Abstract
Background: P38 mitogen activated protein kinase (MAPK) α modulates microglia-mediated inflammatory responses and a number of neuronal physiological processes.
Objective: To evaluate pre-clinically the pharmacological effects in the brain of p38 MAPKα inhibition with a brain-penetrant specific chemical antagonist.
Methods: VX-745, a blood-brain barrier penetrant, highly selective p38 MAPKα inhibitor, and clinical stage investigational drug, was utilized. Initially, a pilot study in 26-month-old Tg2576 mice was conducted. Subsequently, a definitive dose-response study was conducted in aged (20–22 months) rats with identified cognitive deficits; n = 15 per group: vehicle, 0.5, 1.5, and 4.5 mg/kg VX-745 by oral gavage twice daily for 3 weeks. Assessments in aged rats included IL-1β, PSD-95, TNFα protein levels in hippocampus; and Morris water maze (MWM) test for cognitive performance.
Results: Drug effect could not be assessed in Tg2576 mice, as little inflammation was evident. In cognitively-impaired aged rats, VX-745 led to significantly improved performance in the MWM and significant reduction in hippocampal IL-1β protein levels, though the effects were dissociated as the MWM effect was evident at a lower dose level than that required to lower IL-1β. Drug concentration-effect relationships and predicted human doses were determined.
Conclusions: Selective inhibition of p38 MAPKα with VX-745 in aged rats reduces hippocampal IL-1β levels and improves performance in the MWM. As the two effects occur at different dose levels, the behavioral effect appears to be via a mechanism that is independent of reducing cytokine production. The predicted human doses should minimize risks of systemic toxicity.
Keywords: Aged rats, Alzheimer’s disease, amyloid plaque, cognition, IL-1β , p38 MAPKα , Tg2576 mouse
INTRODUCTION
Human genetic and other biologic data indicate major drivers of Alzheimer’s disease (AD) include dysregulated microglia [1, 2] and neuroinflammation [3, 4]. The intracellular signal transduction enzyme p38 mitogen-activated protein kinase alpha (MAPKα) is a modulator of both [5]; combined with potential biological activities within neurons, p38 MAPKα is a recognized therapeutic target for AD [6]. However, the effects of inhibiting p38 MAPKα in chronic animal models of AD are not fully understood due to previous unavailability of blood-brain barrier penetrant p38 antagonists [6].
P38 MAPKα has been best characterized as a regulator of pro-inflammatory cytokine (IL-1β and TNFα) production from macrophages [7] in the periphery and microglia in the central nervous system (CNS). In the CNS, p38 MAPKα has long been considered a therapeutic target for a range of neuroinflammatory conditions, including AD [8, 9]. P38 MAPKα is also expressed in neurons [10], where the IL-1β-p38 MAPK system has been implicated in regulating memory formation through effects on long-term potentiation/depression [11, 12]; neuronal p38 MAPK has also been implicated in tau phosphorylation [13], as a mediator of the toxic effects of oligomeric amyloid-β (Aβ) [14], and as a regulator of synaptic function/organization [15, 16]. Moreover, recent human genetic data indicate that microglial dysfunction and dysregulated inflammation are major drivers of late-onset AD; and p38 MAPKα appears to modulate signaling pathways downstream of the microglial receptors implicated in late-onset AD [17].
However, there are conflicting data with anti-inflammatory strategies in AD animal models [18]; and the evidence for p38 MAPKα have been primarily based on in vitro observations as until recently blood-brain barrier selective p38 MAPKα antagonists have not been available for study in vivo. Munoz and colleagues [19] reported on a selective brain-penetrant p38α inhibitor that reduced the proinflammatory response (IL-1β, TNFα overproduction), synaptophysin loss, and Y-maze behavioral deficits after 4-week intracerebroventricular human oligomeric Aβ infusion. More recently, the laboratories of Watterson and Van Eldik have reported on in vivo results with “probe” and “tool” brain-penetrant p38 MAPKα inhibitors in which they demonstrated amelioration of synaptic and cognitive dysfunction induced by direct hippocampal infusion of Aβ [20] and reduction of lipopolysaccharide-induced neuroinflammation [21]. However, these compounds do not appear to be in pharmaceutical development, perhaps due to lack of appropriate toxicologicproperties.
VX-745 [22, 23] is a highly selective, orally bioavailable, small molecule p38 MAPKα antagonist with a whole blood IC50 for cytokine production from human peripheral blood mononuclear cells of 60–80 ng/mL and an IC50 for cytokine signaling approximately half that for cytokine production (VX-745 Investigator Brochure). When tested against a panel of 119 protein kinases, VX-745 was at least 100-fold more potent for binding for p38 MAPKα for all but two other kinases (p38 MAPKβ, ABL2), both of which were still less strongly bound (50- and 70-fold lower, respectively) [24]; in a separate study, in enzymatic assays against a panel of 200 kinases, only p38 MAPKα and p38 MAPKβ were inhibited to greater than 80% by VX-745 at 1μM concentration (potency against p38 MAPKβ 10–20 fold less) [25]. In rats after single doses of VX-745, total (bound and unbound) brain drug concentrations were 1.7-fold higher than in plasma; while in dogs at steady-state during two weeks of dosing, total brain concentrations were also 1.7-fold higher than plasma concentrations and cerebrospinal fluid concentrations were 2-fold higher than free plasma drug concentrations (VX-745 Investigator Brochure). VX-745 is an investigational drug that has been evaluated in animal models of peripheral inflammatory disorders and clinically in rheumatoid arthritis (RA), though abandoned in the early 2000 s for that indication in favor of a non-blood-brain barrier penetrant compound. In a 3-month treatment duration phase 2a clinical study [26], VX-745 was generally well tolerated at a dose of 250 mg orally twice a day and demonstrated a statistically significant effect on America College of Rheumatology 20% response (ACR20) rate, the standard measure of drug efficacy in RA clinical trials. As generally seen with systemically administered p38 MAPK inhibitors, the most common adverse event in that study was transient elevation in liver transaminases; which was seen in 10–15% of patients. VX-745 has completed a full set of repeat dose chronic toxicology studies in ratsand dogs.
Given the increasing recognition of the role of inflammation/microglial dysfunction in AD, the potential role of neuronal p38 MAPKα, and the opportunity afforded by VX-745 for assessing in vivo pre-clinically and in the clinic the effects of selective p38 MAPKα inhibition, pre-clinical studies of VX-745 were undertaken. The objectives of these studies were to (1) demonstrate pharmacological activity in the brain; (2) define doses to be evaluated in clinical studies in AD; and (3) demonstrate potential to target the brain at lower doses than that required for peripheral disorders, a means to reduce risk of liver transaminase elevation that has otherwise hindered the development of p38 MAPK antagonists. As the primary pharmacology of p38 MAPK antagonists is generally considered to be inhibition of IL-1β and/or TNF-α production, to meet these objectives studies were conducted in models in which there was literature evidence of increased concentrations of these cytokines. After a pilot study in aged Tg2576 mice failed to show significantly increased levels of these cytokines, a definitive dose-response study was conducted in aged rats where increased concentrations of IL-1β in the hippocampus have been well documented [27] and argued to be an important contributor to the development of cognitive deficits in these animals [33].
MATERIALS AND METHODS
Rodents
All studies, including all assessments, were performed at Charles River Laboratories, Finland. All animal experiments were carried out according to the National Institute of Health (NIH) guidelines for the care and use of laboratory animals, and approved by the National Animal Experiment Board, Finland. In the pilot study, highly aged (26 months) Tg2576 transgenic mice purchased from Taconic received either vehicle (1% pluronic F108; n = 6), and or 3 mg/kg VX-745 (n = 5) by oral gavage for 2 weeks. In the definitive study, aged (20–22 months) Fischer rats with identified cognitive deficits received vehicle (1% pluronic F108) or 0.5, 1.5, and 4.5 mg/kg VX-745 by oral gavage BID×3 weeks (=15 per group); also, n = 15 for vehicle-treated young (2-3 months of age) Fischer rats. The Tg2576 mouse model harboring Swedish mutation at amino acid 695 in the human amyloid-β protein precursor (AβPP) and aged rat model have been previously described [28, 29].
Morris water maze (MWM) test
Water maze task was performed as designed by Morris [30]. Before the compound treatment was started, the visible platform pre-training was performed to determine whether any non-cognitive performance impairments (e.g., visual impairments and/or swimming difficulties) were present and to balance treatment groups by cued performance. After completion of cued trials, acquisition (place) trials were conducted once daily on days 8–11 and days 15–18 to determine the rat’s ability to learn the spatial relationship between distant cues and the submerged escape platform which remained in the same location for all place trials. The starting points were randomized (NW is not used). The rats received four trials (15 min apart, 60 s maximum for each trial) each day for 4 days/week. Latency and path length (distance) were recorded.
A single probe trial was conducted 24 h after the last place trial to evaluate memory retention, in which the time spent in target quadrant and target platform annulus (36-cm diameter circular area surrounding platform), and crosses over the target platform position were measured.
Laboratory analyses
IL-1β and TNF-α ELISA measurements in the Tg2576 mouse study were performed from cortical samples according to the manufacturer’s instructions using R&D Systems kits MLB00B (lot 277809) and MTA00 (lot 278130), respectively. The samples were homogenized into Millipore EZBrain42 kit lysis buffer and protease inhibitors (kit lot 1777753). Cortex and hippocampal soluble extracts from the aged rat study were analyzed for PSD95 (post-synaptic protein) content using an ELISA kit (Cusabio Biotech Ltd., #CSB-EL006938RA, Lot O31154387), while the levels of Il-1β and TNFα were analyzed only from hippocampus (R&D Systems, #RLB00, Lot 308544). Plasma samples were processed using acetonitrile precipitation and then analyzed for VX745 drug concentration by LC-MS/MS.
Immunohistochemistry
Twenty-μm-thick coronal sections were prepared with a cryostat and mounted on SuperFrost Plus glass slides from the fixed, cryoprotected, and frozen hemispheres. Selected sections were then thawed and air-dried. After blocking the internal peroxidase activity and unspecific binding, and washes, sections were reacted overnight at room temperature with either anti-Aβ (mouse anti-Aβ [4–10], the Genetics Company AB02, 1:20,000, clone W0-2) or anti-CD11b (rat anti-CD11b, AbD Serotec Inc. MCA711, 1:500). Thereafter the sections were incubated with proper biotinylated secondary antibody and avidin-biotin complex (Vectastain Elite kit, Vector Laboratories, Burlingame, CA) for 2 h each. The peroxidase containing avidin-biotin complex was visualized using nickel-enhanced DAB as a substrate. Finally, the sections were rinsed, dehydrated, coverslipped, and examined with a Leica 3000RB microscope, and analyzed by ImagePro Plus software. The results were expressed as area fraction (stained areatot/measured areatot, expressed in % ) and reported as mean±SEM among the tissue sections analyzed from each individual transgenic mouse. Ventral cortex and dorsal hippocampus were analyzed from the coronal sections (at the AP level of dorsal hippocampus).
Statistics
For the Tg2576 mouse study and the MWM test results, statistical analysis was performed using StatsDirect statistical software. All values are presented as mean±standard error of mean (SEM). The actual numerical value is provided for p-values less than or equal to 0.1, while values more than 0.1 are simply reported as “ns” (not significant). For the MWM test differences among means were analyzed by using Student’s t-test and one-way-ANOVA followed by Dunnet’s test for the comparison of the active dose of VX-745 to vehicle-treated rats. MWM acquisition data for distance and latency were presented as original scores and as normalized to day 8 performance (day 8, 100% ). Because of distribution pattern of the ELISA results, hippocampal IL-1β and PSD-95 results were evaluated with the Kruskal-Wallis test, followed by the Mann-Whitney rank sum test for the comparison of the active dose group to thevehicle-treated rats.
RESULTS
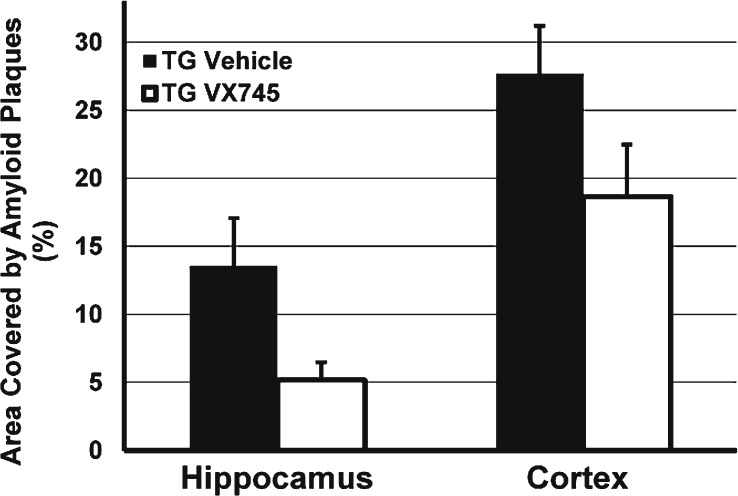
An initial study was performed in 26-month-old Tg2576 mice as prior reports had indicated that inflammation was increased in this model with aging [31]. However, despite aging, in this study minimal inflammation was evident in the brain of the vehicle-treated Tg2576 mice. As a result, levels of the measures assessed (CD11b, TNFα, IL-1β) were either below or near the limit of detection, and there was no means to assess the effect of p38 MAPKα inhibition (data not shown). Surprisingly, there was substantially lower amyloid plaque load by immunohistochemistry in 3 mg/kg VX-745-treated transgenic animals compared to vehicle treated transgenic mice (see Fig. 1), a reduction that approached statistical significance in the hippocampus (5.2% ±1.3 versus 13.6% ±3.5 in vehicle-treated animals; p = 0.069; unpaired two-sided t-test) despite the small numbers of mice: n = 5 for VX-745 and n = 6 for vehicle-treated animals. In addition, amyloid plaque load was numerically lower in the cortex of VX-745 treated animals (18.7% ±3.8 versus 27.7% ±4.8 in vehicle – treated animals; p = not significant).
Fig.1.
Effects of VX-745 on the area of amyloid plaque in the hippocampus and cortex of Tg2576 mice. Expressed as Mean (±SEM) percentage of total area by immunohistochemistry staining for Aβ. Mice treated with VX-745 (3 mg/kg) demonstrated a statistical trend toward decreased number of amyloid plaques in the hippocampus (p = 0.069, unpaired two-sided t-test), compared to vehicle treated transgenic mice. In addition, amyloid plaque load was numerically lower in the cortex of VX-745-treated lower (p = not significant).
With the absence of modifiable inflammation in the Tg2576 mouse (and literature indicating transgenic AD mice do not have a pro-inflammatory phenotype [32]), a larger dose-response study was conducted in aged rats, where the inflammatory milieu more closely mimics that seen in age-related cognitive decline in humans [33]. 20–22-month-old rats were screened with the visible platform in the MWM test, and animals with cognitive impairment were randomized to receive vehicle or one of three dose groups of VX-745 (n = 15/group); the mid-dose of 1.5 mg/kg, was chosen to match by allometric scaling the dose utilized in the Tg2576 mice; and 3-fold lower and 3-fold higher doses were also evaluated. A fifth dose group consisted of young rats that received vehicle.
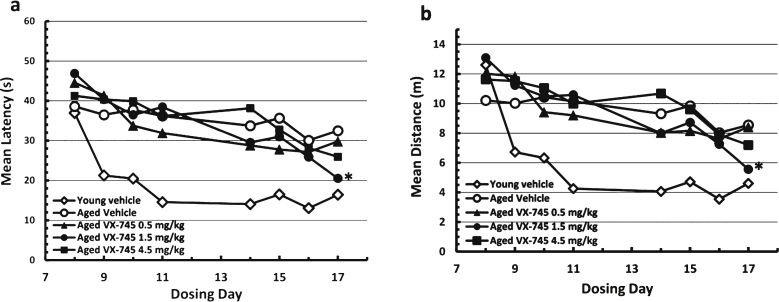
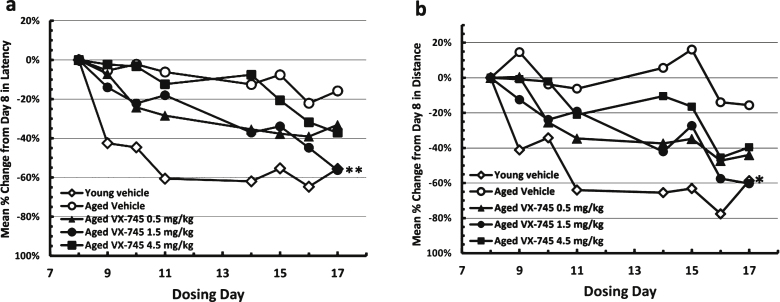
On the MWM test, young rats re-learned the avoidance task quickly (Fig. 2). Aged vehicle-treated rats appeared cognitively impaired, exhibiting longer times and distances on the initial re-testing at day 8, and little performance improvement through two weeks of testing with continued dosing. Relative to vehicle-treated aged rats, swim time and distance decreased at a greater rate in the VX-745-treated aged rats, which is evidence of improved cognitive performance. For both latency and distance, the 1.5 mg/kg dose of VX-745 had the greatest effect, with performance in this group of rats approximating that of young vehicle-treated rats on the last test day. Although the ANOVA revealed only a trend toward a group effect on latency (p = 0.08) on day 17, VX-745 1.5 mg/kg treated rats had significantly decreased latency values on day 17 when compared separately to vehicle treated aged rats (Dunnet’s post-hoc test p = 0.042; t-test: 0.013). For distance, the ANOVA revealed no main group effect (p = 0.1) on day 17. However, VX-745 1.5 mg/kg treated rats had significantly decreased distance values on day 17 when compared to vehicle treated aged rats separately (t-test: 0.019). In addition, VX-745 1.5 mg/kg treated rats had increased distance values on day 8 (t-test: 0.018) indicating that the performance on the first day was poorer compared to vehicle treated aged rats. For this reason, the data were also analyzed after normalization by comparing change in latency and distance from first test day to last test day (Fig. 3). When normalized latency data were analyzed, ANOVA revealed a significant main group effect (p = 0.047) on day 17, and VX-745 1.5 mg/kg treated rats had significantly decreased latency values on day 17 when compared separately to vehicle treated aged rats (Dunnet’s post-hoc test p = 0.018;t-test p = 0.007). When normalized distance data were analyzed, ANOVA revealed a strong trend toward a main group effect (p = 0.06) on day 17 and VX-745 1.5 mg/kg treated rats had significantly decreased distance values on day 17 when compared separately to vehicle treated aged rats (Dunnet’s post-hoc test p = 0.029; t-test: p = 0.012). No effects were seen on probe parameters (data not shown).
Fig.2.
Morris water maze test results during acquisition phase. Results in vehicle-treated young rats and aged rats treated with 0.5, 1.5, or 4.5 mg/kg VX-745 shown as mean (±SEM) Latency (a) and mean (±SEM) Distance (b) by Day of testing. *p < 0.05, for 1.5 mg/kg VX-745 versus aged-vehicle treated rats at last day of testing.
Fig.3.
Normalized Morris water maze test results during acquisition phase. Results in vehicle-treated young rats and aged rats treated with 0.5, 1.5, or 4.5 mg/kg VX-745 shown as Latency (c) and Distance (d) as percentage change from initial testing results at day 8. **p < 0.01 and *p < 0.05 by t-test for latency and distance, respectively, for 1.5 mg/kg VX-745 versus aged-vehicle treated rats at last day of testing.
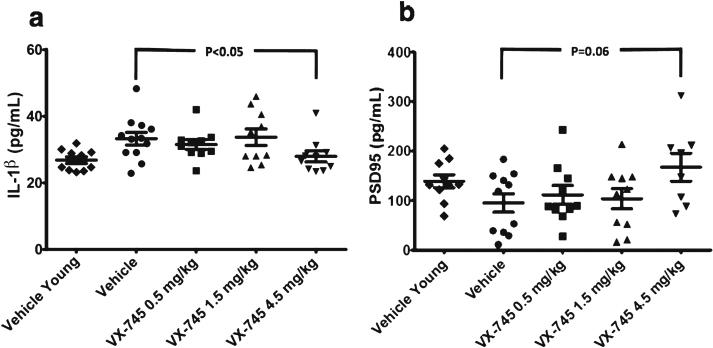
IL-1β protein levels in hippocampal homogenate by ELISA were higher, and of PSD95 were lower in aged-rats compared to young rats; both showed reversion toward young rat protein levels with 4.5 mg/kg VX-745 (Fig. 4). The Kruskal-Wallis test revealed a main group effect for IL-1β (p = 0.02), but not for PSD95 (p = not significant). The 4.5 mg/kg VX-745 demonstrated significantly reduced IL-1β levels (p = 0.038 by Mann-Whitney rank sum test) and a trend toward increased PSD95 levels (p = 0.063) compared to vehicle-treated aged rats. TNFα levels were below limit of detection in all samples analyzed.Cortical levels of PSD95 were highly variable and no differences were apparent between groups, including between vehicle-treated aged and young rats (not shown).
Fig.4.
Protein levels of IL-1β (a) and PSD95 (b) in hippocampal homogenate. Obtained at sacrifice at end of treatment in vehicle-treated young rats and aged rats treated with vehicle or 0.5, 1.5, or 4.5 mg/kg of VX-745. 4.5 mg/kg VX-745 demonstrated significantly reduced IL-1β levels (p = 0.038 by Mann-Whitney rank sum test) and a trend toward increased PSD95 levels (p = 0.06) compared to vehicle-treated aged rats.
Plasma drug levels of VX-745 increased in a dose-proportional manner (Table 1). Drug concentration effects were consistent with in vitro activities of VX-745, with 4.5 mg/kg (which demonstrated a significant effect on hippocampal IL-1β levels) being the only group that exceeded the whole blood IC50 for suppression of cytokine production, while 1.5 mg/kg achieved plasma concentrations that approximated the IC50 for inhibition of cytokine signaling. PK/PD analyses had previously demonstrated that 10 mg/kg dose level in rat adjuvant arthritis model approximates 250 mg orally twice-daily in human RA (VX-745 Investigator Brochure). Comparison of drug concentration-effect correlations in this study with those in the rat adjuvant arthritis and the RA clinical study provides predicted human AD doses: 125 mg orally twice-a-day (i.e., one-half of 250 mg RA dose) for suppression of cytokine production and 40 mg orally twice-a-day (i.e., one-third of dose for suppression of cytokine production) for cognitive effects.
Table 1.
Plasma concentration in ng/mL of VX-745 obtained at sacrifice 1 h after last dose of study drug administration
| VX-745 Dose | Median (Range) | Mean (SD) |
| 0.5 mg/kg | 12.4 (10.1–25.1) | 13.4 (4.5) |
| 1.5 mg/kg | 33.6 (14.4–42.7) | 31.4 (8.5) |
| 4.5 mg/kg | 92.1 (71.5–318) | 126.9 (84) |
DISCUSSION
The reduction in hippocampal levels of the cytokine IL-1β, as well the strong trend on hippocampal levels of the synaptic integrity marker PSD95, demonstrate pharmacological activity of VX-745 in the brain at a dose level that is consistent with its preferential distribution to the brain. In addition, improvement in performance in the MWM test was demonstrated. Importantly, the 1.5-4.5 mg/kg dose range that produced these effects is lower than the 10 mg/kg dose for optimal therapeutic activity for VX-745 in the rat adjuvant arthritis model [23]; and the predicted human doses for either cognitive or anti-inflammatory activity in brain are 5- and 2-fold lower than the 250 mg dose that has been given previously to patients with RA for up to three months (doses up to 750 mg have also been administered for up to four weeks in patients with RA). Taken together, these results provide support for evaluating VX-745 for CNS disorders in the clinic and suggest that VX-745 would have an adequate therapeutic window for such indications.
The improvement in performance in the MWM test occurred at a dose level, 1.5 mg/kg, which did not have an apparent effect on hippocampal IL-1β levels. As the primary pharmacologic activity of p38 MAPK inhibitors is generally considered to be suppression of cytokine production this finding is unexpected. However, it consistent with evidence that inflammation may have both beneficial and deleterious effects [34, 35], and that the goal of therapeutic intervention should be to modulate inflammation rather than full suppression. An alternate explanation that would explain the behavioral effect being at a dose level that did not have an effect on IL-1β production would be that the behavioral effect was mediated by inhibition of IL-1β signaling in neurons rather than inhibition of production of IL-1β from microglia. Moreover, as the effects of IL-1β on long-term potentiation in neurons appear to have both p38 MAPK-dependent and independent components [11] inhibition of IL-1β signaling in the neuron could explain the “U-shaped” dose response (i.e., mid-dose level performs better than either low or high dose level) in the MWM test: the greater potency of VX-745 on cytokine signaling versus cytokine production may allow the mid-dose level to selectively inhibit p38 MAPK dependent deleterious effects of IL-1β signaling in neurons while preserving otherwise positive effects of IL-1β by being below the dose level that impacts more broadly IL-1β action by decreasing the amount of IL-1β in the hippocampus.. In contrast, the high dose level by suppressing IL-1β production would reduce both deleterious and beneficial effects of IL-1β, leading to a more neutral behavioral effect.
One of the concerns regarding inhibiting microglial-driven inflammation has been that it might increase amyloid plaque burden as in certain transgenic models increasing inflammation (e.g., with IL-1β overexpression) reduces amyloid plaque load in AβPP overproducing transgenic mice [36]. Moreover, activation (rather than inhibition) of p38 MAPKα in CX3CR1 (or its ligand, CX3CL1) deficiency in AβPP transgenic mice is associated with increased microglial phagocytic activity and reduced amyloid plaque load relative to the AβPP transgenic mice [37, 38]. While the size of the Tg2576 study in the current report precludes definitive conclusions, the strong trend toward reduction indicates that p38 MAPKα inhibition with VX-745 at the dose utilized at a minimum does not increase amyloid plaque load. One reason for the discrepancy between these results and the CX3CR1/CX3CL1 deficiency result is that in vitro only modest p38 MAPK activation is required for increasing Aβ uptake [39] and the low dose of VX-745 utilized in the current study may have preserved microglial phagocytic activity. In addition, the transgenic mouse experiments are generally read out when mice are young (<12 months) when the microglial phenotype tends to be biased toward the M2 (pro-phagocytic) phenotype [32], and so increasing microglial numbers would be predicted to reduce amyloid plaque load through increasing phagocytosis of amyloid plaques. The current study was conducted in advanced age mice where the microglial phenotype would be expected to bias away from the phagocytic phenotype and toward the more toxic (“M1”) phenotype. Inhibition of p38 MAPKα in the advanced-age context may then promote the switch to the phagocytic phenotype, which could not have occurred in younger mice since their microglia already are in that phenotype. Consistent with that hypothesis, blocking pro-inflammatory cytokines with either anti-IL1 antibody or a TNF synthesis inhibitors in aged (15–17-month-old) mice reduces both amyloid plaque burden and tau pathology [40, 41]. Regardless, further animal studies of VX-745 to evaluate pre-clinically the effects on amyloid plaque load were not pursued to confirm the trends because of the differences between the mouse and human innate immune systems [42], which would in any case make difficult to translate mouse results to human AD. Instead, it is planned to utilize amyloid plaque load by PET scan in initial clinical studies of VX-745 in AD to monitor for either beneficial or adverse effects on amyloid plaque load.
The development of p38 MAPKα inhibitors for peripheral inflammatory disorders has been hindered by systemic toxicity risks. With VX-745 and its preferential distribution to the brain, the concentration-effect analysis indicates that pharmacologic activity may be achieved in the brain with doses below those required for pharmacologic activity in the periphery. Moreover, toxicity risks could be further reduced through utilizing doses that inhibit p38 MAPKα dependent signaling, which in the case of VX-745 occurs at lower concentration than those required for suppression of cytokine production. Targeting cytokine signaling rather than cytokine production may provide an additional opportunity for p38 MAPKα inhibition as inhibition of cytokine production in RA clinical studies was not maintainable with long-term dosing, possibly due to a bypass-signaling pathway for cytokine production that is activated via feedback loops [43, 44].
During the revision of this manuscript an article was published from the groups of Watterson and Van Eldik [45] on the potential of p38 MAPKα inhibitors. They present the discovery and profiling of a novel brain-penetrant selective p38 MAPKα antagonist that has similar kinase selectivity profile as VX-745, MW150. MW150, when administered in preventive mode (from week 8 to age 3-4 months) to AβPP-PS1 mice, improved performance in radial water maze and contextual fear conditioning tests. In addition, 2-week administration to humanized AβPP-PS1 knock-in mice after development of cognitive deficits at age 11 months led to reversal of radial water maze deficits and “behavior indistinguishable from wild-type mice”. This latter finding is similar to the VX-745 MWM results reported in the current report. The combined result with distinct selective p38 MAPKα inhibitors indicates that targeting this mechanism has the potential to improve cognitive performance in the clinic, a clinical effect that is substantially more readily ascertained than prevention of cognition decline. MW150 is apparently otherwise undergoing non-clinical IND enabling evaluation.
In conclusion, selective inhibition of p38 MAPKα with VX-745 in aged rats reduces hippocampal IL-1β levels and improves performance in the MWM test. As the two effects occur at different dose levels, the behavioral effect appears to be via a mechanism that is independent of reducing cytokine production. Combined with its preferential distribution to the brain, VX-745 appears to have the potential to be clinically active at doses that would minimize the risks for systemic toxicity that have otherwise limited the development of p38 MAPK inhibitors. Finally, with the prior clinical experience and understanding of dose-response, VX-745 provides a unique opportunity to directly explore the effects in patients of p38 MAPKα inhibition specifically, and of suppressing neuroinflammation generally in human AD and other inflammatory disease of the brain.
ACKNOWLEDGMENTS
Jukka Puoliväli (JP), PhD, Marc Cerrada-Gimenez (MC-G), PhD, and Susanna Saario, PhD at Charles River Laboratories Finland for direction of all study activities; and to JP and MC-G for conducting initial analysis of results.
The author is the founder and managing member of EIP Pharma, LLC, a private company based in Cambridge, Massachusetts, USA. Funding was provided to EIP Pharma by the author. EIP Pharma used the funding to contract with Charles River Laboratories to conduct the studies. Subsequent to the completion of the pre-clinical studies, EIP Pharma licensed VX-745 from an originator and is preparing to clinically develop the compound for Alzheimer’s disease.
The author’s disclosure is available online (http://j-alz.com/manuscript-disclosures/15-0277r1).
REFERENCES
- 1.Aguzzi A, Barres BA, Bennett ML. Microglia: Scapegoat, saboteur, or something else? Science. 2013;339:156–161. doi: 10.1126/science.1227901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gandy S, Heppner FL. Microglia as dynamic and essential components of the amyloid hypothesis. Neuron. 2013;78:575–577. doi: 10.1016/j.neuron.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meraz-Rios MA, Toral-Rios D, Franco-Bocanegra D, Villeda-Hernandez J, Campos-Pena V. Inflammatory process in Alzheimer’s Disease. Front Integr Neurosci. 2013;7:59. doi: 10.3389/fnint.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin WST. Neuroinflammatory cytokine signaling and Alzheimer’s disease. N Engl J Med. 2013;368:770–771. doi: 10.1056/NEJMcibr1214546. [DOI] [PubMed] [Google Scholar]
- 5.Bachstetter AD, Xing B, de Almeida L, Dimayuga ER, Watterson DM, Van Eldik LJ. Microglial p38alpha MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Abeta) J Neuroinflammation. 2011;8:79. doi: 10.1186/1742-2094-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munoz L, Ammit AJ. Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology. 2010;58:561–568. doi: 10.1016/j.neuropharm.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein DM, Kuglstatter A, Lou Y, Soth MJ. Selective p38α inhibitors clinically evaluated for the treatment of chronic inflammatory disorders. J Med Chem. 2010;53:2345–2353. doi: 10.1021/jm9012906. [DOI] [PubMed] [Google Scholar]
- 8.Bachstetter AD, Van Eldik LJ. The p38 MAPK family as regulators of proinflammatory cytokine production in degenerative diseases of the CNS. Aging Dis. 2010;1:199–211. [PMC free article] [PubMed] [Google Scholar]
- 9.Correa SA, Eales KL. The role of p38 MAPK and its substrates in neuronal plasticity and neurodegenerative disease. J Signal Transduct. 2012;2012:649079. doi: 10.1155/2012/649079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing B, Bachstetter AD, Van Eldik LJ. Inhibition of neuronal p38α, but not p38β, provides neuroprotection against three different neurotoxic insults. J Mol Neurosci. 2015;55:509–518. doi: 10.1007/s12031-014-0372-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neurosci Biobehav Rev. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Barrientos RM, Frank MG, Watkins LR, Maier SF. Aging-related changes in neuroimmune-endocrine function: Implications for hippocampal-dependent cognition. Horm Behav. 2012;62:219–227. doi: 10.1016/j.yhbeh.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Liu L, Barger SW, Griffin WS. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23:1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li S, Jin M, Keoglsperger T, Shepardson NE, Shankar GM, Selkoe DJ. Soluble Aβ oligomers inhibit long-term potentiation through a mechanism involving excessive activation of extrasynaptic NR2B-containing NMDA receptors. J Neurosci. 2011;31:6627–6638. doi: 10.1523/JNEUROSCI.0203-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez F, Soon I, Li Z, Kuan TC, Min DH, Wong ES, Demidov ON, Paterson MC, Dawe G, Bulavin DV, Xiao ZC. Wip1 phosphatase positively modulates dendritic spine morphology and memory processes through the p38 MAPK signaling pathway. Cell Adh Migr. 2012;6:333–343. doi: 10.4161/cam.20892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong L, Prieto GA, Kramar EA, Smith ED, Cribbs DH, Lynch G, Cotman CW. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1β via p38 mitogen-activated protein kinase. J Neurosci. 2012;32:17714–17724. doi: 10.1523/JNEUROSCI.1253-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katholnig K, Kaltenecker CC, Hayakawa H, Rosner M, Lassnig C, Zlabinger GJ, Gaestel M, Muller M, Hengstschlager M, Horl WH, Park JM, Saemann MD, Weichhart T. p38α senses environmental stress to control innate immune responses via mechanistic target of rapamycin. J Immunol. 2013;190:1519–1527. doi: 10.4049/jimmunol.1202683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birch AM, Katsour L, Sastre M. Modulation of inflammation in transgenic models of Alzheimer’s disease. J Neuroinflammation. 2014;11:25. doi: 10.1186/1742-2094-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munoz L, Ranaivo HR, Roy SM, Hu W, Craft JM, McNamar LK, Chico LW, Van Eldik LJ, Watterson DM. A novel p38α MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer’s disease animal model. J Neuroinflammation. 2007;4:21. doi: 10.1186/1742-2094-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watterson DM, Grum-Tokars VL, Roy SM, Schavocky JP, Bradaric BD, Bachstetter AD, Xing B, Dimayuga E, Saeed F, Zhang H, Staniszewski A, Pelletier JC, Minasov G, Anderson WF, Arancio O, Van Eldik LJ. Development of novel in vivo chemical probes to address CNS protein kinase involvement in synaptic dysfunction. PLoS One. 2013;8:e6626. doi: 10.1371/journal.pone.0066226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bachstetter AD, Watterson DM, Van Eldik LJ. Target engagement analysis and link to pharmacodynamic endpoint for a novel class of CNS-penetrant and efficiacious p38α MAPK inhibitors. J Neuroimmune Pharmacol. 2104;9:454–460. doi: 10.1007/s11481-014-9543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duffy J, Harrington EM, Salituro FG, Cochran JE, Green J, Gao H, Bemis GW, Evindar G, Galullo VP, Ford PJ, Germann UA, Wilson KP, Bellon SF, Chen G, Taslimi P, Jones P, Huang C, Pazhanisamy S, Wang YM, Murcko MA, Su MS. The discovery of VX-745: A novel and selective p38α kinase inhibitor. ACS Med Chem Lett. 2011;2:758–763. doi: 10.1021/ml2001455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haddad JJ. VX-745. Vertex Pharmaceuticals. Curr Opin Investig Drugs. 2002;2:1070–1076. [PubMed] [Google Scholar]
- 24.Fabian MA, Biggs WH, Treiber DK, Atteridge CE, Azimioara MD, Benedetti MG, Carter TA, Ciceri P, Edeen PT, Floyd M, Ford JM, Galvin M, Gerlach JL, Grotzfeld RM, Herrgard S, Insko DE, Insko MA, Lai AG, Lélias JM, Mehta SA, Milanov ZV, Velasco AM, Wodicka LM, Patel HK, Zarrinkar PP, Lockhart DJ. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 25.Verkaar F, van der Doelen AA, Smits JFM, Blankesteijn WM, Zaman GJ. Inhibition of Wnt/β-catenin sigaling by p38 MAPK Kinase inhibitors is explained by cross-reactivity with casein kinase Iδ/ɛ . Chem Biol. 2011;18:485–494. doi: 10.1016/j.chembiol.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Weisman M, Furst D, Schiff M, Kauffman R, Merica E, Martin-Munley S (2002) A double blind study of VX-745, an oral p38 mitogen activate protein kinase inhibitor, in patients with rheumatoid arthritis. EULAR conference 2002, Abstract FRI0018, http://www.abstracts2view.com/eular/view.php?nu=EULAR2L1_2002FRI0018, Accessed March 21, 2015
- 27.Murray CA, Lynch MA. Evidence that increased hippocampal expression of the cytokine interleukin-1 beta is a common trigger for age- and stress-induced impairment in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapman PF, White GL, Jones MW, Cooper-Blackseter D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 29.Bolognin S, Buffeli M, Puolivalia J, Iqbal K. Rescue of cognitive-aging by administration of a neurogenic and/or neurotropic compound. Neurobiol Aging. 2104;35:2134–2146. doi: 10.1016/j.neurobiolaging.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 31.Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW. Exercise alters the immune profile in Tg2576 mice toward a response coincident with improved performance and decreased amyloid. J Neuroinflammation. 2008;5:13. doi: 10.1186/1742-2094-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilcock DM, Zhao Q, Morgan D, Gordon MN, Everhar A, Wilson JG, Lee JE, Colton CA. Diverse inflammatory responses in transgenic mouse models of Alzheimer’s disease and the effect of immunotherapy on these responses. ASN Neuro. 2011;5:249–258. doi: 10.1042/AN20110018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lynch MA. Age-related neuroinflammatory changes negatively impact on neuronal function. Front Aging Neurosci. 2010;1:6. doi: 10.3389/neuro.24.006.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santello M, Volterra A. TNFα in synaptic function: Switching gears. Trends Neurosci. 2012;35:638–647. doi: 10.1016/j.tins.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Matousek SB, Ghosh S, Shaftel SS, Kyrkanides S, Olschowka JA, O’Banion MK. Chronic IL-1β-mediated neuroinflammation mitigates amyloid pathology in a mouse model of Alzheimer’s disease without inducing overt neurodegeneration. J Neuroimmune Pharmacol. 2012;7:156–164. doi: 10.1007/s11481-011-9331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh S, Wu MD, Shaftel SS, Kyrkanides S, La Ferla FM, Olshowka JA, O’Banion MK. Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer’s mouse model. J Neurosci. 2011;33:5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee S, Xu G, Jay TR, Bhatta S, Kin KW, Jung S, Landreth GE, Ransohoff RM, Lamb BT. Opposing effects of membrane-anchored CX3CL1 on amyloid and tau pathologies via the p38 MAPK pathway. J Neurosci. 2014;10:12538–12546. doi: 10.1523/JNEUROSCI.0853-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CXCR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer’s disease mouse models. Am J Pathol. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, Lohmann S, Piorkowska K, Gafner V, Atwal JK, Maloney J, Chen M, Gogineni A, Weimer RM, Mortensen DL, Friesenhahn M, Ho C, Paul R, Pfeifer A, Muhs A, Watts RJ. An effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulment of Aβ. J Neurosci. 2012;32:9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitazawa M, Cheng D, Tuskamoto MR, Koike MA, Wess PD, Vasilevko V, Cribbs DSH, LaFerla FM. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J Immunol. 2011;187:6539–6549. doi: 10.4049/jimmunol.1100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tweedie D, Ferguson RA, Fishman K, Fishman K, Frankola KA, Van Praag H, Holloway HW, Luo W, Li Y, Carracciolo L, Russo I, Barlati S, Ray B, Lahiri DK, Bosetti F, Greig NH, Rosi S. Tumor necrosis factor-α synthesis inhibitor 3,6’-dithiothalidomide attenuates markers of inflammation, Alzheimer pathology and behavioral deficits in animal models of neuroinflammation and Alzheimer’s disease. J Neuroinflammation. 2012;9:106. doi: 10.1186/1742-2094-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mestas J, Hughes CCW. Of mice and not men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 43.Clark AR, Dean JL. The p38 MAPK pathway in rheumatoid arthritis: A sideways look. Open Rheumatol J. 2012;6:209–219. doi: 10.2174/1874312901206010209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hua F, Henstock PV, Tang B. ERK activation by GM-CSF reduces effectiveness of p38 inhibitor on inhibiting TNFalpha release. Int Immunopharmacol. 2010;10:730–737. doi: 10.1016/j.intimp.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Roy SM, Grum-Tokars VL, Schavocky JP, Saeed F, Staniszewski A, Teich AF, Rancio O, Bachstetter AD, Webster SJ, Van Eldik LJ, Minasov G, Anderson WF, Pelletier JC, Watterson DM. Targeting human central nervous system protein kinases: An isoform selective p38αMAPK inhibitor that attenuates disease progression in Alzheimer’s disease mouse models. ACS Chem Neurosci. 2015;15:666–680. doi: 10.1021/acschemneuro.5b00002. [DOI] [PMC free article] [PubMed] [Google Scholar]