Abstract
Background: Urinary problems, including urinary frequency, urgency, and nocturia are some of the non-motor symptoms that correlate most with poor quality of life in Parkinson’s disease. However, the mechanism behind these symptoms is poorly understood, in particular regarding peripheral bladder pathophysiology following dopamine degeneration.
Objective: In this study, we compared the contractile responsiveness of urinary bladder from the 6-OHDA unilateral rat model of Parkinson’s disease with that of normal untreated animals.
Methods: The contractility of the urinary detrusor muscle was evaluated in bladder strip preparations using electrical field stimulation, and muscarinic and purinoceptor stimulations in an vitro organ bath setup.
Results: Our data show that the overall contractile response following electrical field stimulation was significantly higher (43% at maximum contraction by 20–40 Hz stimulation) in the 6-OHDA-lesioned rats as compared to control animals. This increase was associated with a significant increase in the cholinergic contractile response, where the muscarinic agonist methacholine produced a 44% (at 10 −4 M concentration) higher response in the 6-OHDA-treated rats as compared to controls with a significant left-shift of the dose response. This indicates an altered sensitivity of the muscarinic receptor system following the specific central 6-OHDA-induced dopamine depletion. In addition a 36% larger contraction of strips from the 6-OHDA animals was also observed with purinoceptor activation using the agonist ATP (5×10 −3 M) during atropine treatment.
Conclusions: Our data shows that it is not only the central dopamine control of the micturition reflex that is altered in Parkinson’s disease, but also the local contractile function of the urinary bladder. The current study draws attention to a mechanism of urinary dysfunction in Parkinson’s disease that has previously not been described.
Keywords: Urinary bladder pathophysiology, detrusor muscle, parasympathetic nervous system, muscarinic receptor
INTRODUCTION
Parkinson’s disease (PD) is characterized by the appearance of the motor symptom bradykinesia and at least one of tremor, rigidity and postural imbalance. However, the PD symptomatology also includes non-motor features, and clinical research in the last decades has focused on characterizing and understanding the impact of non-motor symptoms like depression, cognitive impairment and impaired autonomic function on the patient’s quality of life [1–3]. Dysfunction in the autonomic nervous system includes gastrointestinal and urinary problems. These problems are two of the most prevalent and debilitating non-motor symptoms [3]. Problems with micturition, so called lower urinary tract symptoms (LUTS), affect as many as three out of four patients with early-to-moderate PD [4]. The most common urinary problems in PD are nocturia, which more than 80% of the patients are affected by, followed by frequency (≈70% ) and urgency (≈65% ) [5]. Among patients affected by LUTS up to 39% suffer from severe LUTS, showing several of the LUTS symptoms. The urinary problems also worsen as the disease progresses. Barone and colleagues showed that around 43% of the PD patients at Hoehn and Yahr stage 1 exhibited urinary problems, while at stage 4 up to 90% suffered from these symptoms [1]. Furthermore, common PD treatment has an unpredictable effect on the urinary symptoms, where some patients get symptomatic relief, while others are unaffected or even experience worsening of the symptoms with treatment [6–8].
Although these problems are very disabling for the patients, only limited research has been conducted to better understand the mechanisms leading to the overactive bladder in PD. It is know that the midbrain dopamine neurons control the micturition reflex through the pontine micturition center (PMC) in the brainstem by D1 and D2 receptor activation, directly by innervation from the ventral tegmental area (VTA) and indirectly from the substantia nigra (SN) via striatal and globus pallidal loops [4]. This dopamine effect has been demonstrated in rodents where D1 receptors activation tonically inhibits the reflex, while D2 receptor activation facilitates the same micturition reflex [9]. Similarly, in the 6-OHDA lesion model of PD, which shows similar urinary bladder overactivity as seen in human PD patients [10–13], D1 receptor agonists reverse bladder hyperactivity, and D2 receptor agonists further aggravate the urinary detrusor activity [13]. Dopamine degeneration of cells and fibers in the midbrain and striatum, following 2–4 weeks after intracerebral injections of 6-OHDA, not only increase spontaneous activity in the bladder but also lead to several LUTS, including reduced bladder capacity and micturition volume and increased micturition frequency, which are all observed in PD patients [11, 13–15]. These findings suggest that the classical 6-OHDA-lesion model of Parkinson’s disease is also relevant for studying the mechanism of urinary dysfunction in PD patients.
However, the limited studies published until today, to our knowledge, only focus on the effect of central dopamine lesions in the basal ganglia on the bladder function, rather than if changes occur locally in the urinary bladder. Therefore, in the current study we have investigated the contractile function of isolated bladder strips from normal and 6-OHDA-lesioned rats, in response to electric (electrical field stimulation, EFS) and pharmacological stimulation by muscarinic and purinergic agonists and antagonists. We show for the first time that the contractile response to electric and pharmacological stimulation is significantly altered locally in the Parkinsonian bladder. We believe this will add to an expanded understanding of the mechanisms of urinary bladder dysfunction in PD.
MATERIALS AND METHODS
Animals
A total of 38 adult male Sprague-Dawley (Charles-River GmbH, Germany) rats, with an age and weight of 12–20 weeks and 340–675 g, respectively, were used in the present study. The animals were housed under 12 h light - 12 h dark conditions with free access to fresh water and food. All handling and surgical procedures were approved by the local ethics committee for laboratory animals (Jordbruksverket: Dnr: 286–2012), which is in accordance with EU legislation (Directive 2010/63/EU).
Experimental design
The contractility of the urinary bladders was evaluated in 25 normal control rats (“Control”; average weight: 431±21 g), and 13 animals that received a unilateral 6-OHDA lesion in the medial forebrain bundle (MFB; “6-OHDA”; average weight: 448±11 g) to achieve a severe lesion of the dopaminergic pathway. Healthy untreated rats, in contrast to sham-operated animals, were used as controls in the current study as Soler and colleagues showed no significant functional differences between these groups at the tested time point [14]. The control and 6-OHDA lesioned rats (taken at 26–29 days post lesion) were deeply anesthetized by an over-dose of pentobarbital, the bladders were removed, and two full thickness tissue strips were prepared from each bladder for in vitro organ bath measurements. First, the viability (responsiveness) of the prepared strips was evaluated using a high K + Krebs solution (see In-vitro organ baths section), where a contractile response of >8 mN was set as a criterion for a viable bladder strip. This yielded an inclusion of 34 strips (from 20 rats) in the control group, and 19 strips (from 13 rats) in the 6-OHDA lesion group. In the included bladder strips the contractile muscle responses were evaluated following EFS, and administration of the muscarinic agonist methacholine and the purinergic agonist ATP with or without agonists as described below (see In-vitro organ baths section).
Following the removal of the bladder of the 6-OHDA lesioned rats for the in vitro measurements, the animals were transcardially perfused with PFA and the brain was removed and processed for immunohistochemistry.
6-OHDA lesion surgery
The unilateral DA lesion was carried out under 1-2% isoflurane (in air; Forene Abbott, Wiesbaden, Germany) general anesthesia. Additional local anesthesia (Marcain 2.5 mg/ml, AstraZeneca AB, Södertälje, Sweden), was applied in the area of the incision in the scalp, and following surgery Romefen (5 mg/kg; VET, Merial, Lyon, France) was used as post-analgesia. Briefly, in order to achieve a severe dopamine lesion in the nigrostriatal pathway, the rats were placed in a stereotaxic frame (Kopf Instruments, Tujunga, CA), and 14μg 6-OHDA (Sigma-Aldrich AB, Stockholm, Sweden) in 4μl ascorbic acid (0.02% in saline) was injected intracerebrally at one site in the right or left MFB with the coordinate (according to the bregma): anterior-posterior: −4.4 mm; medial-lateral:±1.2 mm; dorsal-ventral: −7.8 mm below the dura. The tooth bar was set to −2.4mm for the injection. The toxin was injected into the brain at a rate of 1μl/ml, and the needle was kept in place for an additional 3 min before slowly being retracted. Thirty minutes prior to the 6-OHDA injections the selective noradrenaline reuptake inhibitor desipramine (25 mg/kg, Sigma Aldrich AB) was intraperitonally injected to protect the noradrenergic neurons from the 6-OHDA toxicity.
In vitro organ bath experiments
At 26–29 days post 6-OHDA injections, the rats were given an overdose of sodium pentobarbital (100–200 mg/kg, APL, Stockholm, Sweden) and the urinary bladder was dissected out. Two approximately 6×3 mm tissue strips were cut out from the excised bladders, and each strip was mounted in an organ bath (Linton Instrumentation, Norfolk, UK); one end attached to a mounting hook and the other to a force transducer (TSD125C, Biopac systems Inc., Goleta, CA). Each bath contained Krebs solution [NaCl, 118 mM; KCl, 4.6 mM; KH2PO4, 1.15 mM; MgSO4 (anhydrous), 1.15 mM; NaHCO3, 25 mM; CaCl2, 1.25 mM; and glucose, 5.5 mM], gassed by 95% O2 and 5% CO2, and heated to 37°C. The tissue strips were stretched to a basal tension of around 5 mN (average baseline for all animals: 4.9±0.10 mN [Controls: 4.9±0.13 mN; 6-OHDA-lesioned animals: 4.9 ± 0.12 mN)], and left to equilibrate for 45 minutes.
Following the equilibration phase, the strip was first challenged with exchanging the normal Krebs solution in the baths with a high K + Krebs solution (containing 124 mM K + obtained by exchanging Na + for equimolar amounts of K +), in order to evaluate the viability of the bladder tissue. Strips with a maximum response of >8 mN from the baseline were considered viable, and were selected for further measurements. Following three washes with normal Krebs and a recovery period of 10–15 min each strip (Control: 25 strips and 6-OHDA: 19 strips) was challenged with EFS at 1, 2, 5, 10, 20 and 40 Hz (Stimulator: STM100C; Linton, Welwyn Garden City, UK) at supra-maximal voltage; delivered as square wave pulses with a duration of 0.8 ms, until the peak response was obtained. Following an additional 15 min recovery period after the EFS, the muscarinic agonist methacholine (MeCH; Sigma-Aldrich, St Louis, MO, USA) was cumulatively applied to each bath at concentrations 10 − 8, 10 − 7, 10 − 6, 10 − 5, 10 − 4 and 10 − 3 M (Control: 27 strips and 6-OHDA: 12 strips). Following three washes with normal Krebs and a recovery period of 15 min each strip was challenged with the purinergic agonist ATP (Sigma-Aldrich) at cumulative concentrations starting at 10 − 8, 10 − 7, 10 − 6, 10 − 5, 10 − 4, 10 − 3 and 5×10 − 3 M (Control: 27 strips and 6-OHDA: 12 strips). The contractile responses to EFS, methacholine and ATP were also evaluated at 20 min following the administration of the muscarinic receptor antagonist atropine (10 − 6 M). In 7 control strips and 7 strips from 6-OHDA lesioned animals the EFS was also evaluated in the presence of the non-selective alpha-adrenergic antagonist phentolamine (10 − 5 M; Sigma-Aldrich) together with atropine and suramin (a purinergic P2 antagonist; 10 − 5 M; Sigma-Aldrich).
At the end of each experiment, after the contractile measurements, the strips were removed from the organ baths, briefly dried and weighed on a scale.
The contractile response of the bladder strip was recorded and analyzed using the MP100WSW data acquisition system with Acknowledge Software v 3.8 (Biopac systems Inc., Goleta, CA). The basal tension was measured as an average over 5–15 seconds prior to each EFS frequency, and to first doses of methacholine and ATP, while the maximum response was measured at the absolute peak.
Histological analysis
Following the removal of the bladder, and during deep anesthesia after administering an overdose of sodium pentobarbital, all 6-OHDA-lesioned animals were transcardially perfused with 4% PFA in 0.1 PBS, as previously described [16]. Briefly, a needle was inserted in the right ventricle, and 20–40 ml of room tempered 0.9% NaCl, followed by 150–200 ml ice cold 4% PFA solution in 0.1M phosphate buffer (PB) was infused for 8–10 minutes. The brains were removed, postfixed in the same fixative overnight and transferred into 25% Sucrose (in 0.1M PB) for at least 24 h before they were cut into 35μm thick slices (in 7 series) using a cryotome (Leica, CM1950; Leica Microsystems, Nussloch, Germany). The sections were finally stored in cryoprotectant until further use.
Immunohistochemistry
Free-floating sections of the brain were stained according to a previously described protocol [16]. Briefly, the sections were first quenched in 3% hydrogen peroxide (H2O2) and 10% methanol in PBS. The sections were then pre-incubated for one hour in 5% appropriate normal serum (Vector Laboratories, Burlingame, CA) and 0.25% triton-x in PBS, followed by an overnight incubation with the primary antibody in the same solution. To detect the dopamine fibers in the striatum, the mouse anti-tyrosine hydroxylase (1:2000, MAB318, Merck Millipore, Billerica, MA) antibody was used. The primary antibody incubation was followed by a one-hour incubation in appropriate biotinylated secondary antibody (1:200, BA2001, Vector Laboratories). This step was followed by one hour in an avidin-biotin-complex solution (ABC Elite, Vector Laboratories), and finally the staining was visualized by 3,3′-diaminobenzidine and 0.01% H2O2. The sections were then mounted on glass slides and cover slipped using DEPEX. All steps were performed at room temperature, and between each step the sections were rinsed in PBS.
TH-positive fiber density measurements in the striatum
In order to evaluate the dopamine lesion severity in the striatum, the mean optical density at 5 striatal levels, corresponding to approximately +1.6 +1.0, + 0.3, −0.3 and −0.90 mm from the bregma [17], was measured using the ImageJ v1.48 software for MacOS X platform (NIH, USA; http://imagej.nih.gov/ij/). The striatum was outlined as previously described [18] and the corpus callosum was used as background staining. The images were obtained by scanning the slides in a photo copier (Toshiba e-STUDIO656SE).
Statistical analysis
Statistical analyses were performed using a two-way repeated measure ANOVA, followed by the Bonferroni multiple comparison test, or an unpaired t-test where appropriate. The EC50 values of the methacholine response were calculated out of the percent of individual maximal response. All calculations were conducted using the GraphPad Prism program for Mac OS X (GraphPad Software Inc, San Diego, CA, USA), and the data in the study are presented as the mean±S.E.M., with a level of significance set to p < 0.05.
RESULTS
In the present study we evaluated the contractile responses of the urinary bladder to EFS and muscarinic and purinoceptor stimulation, in normal control rats (Control) and in animals four weeks post 6-OHDA-lesion in the MFB (6-OHDA). Visual inspection and fiber density measurements of the striatum revealed more than 95% dopamine fiber loss in 11 of 13 animals (average TH fiber density: 3.5±2.3% compared to the intact side; Fig. 1A), while two animals showed only a partial dopamine lesion (67.6 and 48.0% compared to the intact side) and were therefore excluded from further data analysis.
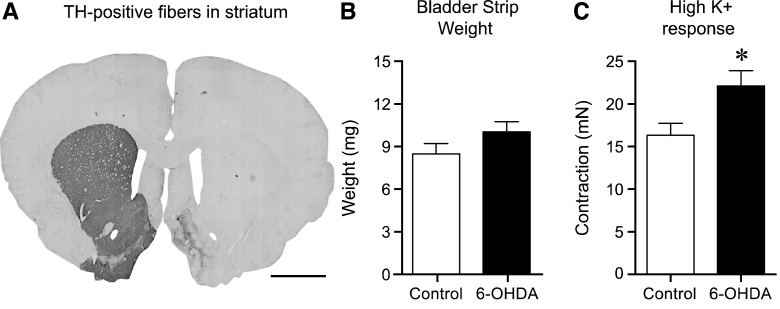
Fig.1.
The effect of 6-OHDA lesion on striatal tyrosine hydroxylase (TH)-positive fibers, bladder weight and contractile response to high K + Krebs. Following unilateral 6-OHDA lesion TH-positive fibers in the striatum were >95% abolished as compared to the untreated side (A). The bladder strip weight in the 6-OHDA-lesioned animals showed a trend to be larger (10.0±0.8 mg, n = 17) as compared to normal (8.4±0.8 mg, n = 34), however this 20% increase was not significant (p = 0.20, unpaired t-test; B). Testing the viability of the strips by using high K + Krebs solution revealed a significantly (36% ) higher response in the 6-OHDA-lesioned animals as compared to the untreated controls; 22.1±1.8 mN (n = 34) vs. 16.2±1.5 mN (n = 17) respectively (unpaired t-test p = 0.022; C). Scale bar in Panel A represents 2 mm. * = significantly different from control.
Interestingly, during dissection, the appearance of the urinary bladders of the 6-OHDA rats were somewhat altered. The tissue was more rigid and in some cases had a reddish tone, as if being inflamed. The observed difference in appearance of bladder from 6-OHDA-lesioned and control rats did not translate into significantly different strip weights (control strips weighed 8.4±0.8 mg, and strips from 6-OHDA-treated animals 10.0±0.8 mg, p = 0.20; Fig. 1B). However, the tissue strip viability test using high K + Krebs solution revealed a significantly higher response in the 6-OHDA-lesioned animals as compared tonormal controls reaching 22.1±1.8 mN and 16.2±1.5 mN, respectively (36% increase; p = 0.022; Fig. 1C).
Effect of EFS on the contraction of bladder strips from controls and 6-OHDA-lesioned rats
In order to evaluate the contractions in the bladder strips, we first employed EFS in increasing frequencies from 1 to 40 Hz. Bladder strips from6-OHDA-lesioned rats had an overall significantly higher contractile response to the stimulation compared to control animals, with the largest difference at 40 Hz (Fig. 2A and B). Moreover, the maximal measured contractile responses of the stimulation was evident at 20–40 Hz, where the bladder strips from the 6-OHDA-induced animals (15.7±1.7 mN) showed a significant increase (unpaired t-test, p = 0.023) of 43% as compared to control strips (11.0±1.2 mN). In the presence of atropine (10 − 6 M), the EFS response in strips from lesioned animals and controls decreased to 10.5±1.6 mN and 7.2±1.1 mN at 40 Hz, respectively, representing a 33% and 31% reduction in both the bladder strips from the 6-OHDA-lesioned rats and the normal untreated controls, respectively (Fig. 2C). However, the statistical difference between strips from controls and 6-OHDA-treated animals remained at 40 Hz (7.2±1.1 mN and 10.5±1.6 mN,respectively).
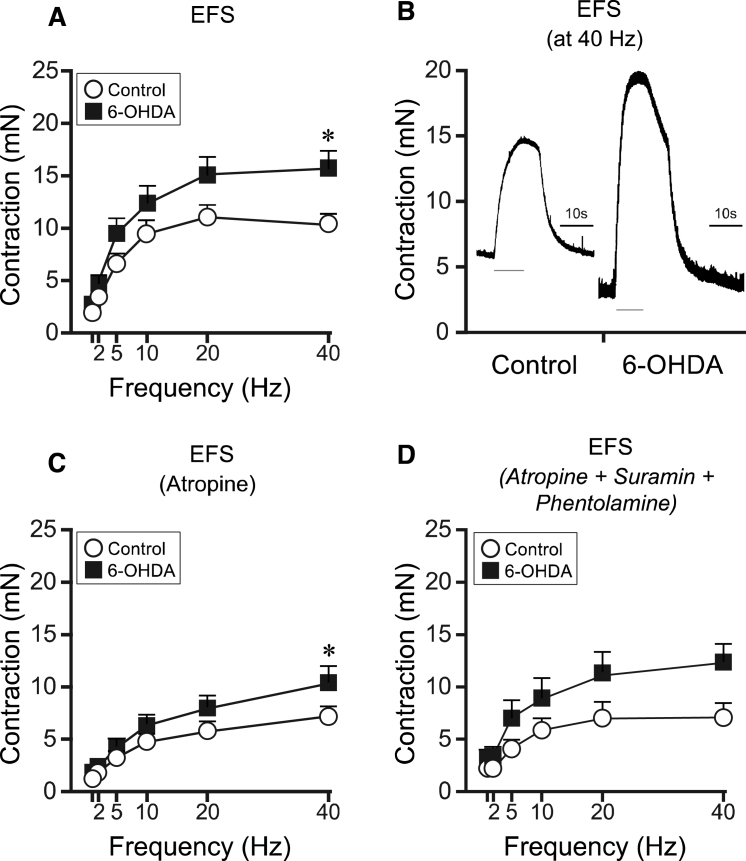
Fig.2.
EFS-induced bladder responses in bladder strips in controls and 6-OHDA-lesioned rats. Following EFS the bladder strips from 6-OHDA-lesioned rats (n = 16) showed an overall significantly higher contractile response as compared to controls (n = 26; A, B). In the presence of atropine the EFS response was reduced in both groups by 31% and 33% for controls and 6-OHDA respectively (C). However a significant difference could still be observed at 40 Hz. Following administration of phentolamine (10 − 5M) together with suramin and atropine, no change in contraction in the control (n = 7) as compared to 6-OHDA (n = 7) was observed (p = 0.094; D). The grey thin line under the responses in panel C represent the duration of the electric stimulation. * = significantly different from control group. [Two-way repeated ANOVAs; (A) interaction: F(5, 200) = 3.86, p = 0.00023, Group: F(1, 40) = 4.15, p = 0.048; (C) interaction: F(5, 200) = 2.86, p = 0.016, Group: F(1, 40) = 1.96, p = 0.17; (D) interaction: F(5, 60) = 1.98, p = 0.094, Group: F(1, 12) = 3.09, p = 0.10. All ANOVAS are followed by a Bonferroni multiple comparison test].
Following administration of the non-selective alpha-adrenergic antagonist phentolamine (10 − 5 M) in combination with suramin (10 − 6 M) and atropine (10 − 6 M), in strips from four controls and four 6-OHDA lesioned rats, no obvious change was observed as compared to atropine alone, compared to all animals (Fig. 2D). The difference between the two groups did however not reach significance in this small number of animals (p = 0.094). Testing suramin with atropine alone did also not cause any change in EFS response (data not shown). Importantly, however, the EFS without antagonists displayed in these small number of animals had the same pattern as observed when comparing all animals, with 34% higher contractile response at 40 Hz in the bladder strips from the 6-OHDA-lesioned animals (15.6±2.2 mN) as compared to untreated controls (11.6±1.3 mN).
Effect of the muscarinic receptor stimulation of bladder strips from controls and 6-OHDA-lesioned rats
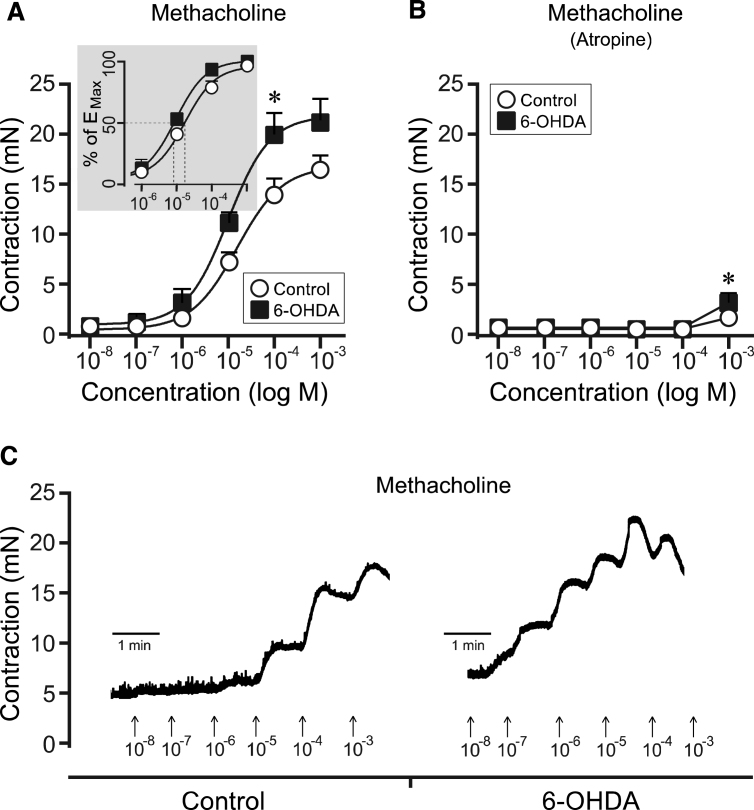
In the next phase we evaluated the muscarinic response in the bladder strips after cumulative administration of increasing concentrations of the non-selective muscarinic agonist methacholine (10 − 8 M to 10 − 3 M). These data show a clear overall difference between the two groups, with significance at the 10 − 4 M methacholine concentration reaching a contractile response of 13.9±1.7 mN in untreated controls and 20.0±2.0 mN in the 6-OHDA-lesioned animals (Fig. 3A and C). This difference could also be observed as a significant left shift of the EC50 value in the 6-OHDA (9.9×10 − 6 ±1.6×10 − 6 M) as compared to the normal control rats (4.1×10 − 5±1.4×10 − 5 M M; unpaired t-test with Welch’s correction, p = 0.040). Furthermore, the contractile response to methacholine was evaluated in the presence of the muscarinic antagonist atropine (10 − 5 M). The contractions were almost completely abolished in both groups (Fig. 3B). At the highest concentration of methacholine (10 − 3 M) a weak contraction was observed and was significantly larger in strips from 6-OHDA lesioned animals: 1.7±0.3 M in control strips versus 3.2±0.6 M in the bladder strips from the 6-OHDA group.
Fig.3.
Methacholine-induced responses in bladder strips from controls and 6-OHDA-lesioned rats. Following cumulative administration of methacholine the bladder strips from the 6-OHDA-lesioned rats (n = 10) showed an overall significantly increased contractile response as compared to controls (n = 27; A). This response could specifically be observed at a concentration of 10 − 4 M (A, C). The EC50 showed also a significant left-shift in the 6-OHDA group as compared to controls (Insert, Panel A) Further, in the presence of atropine (10 − 6 M) the methacholine response was almost completely blocked (B). Significance was nevertheless still observed at the highest dose administered (10 − 3 M). * = significantly different from control. [Two-way repeated ANOVAs; (A) interaction: F(5, 175) = 2.71, p = 0.022, Group: F(1, 35) = 5.24, p = 0.028; (B) interaction: F(5, 175) = 3.93, p = 0.0021, Group: F(1, 35) = 5.79, p = 0.022; All ANOVAS are followed by a Bonferroni multiple comparison test].
Effect of purinoceptor stimulation of bladder strips from controls and 6-OHDA-lesioned rats
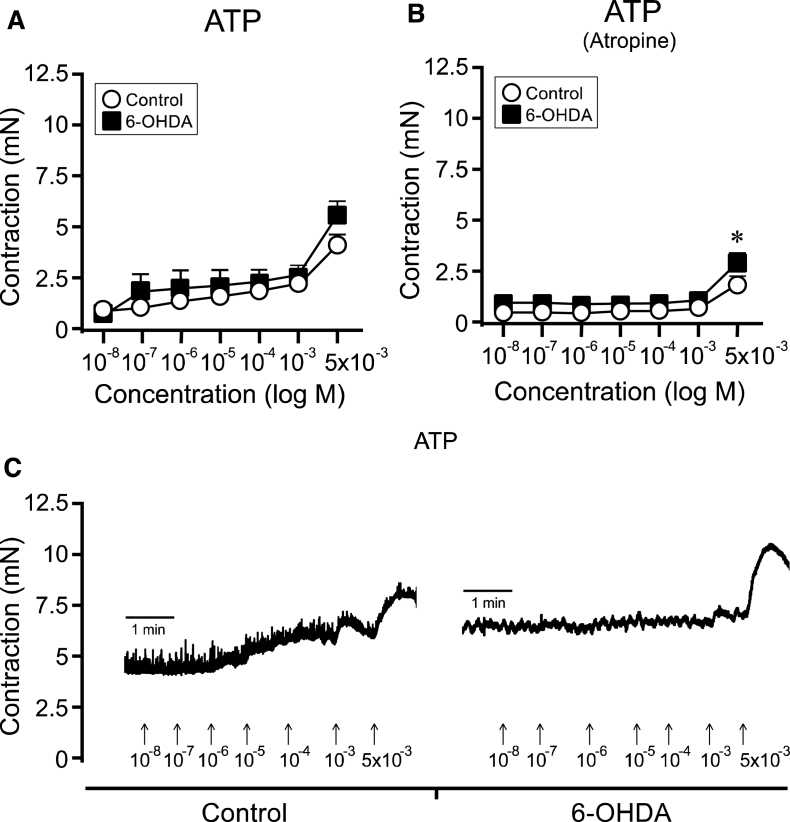
Finally, we evaluated the purinoceptor response in the bladder strips using increasing concentrations of the purinergic agonist ATP (10 − 8 to 5×10 − 3 M). The two-way ANOVA did not reveal significant differences between control strips and 6-OHDA strips (Fig. 4A and C). When the effect of ATP-induce cholinergic transmission were inhibited by adding atropine (10 − 5 M), a stronger ATP response in the 6-OHDA-lesioned animals became evident as there was significant overall group difference effect. At the highest dose applied (5×10 − 3 M), the response to ATP was 60% higher in the Parkinsonian animals (6-OHDA: 2.9±0.3 mN) as compared to normal untreated controls (1.8±0.3 mN;Fig. 4B).
Fig.4.
ATP-induced bladder responses in bladder strips from controls and 6-OHDA-lesioned rats. Cumulative administration of ATP showed a trend to yield an overall higher contraction in the 6-OHDA-lesioned rats (n = 10) as compared to controls (n = 27; A). This was evident in particular at the highest concentration (5×10 − 3 M; A, C). A significant difference was observed following atropine administration, where a higher response was shown in the bladder strips from the 6-OHDA-lesioned animals (n = 10) as compared to control animals (n = 26; B). * = significantly different from control group. [Two-way repeated ANOVAs; (A) interaction: F(6, 210) = 1.69, p = 0.12, (B) interaction: F(6, 204) = 1.85, p = 0.092, Group: F(1, 34) = 8.40, p = 0.0065; All ANOVAS are followed by a Bonferroni multiple comparison test].
DISCUSSION
Non-motor symptoms in PD, in particular urinary bladder dysfunction has a significant impact on the patient’s quality of life. Regularly used PD treatments, in addition, have unpredictable symptomatic effects on the urinary symptoms, and could in some patients even worsen the problems [6–8]. The mechanism behind the urinary symptoms is poorly understood, and needs to be further studied in order to develop better treatments.
In the present study we have measured the contractility of tissue strips in an in vitro organ bath setup to investigate the local contractile function of the urinary bladder in the unilateral 6-OHDA rat model and compare it with normal un-lesioned animals. Interestingly, our data show that the Parkinsonian rat urinary bladders are significantly more responsive to high K +, EFS, to direct stimulation of muscarinic receptors by the non-selective agonist methacholine, and to ATP purinoceptor activation (after inhibition of the muscarinic response). Overall, Parkinsonian rat urinary bladder walls displayed approximately a 40% increase in the contractile response of the detrusor muscle. Furthermore the potency of methacholine was increased in bladder strips from Parkinsonian rats.
Previous in vivo and in situ studies have shown that the micturition patterns are significantly changed in the 6-OHDA rat model, including parameters such as decreased voiding volume and bladder capacity, as well as increased micturition frequency and spontaneous activity of the bladder muscle [11, 13, 14]. The same changes characterize bladder function in PD patients with LUTS [19, 20], which in turn validates this as a PD rat model for studying PD urinary dysfunction. However, the uniqueness of the current study is that we here show that the local response of the urinary bladder, without direct involvement of the central nervous system, is altered following a chronic dopamine loss in basal ganglia. This demonstrates that changes have occurred locally in the urinary bladder muscle or in its nerve innervations, something which can have implications for how this condition is besttreated.
First, we observed a change in the texture and redness of the bladder tissue in the 6-OHDA lesioned rats. This was associated with a trend of increased bladder strip weight in the Parkinsonian animals as compared to the normal controls. Significant increases in bladder weight in 6-OHDA animals at 28 days post lesion have in fact been previously reported by Soler and colleagues [14]. This weight increase may indicate a thickening of the bladder wall in the Parkinsonian animals. An increased muscle layer may therefore be a plausible hypothesis that partly accounts for the observed increase in contractile responses in the bladder strips of the 6-OHDA-leisonsed rats. Hypertrophy of the smooth muscle cells has also been reported in patients with other syndromes (overactive bladder; OAB) related to hyperactive bladder [21]. However, the redness of the bladder may rather indicate an inflammatory process in the bladder, and maybe caused by the increased spontaneous activity of the rat PD bladder [11, 13, 14]. Inflammation in the urinary bladder in rats has, however, been shown to decrease the maximal contraction both in vivo and in vitro [22–24]. The redness may therefore have other explanations as contraction was increased in the bladder of 6-OHDA animals.
In the present study we showed that the contractile response of the 6-OHDA urinary bladder is higher following both high K + and in response to nerve stimulation using EFS. The here employed electric stimulation protocol have previously been demonstrated to strictly excite the nerves, thus releasing neurotransmitter, and not to cause a direct contractile effect on the smooth muscle [25]. These increases in contractile bladder response may be caused by a number of local changes, including muscular bladder wall hypertrophy as previously mentioned, but also increased responsiveness in receptor or in the postsynaptic machinery. When the high K + response is normalized to the strip weight (data not shown), or the EFS response to either strip weight or K + response (data not shown), the contraction of 6-OHDA bladder strips was not higher than in control strips, suggesting that the efficacy of the receptor mediated contraction is not increased and that detrusor muscle hypertrophy is the more likely explanation of the increase in contraction in the PD rat bladder. In order to better understand which components were relevant for the increased EFS response, we further looked at the contractility following pharmacological stimulation with the muscarinic agonist methacholine. We found a similar difference in contractility, as with EFS in response to the agonist treatment. However, the potency of methacholine was increased indicating that the 6-OHDA rat bladder is hypersensitive to muscarinic stimulation. Cholinergic transmission is the most important pathway in bladder contraction and the muscarinic M3 receptor is the main receptor involved in the contraction [26–28]. Applying the muscarinic agonist methacholine we clearly showed a significantly higher cholinergic response with >40% in the Parkinsonian animals. This was associated with the left shift of the EC50 in the methacholine response, which may in addition to detrusor muscle hypertrophy suggest that pre- and/or postsynaptic changes in the cholinergic system are present locally in the bladder following chronic central dopamine degeneration by 6-OHDA.
In other conditions with local bladder pathology there is a patchy denervation of the local cholinergic system, which can cause supersensitive responses to acetylcholine [29–31]. However, bladder histopathology needs to be further studied in bladders from 6-OHDA-lesioned animals to determine if similar mechanisms explain the increase in bladder contractility. Another change that has been observed in OAB syndromes is dysfunctional electric coupling of the smooth muscle bundles in the detrusor muscle [32]. Normally the bundles are weakly coupled and aberrant electrical impulses therefore rarely lead to detrusor contraction. In the overactive bladder, however, these connections are more tightly coupled. To our knowledge increased local bladder contractility has not previously been described in PD or models of PD, so it is not known if similar mechanisms are at work.
Partial blockade of EFS-evoked contractions by a muscarinic antagonist, revealing the atropine resistant part of the response, suggests involvement of other co-transmitters besides acetylcholine, which may also be involved in the increased contractile responses observed in the Parkinsonian rat bladders. The purinergic system is one of the other transmitter systems that could play a role in the contractile response of the bladder. The purinoceptor response is however to a much lesser extent involved in the contraction of the detrusor muscle [33]. The responses to ATP in the Parkinsonian animals were significantly altered, in particular following muscarinic receptor blockage by atropine, which may also suggest that this system could be affected by the central dopamine denervation by 6-OHDA. It has previously been shown that ATP can act in concert with acetylcholine to facilitate detrusor muscle contraction [24, 34]. It has also been reported that purinergic P2X1 receptors can be desensitized by low concentrations of ATP and mask the true potency of an agonist [35]. In our study we particularly see significant changes in the ATP response at the highest dose administered (5×10 − 3 M), and after blocking the cholinergic transmission by atropine. It may be argued that a desensitization may have occurred by the cumulative ATP administration. However, since both the controls and the 6-OHDA-lesioned rats were treated in the same way, the comparison and the statically differences are still valid.
Another network that may account for some of the altered effects in the 6-OHDA-lesioned animals is the noradrenergic transmitter system. The adrenergic system has both a relaxatory and excitatory function on the urinary bladder function [36], where alpha-adrenergic receptors evoke contraction and beta-adrenergic receptors induce relaxation [33, 37]. Our data showed that EFS-induced contraction, following the challenge with phentolamine, did not change. This indicates that the noradrenergic system is likely less involved in the observed local increase in urinary bladder contractile response in rats. Kitta and colleagues have, however, showed that the increased spontaneous bladder activity in the 6-OHDA-lesion model can be reverse by alpha2A adrenoceptor antagonists when infused directly into the central nervous system [11]. This suggests that at least the adrenergic transmission is important centrally in hyperactive bladder.
In conclusion, to our knowledge the current study is the first to show altered contractile function caused by local morphological changes and plasticity in the urinary bladder in a rat model of PD. In view of the rather similar degree of potentiation on both muscarinic receptor and purinoceptor responses, the increase may reflect a generalized enhanced contractile ability in the detrusor muscle, at least as the dominating factor, in the 6-OHDA PD rats. In addition, our study also further validates the usefulness of the 6-OHDA-lesioned model when studying PD urinary bladder dysfunction. We believe that the current data shed new light on the mechanisms behind PD bladder dysfunction, which can contribute to the development of new therapeutic innovations for urinary dysfunction in PD patients.
ACKNOWLEDGMENTS
We would like to thank Felix Holmström for his technical support in the project. The current study was funded by Parkinson Research Foundation (Mats Heiman and Ingrid Atteryd Heiman), and by Wilhelm och Martina Lundgrens vetenskapsfond to TC. Reinika Mitra was funded by the ERASMUS programme. The authors have no conflict of interest to report.
REFERENCES
- 1.Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, Bottacchi E, Cannas A, Ceravolo G, Ceravolo R, Cicarelli G, Gaglio RM, Giglia RM, Iemolo F, Manfredi M, Meco G, Nicoletti A, Pederzoli M, Petrone A, Pisani A, Pontieri FE, Quatrale R, Ramat S, Scala R, Volpe G, Zappulla S, Bentivoglio AR, Stocchi F, Trianni G, Dotto PD, group Ps. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24:1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 2.Gallagher DA, Lees AJ, Schrag A. What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord. 2010;25:2493–2500. doi: 10.1002/mds.23394. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Martin P. The importance of non-motor disturbances to quality of life in Parkinson’s disease. J Neurol Sci. 2011;310:12–16. doi: 10.1016/j.jns.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Winge K, Fowler CJ. Bladder dysfunction in Parkinsonism: Mechanisms, prevalence, symptoms, and management. Mov Disord. 2006;21:737–745. doi: 10.1002/mds.20867. [DOI] [PubMed] [Google Scholar]
- 5.Winge K, Skau AM, Stimpel H, Nielsen KK, Werdelin L. Prevalence of bladder dysfunction in Parkinsons disease. Neurourol Urodyn. 2006;25:116–122. doi: 10.1002/nau.20193. [DOI] [PubMed] [Google Scholar]
- 6.Brusa L, Petta F, Pisani A, Miano R, Stanzione P, Moschella V, Galati S, Finazzi Agro E. Central acute D2 stimulation worsens bladder function in patients with mild Parkinson’s disease. J Urol. 2006;175:202–206. doi: 10.1016/S0022-5347(05)00058-3. discussion 206-207. [DOI] [PubMed] [Google Scholar]
- 7.Brusa L, Petta F, Pisani A, Moschella V, Iani C, Stanzione P, Miano R, Finazzi-Agro E. Acute vs chronic effects of l-dopa on bladder function in patients with mild Parkinson disease. Neurology. 2007;68:1455–1459. doi: 10.1212/01.wnl.0000260605.12506.86. [DOI] [PubMed] [Google Scholar]
- 8.Winge K, Werdelin LM, Nielsen KK, Stimpel H. Effects of dopaminergic treatment on bladder function in Parkinson’s disease. Neurourol Urodyn. 2004;23:689–696. doi: 10.1002/nau.20054. [DOI] [PubMed] [Google Scholar]
- 9.Seki S, Igawa Y, Kaidoh K, Ishizuka O, Nishizawa O, Andersson KE. Role of dopamine D1 and D2 receptors in the micturition reflex in conscious rats. Neurourol Urodyn. 2001;20:105–113. doi: 10.1002/1520-6777(2001)20:1<105::aid-nau12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Andersson KE, Soler R, Fullhase C. Rodent models for urodynamic investigation. Neurourol Urodyn. 2011;30:636–646. doi: 10.1002/nau.21108. [DOI] [PubMed] [Google Scholar]
- 11.Kitta T, Chancellor MB, de Groat WC, Kuno S, Nonomura K, Yoshimura N. Suppression of bladder overactivity by adenosine A2A receptor antagonist in a rat model of Parkinson disease. J Urol. 2012;187:1890–1897. doi: 10.1016/j.juro.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimura N, Miyazato M, Kitta T, Yoshikawa S. Central nervous targets for the treatment of bladder dysfunction. Neurourol Urodyn. 2014;33:59–66. doi: 10.1002/nau.22455. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura N, Kuno S, Chancellor MB, De Groat WC, Seki S. Dopaminergic mechanisms underlying bladder hyperactivity in rats with a unilateral 6-hydroxydopamine (6-OHDA) lesion of the nigrostriatal pathway. Br J Pharmacol. 2003;139:1425–1432. doi: 10.1038/sj.bjp.0705388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soler R, Fullhase C, Santos C, Andersson KE. Development of bladder dysfunction in a rat model of dopaminergic brain lesion. Neurourol Urodyn. 2011;30:188–193. doi: 10.1002/nau.20917. [DOI] [PubMed] [Google Scholar]
- 15.Winge K, Nielsen KK, Stimpel H, Lokkegaard A, Jensen SR, Werdelin L. Lower urinary tract symptoms and bladder control in advanced Parkinson’s disease: Effects of deep brain stimulation in the subthalamic nucleus. Mov Disord. 2007;22:220–225. doi: 10.1002/mds.21253. [DOI] [PubMed] [Google Scholar]
- 16.Carlsson T, Carta M, Munoz A, Mattsson B, Winkler C, Kirik D, Bjorklund A. Impact of grafted serotonin and dopamine neurons on development of L-DOPA-induced dyskinesias in parkinsonian rats is determined by the extent of dopamine neuron degeneration. Brain. 2009;132:319–335. doi: 10.1093/brain/awn305. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; San Diego: 2005. [DOI] [PubMed] [Google Scholar]
- 18.Carlsson T, Winkler C, Lundblad M, Cenci MA, Bjorklund A, Kirik D. Graft placement and uneven pattern of reinnervation in the striatum is important for development of graft-induced dyskinesia. Neurobiol Dis. 2006;21:657–668. doi: 10.1016/j.nbd.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Badri AV, Purohit RS, Skenazy J, Weiss JP, Blaivas JG. A review of lower urinary tract symptoms in patients with Parkinson’s disease. Curr Urol Rep. 2014;15:435. doi: 10.1007/s11934-014-0435-0. [DOI] [PubMed] [Google Scholar]
- 20.Kapoor S, Bourdoumis A, Mambu L, Barua J. Effective management of lower urinary tract dysfunction in idiopathic Parkinson’s disease. Int J Urol. 2013;20:79–84. doi: 10.1111/j.1442-2042.2012.03220.x. [DOI] [PubMed] [Google Scholar]
- 21.Charlton RG, Morley AR, Chambers P, Gillespie JI. Focal changes in nerve, muscle and connective tissue in normal and unstable human bladder. BJU Int. 1999;84:953–960. doi: 10.1046/j.1464-410x.1999.00360.x. [DOI] [PubMed] [Google Scholar]
- 22.Andersson M, Aronsson P, Giglio D, Wilhelmson A, Jerabek P, Tobin G. Pharmacological modulation of the micturition pattern in normal and cyclophosphamide pre-treated conscious rats. Auton Neurosci. 2011;159:77–83. doi: 10.1016/j.autneu.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Andersson MC, Tobin G, Giglio D. Cholinergic nitric oxide release from the urinary bladder mucosa in cyclophosphamide-induced cystitis of the anaesthetized rat. Br J Pharmacol. 2008;153:1438–1444. doi: 10.1038/bjp.2008.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aronsson P, Carlsson T, Winder M, Tobin G. Cyclophosphamide-induced alterations of the micturition reflex in a novel in situ urinary bladder model in the anesthetized rat. Neurourol Urodyn. 2014. 10.1002/Nau22562 [DOI] [PubMed]
- 25.Aronsson P, Andersson M, Ericsson T, Giglio D. Assessment and characterization of purinergic contractions and relaxations in the rat urinary bladder. Basic Clin Pharmacol Toxicol. 2010;107:603–613. doi: 10.1111/j.1742-7843.2010.00554.x. [DOI] [PubMed] [Google Scholar]
- 26.Longhurst PA, Levendusky M. Pharmacological characterization of beta-adrenoceptors mediating relaxation of the rat urinary bladderin vitro. Br J Pharmacol. 1999;127:1744–1750. doi: 10.1038/sj.bjp.0702709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tobin G, Sjogren C. in vitro and in vitro effects of muscarinic receptor antagonists on contractions and release of [3H]acetylcholine in the rabbit urinary bladder. Eur J Pharmacol. 1995;281:1–8. doi: 10.1016/0014-2999(95)00221-6. [DOI] [PubMed] [Google Scholar]
- 28.Uchiyama T, Chess-Williams R. Muscarinic receptor subtypes of the bladder and gastrointestinal tract. J Smooth Muscle Res. 2004;40:237–247. doi: 10.1540/jsmr.40.237. [DOI] [PubMed] [Google Scholar]
- 29.Ekstrom J, Malmberg L. Development of supersensitivity to methacholine in the rat detrusor following either parasympathetic denervation or decentralization. Acta Physiol Scand. 1984;122:175–179. doi: 10.1111/j.1748-1716.1984.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 30.Ekstrom J, Malmberg L. Supersensitivity to methacholine in rat urethra following hypertrophy or disuse. Acta Pharmacol Toxicol (Copenh) 1985;57:297–300. doi: 10.1111/j.1600-0773.1985.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 31.Sibley GN. Developments in our understanding of detrusor instability. Br J Urol. 1997;80(Suppl 1):54–61. [PubMed] [Google Scholar]
- 32.Mok MH, Knight GE, Andrews PL, Hoyle CH, Burnstock G. The effects of cyclophosphamide on neurotransmission in the urinary bladder of Suncus murinus, the house musk shrew. J Auton Nerv Syst. 2000;80:130–136. doi: 10.1016/s0165-1838(00)00085-0. [DOI] [PubMed] [Google Scholar]
- 33.Giglio D, Aronsson P, Eriksson L, Tobin G. characterization of parasympathetic and sympathetic responses in cyclophosphamide-induced cystitis in the rat. Basic Clin Pharmacol Toxicol. 2007;100:96–108. doi: 10.1111/j.1742-7843.2007.00014.x. [DOI] [PubMed] [Google Scholar]
- 34.Burnstock G, Dumsday B, Smythe A. Atropine resistant excitation of the urinary bladder: The possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol. 1972;44:451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rettinger J, Schmalzing G. Desensitization masks nanomolar potency of ATP for the P2X1 receptor. J Biol Chem. 2004;279:6426–6433. doi: 10.1074/jbc.M306987200. [DOI] [PubMed] [Google Scholar]
- 36.Elmer M. Action of drugs on the innervated and denervated urinary bladder of the rat. Acta Physiol Scand. 1974;91:289–297. doi: 10.1111/j.1748-1716.1974.tb05685.x. [DOI] [PubMed] [Google Scholar]
- 37.Ekstrom J. Supersensitivity of the rat urinary bladder following “chemical sympathectomy”. Acta Pharmacol Toxicol (Copenh) 1979;44:377–384. [PubMed] [Google Scholar]