiainen, R. Luukkainen, and K. L. Rinehart. 1992. Isolation and characterization of a variety of microcystins from seven strains of the cyanobacterial genus Anabaena. Appl. Environ. Microbiol. 58:2495-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sivonen, K., and G. Jones. 1999. Cyanobacterial toxins, p. 41-111. In I. Chorus and J. Bartram (ed.), Toxic cyanobacteria in water—a guide to their public health consequences, monitoring and management. E & F. N. Spon, London, United Kingdom.
- 32.Spoof, L., K. Karlsson, and J. Meriluoto. 2001. High-performance liquid chromatographic separation of microcystins and nodularin, cyanobacterial peptide toxins, on C18 and amide C16 sorbents. J. Chromatogr. A 909:225-236. [DOI] [PubMed] [Google Scholar]
- 33.Tandeau de Marsac, N., and J. Houmard. 1988. Complementary chromatic adaptation: physiological conditions and action spectra. Methods Enzymol. 167:318-328. [Google Scholar]
- 34.Tillett, D., E. Dittmann, M. Erhard, H. von Döhren, T. Börner, and B. A. Neilan. 2000. Structural organization of microcystin biosynthesis in Microcystis aeruginosa PCC 7806: an integrated peptide-polyketide synthase system. Chem. Biol. 7:753-764. [DOI] [PubMed] [Google Scholar]
- 35.Utkilen, H., and N. Gjølme. 1995. Iron-stimulated toxin production in Microcystis aeruginosa. Appl. Environ. Microbiol. 61:797-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Westhausen, A. J., and J. N. Eloff. 1985. Effect of temperature and light on the toxicity and growth of the blue-green alga Microcystis aeruginosa (UV-600). Planta 163:55-59. [DOI] [PubMed] [Google Scholar]
- 37.Vezie, C., J. Rapala, J. Vaitomaa, and K. Sivonen. 2002. Effect of nitrogen and phosphorus on growth of toxic and nontoxic Microcystis strains and on intracellular microcystin concentrations. Microb. Ecol. 43:443-454. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe, M., and S. Oishi. 1985. Effects of environmental factors on toxicity of a cyanobacterium (Microcystis aeruginosa) under culture conditions. Appl. Environ. Microbiol. 49:1342-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weckesser, J., C. Martin, and C. Jakobi. 1996. Cyanopeptolins, depsipeptides from cyanobacteria. Syst. Appl. Microbiol. 19:133-138. [Google Scholar]
- 40.Wittmann, C., K. P. Suominen, and M. S. Salkinoja-Salonen. 2000. Evaluation of ecological disturbance and intrinsic bioremediation potential of pulp mill-contaminated lake sediment using key enzymes as probes. Environ. Pollut. 107:255-261. [DOI] [PubMed] [Google Scholar]
- 41.Yoshizawa, S., R. Matsushima, M. F. Watanabe, K.-I. Harada, A. Ichihara, W. W. Carmichael, and K. Fujiki. 1990. Inhibition of protein phosphatases by microcystins and nodularins associated with hepatotoxicity. J. Cancer Res. Clin. Oncol. 116:609-614. [DOI] [PMC free article] [PubMed] [Google Scholar]