Abstract
Candida tropicalis (ATCC 20336) converts fatty acids to long-chain dicarboxylic acids via a pathway that includes among other reactions the oxidation of ω-hydroxy fatty acids to ω-aldehydes by a fatty alcohol oxidase (FAO). Three FAO genes (one gene designated FAO1 and two putative allelic genes designated FAO2a and FAO2b), have been cloned and sequenced from this strain. A comparison of the DNA sequence homology and derived amino acid sequence homology between these three genes and previously published Candida FAO genes indicates that FAO1 and FAO2 are distinct genes. Both genes were individually cloned and expressed in Escherichia coli. The substrate specificity and Km values for the recombinant FAO1 and FAO2 were significantly different. Particularly striking is the fact that FAO1 oxidizes ω-hydroxy fatty acids but not 2-alkanols, whereas FAO2 oxidizes 2-alkanols but not ω-hydroxy fatty acids. Analysis of extracts of strain H5343 during growth on fatty acids indicated that only FAO1 was highly induced under these conditions. FAO2 contains one CTG codon, which codes for serine (amino acid 177) in C. tropicalis but codes for leucine in E. coli. An FAO2a construct, with a TCG codon (codes for serine in E. coli) substituted for the CTG codon, was prepared and expressed in E. coli. Neither the substrate specificity nor the Km values for the FAO2a variant with a serine at position 177 were radically different from those of the variant with a leucine at that position.
When grown on fatty acids, wild-type strains of Candida tropicalis rapidly catabolize fatty acids through the cyclic β-oxidation pathway to acetyl coenzyme A (CoA), which serves as both a carbon and energy source. C. tropicalis can also oxidize fatty acids through an ω-oxidation pathway, in which the terminal methyl carbon is oxidized to a carboxylic acid, yielding a dicarboxylic acid. In ω-oxidation, the fatty acid is converted to a dicarboxylic acid by a three-step pathway. The first step is catalyzed by the hydroxylase complex composed of a cytochrome P450 monooxygenase (CYP52) and a cytochrome P450:NADPH oxidoreductase (NCP) (3, 16). The alcohol produced is further oxidized to an aldehyde by a fatty alcohol oxidase (FAO) and then to the dicarboxylic acid by a fatty aldehyde dehydrogenase (12). In wild-type C. tropicalis strains, β-oxidation predominates over the ω-oxidation pathway so that little dicarboxylic acid (diacid) accumulates during growth on fatty acids. A C. tropicalis strain, H5343 (ATCC 20962), blocked in the β-oxidation pathway by insertional inactivation of the POX4 and POX5 genes (which encode acyl-CoA oxidase, the first step in the β-oxidation pathway) rapidly accumulates diacid (15, 16). However, in fermentations producing diacids from fatty acids, small amounts (ca. 0.5% [wt/wt]) of ω-hydroxy fatty acid (HFA) consistently accumulated in the broth. Although the first step in the ω-oxidation pathway is known to be the major rate-limiting step, the accumulation of HFA indicated that the second step in the pathway was also rate-limiting.
A small number of FAOs from various yeasts (2, 4, 7-13, 19) have been described in the scientific literature. The properties of these enzymes were found to be sufficiently different so that the specific properties of the C. tropicalis strain ATCC 20336 enzymes would need to be determined in order to understand the enzymology involved in the second step in the pathway of diacid production. Two FAO genes from C. tropicalis ATCC 20336 therefore were cloned and characterized, and the two enzymes were then produced separately in Escherichia coli, a host free of background FAO activity. The substrate specificity and kinetic properties of the recombinant enzymes were different from those of previously described FAOs. Because of distinctive differences in the specificities of the two enzymes, analysis of the activities present in C. tropicalis strain H5343 grown on fatty acids revealed that one of the two genes, FAO1, was the major FAO expressed during production of dicarboxylic acids.
MATERIALS AND METHODS
Strains and fermentations.
H5343, a β-oxidation-blocked strain derived from C. tropicalis ATCC 20336 (15), was produced by insertional mutagenesis of the POX4 and POX5 genes, both of which code for acyl-CoA oxidase. Fermentations were performed with high-oleic sunflower fatty acid (HOSFFA) as the substrate, with glucose used as the cosubstrate as described by Anderson and Wenzel (1). The HOSFFA used was approximately 85% oleic acid (84.45% oleic acid, 5.24% linoleic acid, 4.73% stearic acid, 3.87% palmitic acid, 1.71% other fatty acids).
Washing of cells from diacid fermentations.
Due to the high levels of solid diacid in the broth from diacid fermentations, the samples had to be washed extensively to remove the diacid prior to making cell extracts. The washing procedure took advantage of the difference in density between the denser yeast cells and the solid diacid. Samples (20 ml) were centrifuged at approximately 1,500 × g for 5 min at 4°C to pellet the cells, along with a portion of the solid, white-colored diacid. The supernatant solution, which contained suspended diacid, was decanted, and the pellet was washed by resuspension in 40 ml of 50 mM HEPES buffer (pH 7.6), followed by centrifugation at 1,500 × g at 4°C for 1 min. For all washing steps, the HEPES buffer was maintained at 4°C by storage on ice. The supernatant solution, which contained additional suspended diacid, was decanted, and the cells were washed again in 40 ml of buffer. This washing procedure was repeated until the cell pellet was a pale tan color throughout (the color of the yeast cells) and the supernatant solution was essentially clear, indicating that the white-colored diacid had been removed. The number of repeat washings depended upon the amount of diacid present in the original broth sample.
Preparation of cell extracts and microsomes.
The washed cell pellet was resuspended in 10 ml of 50 mM potassium phosphate buffer containing 20% glycerol, pH 7.6 (phosphate-glycerol buffer). Phenylmethylsulfonyl fluoride was added to a final concentration of 1 mM prior to breaking the cells by passing the sample three times through a chilled French pressure cell at approximately 20,000 lb/in2 gauge. Following centrifugation of the broken cell suspension at approximately 37,000 × g for 30 min, the supernatant (cell extract) was decanted and stored at −20°C prior to performing FAO enzyme assays.
Microsomes were prepared from cell extracts by centrifugation at 100,000 × g for 1 h at 4°C. The supernatant was removed, and the microsomal pellet was resuspended in an equivalent amount of phosphate-glycerol buffer and was assayed for catalase and FAO activity. The FAO, which is a membrane-bound protein, was found within the microsomal pellet. Catalase, a soluble enzyme, remained in the supernatant, which allowed the separation of these two enzymes. Cell extracts and microsomes from E. coli were prepared in the same manner.
Cloning of FAO genes.
The protocols for the preparation of genomic DNA that was used for the generation of a C. tropicalis ATCC 20336 genomic library and for screening the library for particular genes were the same as those described by Craft et al. (3).
Generation of a probe for library screening.
By comparing regions of sequence similarity between two C. cloacae FAO genes and a C. tropicalis FAOT gene (19), nondegenerative primers were designed for use in a PCR to prepare an FAO-specific probe that was anticipated to yield a PCR product 1,173 bp in length and encoding a region of the open reading frame (ORF) from 608 to 1,780 bp 3′ of the start of the ORF. The primer pair generated a PCR product approximately 1,200 bp in length, very near the expected size of 1,173 bp.
The PCR product was cloned with a TOPO TA cloning kit (Invitrogen) into Top10F′ strain cells. Plasmid DNA was obtained from cultures with the Qiaprep Miniprep kit (Qiagen) and analyzed for the presence of the insert by cutting the plasmid with EcoRI. Several clones showed the expected insert size of ca.1,200 bp. Two of these clones were sequenced (Sequetech Corp., Mountain View, Calif.), and the DNA sequence was compared to the sequence for FAOT (19). The degree of homology for both clones was very high and was 79% identical to a corresponding region of the FAOT ORF. One clone was selected for preparation of the probe DNA.
Several micrograms of plasmid DNA was digested with EcoRI to release the insert. The digest was electrophoretically separated on a 1.2% low-melting-temperature agarose gel, and the 1,200-bp band was excised. The insert DNA was extracted from the excised gel by using a QIAquick gel extraction kit (Qiagen) and was quantified. The FAO DNA fragment was then labeled with an ECL enhanced chemiluminescence kit (Amersham).
Subcloning and expression of FAO1 and FAO2a in E. coli.
The primers used for PCR of the ORF of the FAO1 and the FAO2a were designed to provide EcoRI and BamHI restriction sites (underlined) in the final PCR product. The restriction sites were added at the 5′ and 3′ ends, respectively. The ATG initiation codon in the forward primer and the dual termination codons in the reverse primers are shown in boldface. The primers for FAO1 were 5′-CCGAATTCGACATGGCTCCATTTTTG-3′ (forward primer) and 5′-CCGGATCCATTACTACAACTTGGCCTTGGT-3′ (reverse primer). The primers for FAO2 were 5′-CCAGTGAATTCAGATGAATACCTTCT-3′ (forward primer) and 5′-CCGGATCCCCGTCTCACTACAACTTG-3′ (reverse primer).
For each gene, the PCR product from three reactions were pooled and purified with the QIAquick-spin PCR purification kit (Qiagen). The DNA was then fractionated on a 1.0% agarose gel. The 2.1-kb bands were removed, and the DNA was extracted with the QIAEX II gel extraction kit (Qiagen). The expression vector pJF118EH (6) was digested with EcoRI and BamHI and was fractionated on a 1.0% agarose gel. The band was excised and purified with the QIAEX II gel extraction kit (Qiagen). The FAO1 and FAO2a PCR products were digested with EcoRI and BamHI and were gel purified in the same manner. Following ligation of FAO1 or FAO2a, 100 μl of Library Efficiency E. coli DH5α (Life Technologies, Inc.) was transformed with 1.5 μl of each ligation reaction mixture. Plasmid DNA was prepared, and the insert size was determined by digestion with EcoRI and BamHI. One positive clone of each gene was selected for further study by enzyme activity analysis. Both FAO genes in these plasmids were sequence confirmed by Sequetech Corp. The FA01 clone was designated FAO1-EC, and the FAO2a clone was designated FAO2-EC.
Construction of a CTG-codon-altered FAO2a gene.
A codon alteration of FAO2a was performed by overlap-extension PCR, which was designed to change the CTG codon, centered at 530 bp 3′ of the start of the ORF, to a TCG codon. Primer sets were designed to cover the region from 208 to 545 bp 3′ of the start of the ORF, yielding a fragment 338 bp in length. A second set of primers was designed to cover the region 510 to 2,069 bp 3′ of the start of the ORF, yielding a fragment 1,560 bp in length.
The PCR products from both sets of PCRs were separated by electrophoresis on a 1% low-melting-temperature agarose gel, and DNA bands of the appropriate size (338 bp and 1,560 bp) were excised. A third PCR was performed using these fragments as a template. This resulted in a PCR fragment that was 1,862 bp in length and covered from 208 to 2,069 bp 3′ from the start of the ORF. This fragment was TOPO-TA cloned, and the resulting plasmid DNA was prepared as described previously in Materials and Methods.
In order to replace the CTG codon in the expression plasmid with the modified sequence, the plasmid was digested with KpnI and MfeI to remove a fragment 470 kb in length, leaving the major portion of the plasmid (6,924 bp) intact. The 1,862-bp fragment was also cut with KpnI and MfeI to remove a fragment 470 kb in length. The 6,924-bp fragment from the plasmid and the 470-bp fragment containing the modified CTG codon were gel purified as described previously. These DNA fragments were ligated with the New England Biolabs Quick Ligation kit. This ligation product was transformed back into DH5α cells. Plasmid preparations from putative clones were screened by restriction analysis using KpnI and MfeI. A positive clone was selected for further study. The 470-bp portion of the CTG-modified FAO2a gene (designated FAO2a′) that was generated by PCR was sequence verified.
Induction of FAO1 and FAO2a.
Overnight cultures were grown at 30°C in 5 ml of Terrific Broth (TB) (Sigma) plus 100-μg/ml ampicillin at 250 rpm. Fifty milliliters of TB plus 100-μg/ml ampicillin was placed in each of two 500-ml baffled flasks. TB plus 100-μg/ml ampicillin was used for both the starter cultures and the cultures that were induced to produce the enzyme. The flasks were inoculated with the overnight cultures to an A600 of approximately 0.2. The cultures were grown at either 30 or 37°C with shaking at 250 rpm. Somewhat higher enzyme activities were observed with cells grown and induced at 30°C. At an A600 of 5 to 6, the cultures were induced with isopropyl β-d-1-thiogalactopyranoside (IPTG) to a final concentration in each culture of 1 mM. The cultures were then allowed to incubate another 3 h postinduction. The cells were harvested by centrifugation. The spent broth was separated from the cell pellets, which were frozen at −20°C for later use.
FAO enzyme assay.
The assay procedure was modified from the protocol of Kemp et al. (12). The assay is a two-enzyme, coupled reaction. 1-Dodecanol was used as the standard substrate for the general assay of FAO. Titration of horseradish peroxidase (HRP) ensured that there was sufficient HRP present to compete with any catalase that may have been present in the cell extracts. In experiments in which the quantity of HRP was varied in the reaction mixture, it was found that 5 μl of this solution in a 1-ml reaction mixture was sufficient to obtain maximal velocity.
The final reaction mixture for the general alcohol oxidase assay consisted of a 500 μl of 200 mM HEPES buffer (pH 7.6), 50 μl of a 10-mg/ml 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) solution in deionized water; 10 μl of a 5 mM solution of 1-dodecanol in acetone, and 5 μl of a 2-mg/ml HRP solution (Sigma catalog no. P8415; approximately 250 U/mg in 50 mM potassium phosphate buffer, pH 7.6). Various amounts of the extract were added, and water was added to a total volume of 1 ml. FAO activity was measured at room temperature at 405 nm. Alcohol oxidase activity was reported as specific activity units per milligram of protein (1 U = 1 μmol of substrate oxidized/min). An extinction coefficient at 405 nm of 18.4 was used for 1 mM radical cation of ABTS and was equivalent to 0.5 mM oxidized substrate. In certain substrate specificity experiments, 200 mM HEPES buffer (pH 7.6) containing 0.5% Triton X-100 was used in place of the 200 mM HEPES buffer in the reaction mixture described above. The detergent aided in solubilization of some of the more water-insoluble substrates tested but had no effect on enzymatic activity.
Protein determination.
The protein concentration in the extracts was determined by utilizing the Bradford protein assay (Sigma Chemical Co.).
Nucleotide sequence accession number.
The GenBank accession numbers for sequences disclosed in this study are as follows: FAO1, AY538780; FAO2a, AY538781; and FAO2b, AY538782. The putative FAO (ORF 6.5671, contig 6-2421) gene from Candida albicans was obtained from the Stanford Genome Technology Center website at http://www-sequence.stanford.edu/group/candida.
RESULTS
Alcohol oxidase activity in fermentation samples.
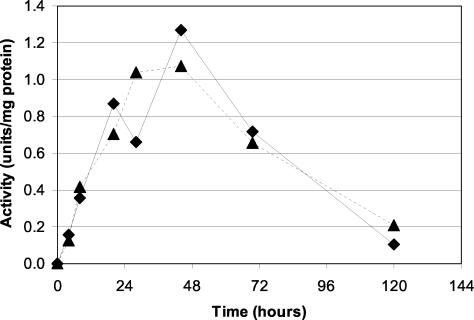
During the course of a fermentation with β-oxidation-blocked strains of C. tropicalis using HOSFFA as substrate, FAO activity generally did not appear until approximately 2 to 4 h postinduction with HOSFFA (Fig. 1). Activity rose rapidly to peak at approximately 30 to 40 h postinduction and then dropped approximately 5- to 10-fold by 100 to 120 h postinduction. Although this trend remained consistent among most of the fermentation runs, the time and magnitude of the peak of activity did vary somewhat.
FIG. 1.
Typical alcohol oxidase activity in laboratory-scale diacid fermentations with HOSFFA as a substrate. Samples from two different HOSFFA fermentations were taken at the times indicated. Extracts were prepared as described in Materials and Methods. The FAO activity was determined for each sample by using 1-dodecanol as a substrate. Duplicate reactions were averaged to determine each of the data points.
Cloning of FAO genes from C. tropicalis ATCC 20336.
Plates of the λ phage library of C. tropicalis were prepared, and lifts of these plates were made onto nitrocellulose membrane filters, following the procedure described previously (3). Putative positive clones were identified by using the probes prepared as outlined in Materials and Methods and by previously described methods (3). The E. coli cells containing these library fragments were grown, and plasmid DNA was prepared. It was known from the sequence information of the probe DNA that at least some of the clones should cut with PvuII and KpnI. Therefore, the library clones were digested with EcoRI, PvuII, and KpnI in single digests and with PvuII and KpnI in a double digest. This allowed the direction of the FAO gene to be determined and its placement within the insert of the pBK-CMV vector to be estimated. The initial primers used in preparing the probe DNA were used to PCR screen the library clones by using purified plasmid DNA of these clones as the template. C. tropicalis genomic DNA was used as the template for the PCR in the control. Following these analyses, eight FAO library clones, designated A1, A4, A5, A6, A8, A9, B5, and B6, were identified as putative positive clones and were partially sequenced with FAO-specific sequencing primers.
When the DNA sequences of the clones were compared to the FAOT sequence (19), the clones fell into two groups with similar, but not identical, nucleotide sequences. Group 1 was composed of clones A4, A8, B5, and B6. Group 2 was composed of A1, A5, A6, and A9.
The FAO gene from clone A8, which was designated FAO1, was double-strand sequenced. In addition to the ORF, which was 2,112 bp in length, there was 1,940 bp of upstream DNA and 242 bp of downstream DNA sequenced. The sequenced regions of clones A4, B5, and B6 were identical to those of clone 8, so no additional sequencing was performed.
The FAO gene from clone A9, which was designated FAO2a, was also double-strand sequenced. In addition to the ORF, which was 2,112 bp in length, there was 1,520 bp of upstream and 523 bp of downstream DNA sequenced. Note that there is a CTG codon centered at bp 530 of the ORF of FAO2. This CTG is designated as leucine in the universal code, but it has been demonstrated that C. tropicalis translates CTG as serine (14, 17, 18). Recent investigations have confirmed that CTG codes for serine in C. tropicalis ATCC 20336 (5). Additional sequencing of the FAO gene from clone A6 demonstrated close but not identical sequence similarity to FAO2a, so double-strand sequencing of the gene was also performed. The results of DNA and amino acid sequence comparisons with other FAO genes (Table 1 and Table 2) showed that the FAO gene from clone A6 was most likely an allele of the FAO2a gene. It was designated FAO2b.
TABLE 1.
DNA sequence comparison between FAO1, FAO2a, or FAO2b genes from C. tropicalis (ATCC 20336) and similar genes from C. tropicalis (NCYC 470), C. cloacae, and C. albicansa FAO genes
| DNA sequence
|
% Identity | |
|---|---|---|
| 1 | 2 | |
| FAO1 | FAO2a | 82 |
| FAO1 | FAO2b | 81 |
| FAO1 | C. tropicalis FAOT | 77 |
| FAO1 | C. albicans FAO | 71 |
| FAO1 | C. cloacae FAO1 | 62 |
| FAO2a | FAO2b | 95 |
| FAO2a | C. tropicalis FAOT | 78 |
| FAO2a | C. albicans FAO | 73 |
| FAO2a | C. cloacae FAO1 | 63 |
| FAO2b | C. tropicalis FAOT | 77 |
| FAO2b | C. albicans FAO | 73 |
| FAO2b | C. cloacae FAO1 | 62 |
| C. tropicalis FAOT | C. cloacae FAO1 | 62 |
| C. tropicalis FAOT | C. albicans FAO | 73 |
| C. cloacae FAO1 | C. albicans FAO | 59 |
| C. cloacae FAO1 | C. cloacae FAO2 | 79 |
1Putative FAO gene (ORF 6.5671, contig. 6-2421) Sequence data were obtained from the Stanford Genome Technology Center website at http://www-sequence.stanford.edu/group/candida.
TABLE 2.
Derived amino acid sequence comparison between FAO1, FAO2a, and FAO2b genes from C. tropicalis ATCC 20336 and similar sequences derived from C. tropicalis NCYC470, C. cloacae, and C. albicansa FAO genes
| Protein
|
% Identity | % Similarity | |
|---|---|---|---|
| 1 | 2 | ||
| FAO1 | FAO2a | 81 | 92 |
| FAO1 | FAO2b | 80 | 91 |
| FAO1 | C. tropicalis FAOT | 82 | 90 |
| FAO1 | C. albicans FAO | 74 | 88 |
| FAO1 | C. cloacae FAO1 | 60 | 76 |
| FAO2a | FAO2b | 97 | 98 |
| FAO2a | C. tropicalis FAOT | 85 | 93 |
| FAO2a | C. albicans FAO | 78 | 88 |
| FAO2a | C. cloacae FAO1 | 59 | 76 |
| FAO2b | C. tropicalis FAOT | 85 | 93 |
| FAO2b | C. albicans FAO | 76 | 88 |
| FAO2b | C. cloacae FAO1 | 59 | 75 |
| C. tropicalis FAOT | C. cloacae FAO1 | 60 | 77 |
| C. tropicalis FAOT | C. albicans FAO | 76 | 87 |
| C. cloacae FAO1 | C. albicans FAO | 55 | 72 |
| C. cloacae FAO1 | C. cloacae FAO2 | 76 | 88 |
Putative FAO gene (ORF 6.5671, contig 6-2421). Sequence data were obtained from the Stanford Genome Technology Center website at http://www-sequence.stanford.edu/group/candida.
The ORF regions of FAO1, FAO2a, and FAO2b from C. tropicalis (ATCC 20336) were compared to analogous regions of the FAOT gene from C. tropicalis (NCYC 470) as well as the FAO1 and FAO2 genes from Candida cloacae (19) and a putative FAO gene from C. albicans (Table 1). The amino acid sequences, which were derived by using the standard universal code, were also compared (Table 2). The two allelic genes, FAO2a and FAO2b, from C. tropicalis (ATCC 20336) were 95% identical by DNA sequence and had 97% identity and 98% similarity by deduced amino acid sequence. The FAO1 and FAO2a genes from C. tropicalis (ATCC 20336) were 82% identical by DNA sequence and had 81% identity and 92% similarity by deduced amino acid sequence. In comparison, the C. cloacae FAO1 and FAO2 genes were 79% identical by DNA sequence and had 76% identity and 88% similarity by deduced amino acid sequence. These results are consistent with the fact that, like C. cloacae, C. tropicalis (ATCC 20336) has two different FAO genes. The putative FAO gene from C. albicans showed 71% identity to the FAO1 gene and 73% to the FAO2a gene from C. tropicalis (ATCC 20336), very near the identity value found for the FAOT gene (73%).
Interestingly, Vanhanen et al. (19) identified only one FAO gene in C. tropicalis strain (NCYC 470). The DNA sequence of FAOT was 77% identical to the FAO1 and FAO2b genes from C. tropicalis (ATCC 20336) and was 78% identical to the FAO2a gene from C. tropicalis (ATCC 20336). The deduced amino acid sequence comparison showed that FAOT had 82% identity and 92% similarity to the FAO1 gene and had 85% identity and 93% similarity to the FAO2a and FAO2b genes from C. tropicalis (ATCC 20336). Although the FAOT gene was most similar to the FAO2a gene from C. tropicalis (ATCC 20336), the dissimilarity was still equivalent to about 49 amino acids out of 704. These data demonstrate that the FAOT gene is as different from the FAO genes from C. tropicalis (ATCC 20336) as they are from each other. Based upon sequence comparisons, it appears that FAO1, FAO2, and FAOT are different genes rather than alleles of one another.
Expression of FAO1 and FAO2a genes in E. coli.
Since the sequence homology data strongly indicated that FAO1 and FAO2 from C. tropicalis (ATCC 20336) were different genes, the uniqueness of the two genes was investigated by cloning and expressing FAO1 and FAO2a individually in E. coli to determine the substrate specificity of the two gene products. The ORFs of both genes were amplified by PCR and cloned into the self-replicating vector pJF118EH (6). This vector, containing either the FAO1 or FAO2a gene, was transformed into E. coli. Expression of these genes in E. coli allowed large quantities of enzyme to be generated in a clean background so that their properties could be more clearly defined. Since it is known that FAO2a has a CTG codon, which is translated as a serine in C. tropicalis but as a leucine in E. coli, an FAO2a construct (designated FAO2a′) was generated having a TCG codon, which codes for serine in both C. tropicalis and E. coli, in place of the CTG codon. In order to determine the effect of this single-amino-acid substitution, the enzymatic properties of both FAO2a and FAO2a′ were determined.
Substrate specificity of FAO1, FAO2a, and FAO2a′.
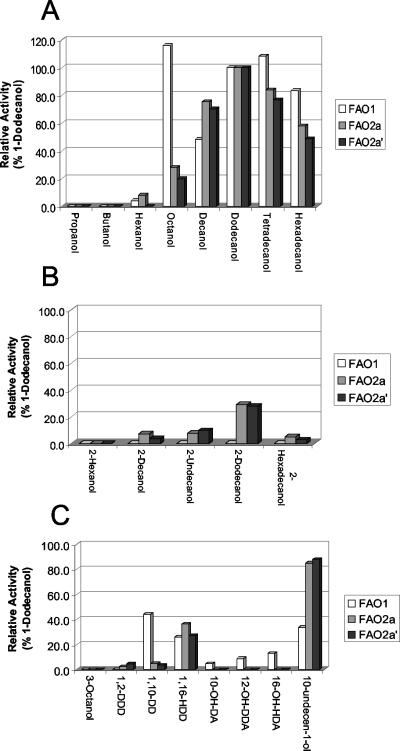
FAO1, FAO2a, and FAO2a′ were tested for their level of activity with various alcohols (Table 3), prepared as 20 mM stock solutions in acetone. Alcohols showing activity with either FAO1 or FAO2 are indicated. Note that the concentration of the alcohol used in the substrate specificity experiment was 200 μM in the final reaction mixture. The same alcohols were used to determine the Km and Vmax of FAO1, FAO2a, and FAO2a′. The substrate specificity profiles of FAO1, FAO2a, and FAO2a′ were reported as percentages of the FAO activity using 1-dodecanol as substrate, with 1-dodecanol arbitrarily set at 100% activity (Fig. 2). The activity of FAO1, FAO2a, and FAO2a′ with 1-alkanols is shown in Fig. 2A. Interestingly FAO1 preferred 1-octanol as substrate, with 1-tetradecanol being the preferred longer-chain alcohol. In contrast, FAO2a and FAO2a′ preferred 1-dodecanol above all other 1-alkanols. There was a big drop in activity between a C8 alcohol and a C6 alcohol with either FAO1, FAO2a, or FAO2a′.
TABLE 3.
Substrate specificity testing of FAO enzymes for alcohols
| Compound tested | Activity detectedb | Compound tested | Activity detecteda | |
|---|---|---|---|---|
| 2-Pentanol | —b | |||
| 2-Hexanol | — | |||
| 2-Decanol | FAO2 | |||
| 2-Undecanol | FAO2 | |||
| 2-Dodecanol | FAO2 | |||
| 2-Hexadecanol | FAO2 | |||
| 3-Octanol | — | |||
| 10-Undecen-1-ol | FAO1/FAO2a | |||
| 1,8-Octanediol | — | |||
| 1,10-Decanediol | FAO1/FAO2a | |||
| 1,16-Hexadecanediol | FAO1/FAO2a | |||
| 1,2-Octanediol | FAO2 | |||
| 1,2-Dodecanediol | FAO2 | |||
| 10-Hydroxydecanoic acid | FAO1 | |||
| 12-Hydroxydodecanoic acid | FAO1 | |||
| 16-Hydroxydodecanoic acid | FAO1 | |||
| Citronellol | — | |||
| Geraniol | — | |||
| Linalool | — | |||
| 1-Phenylpropan-1-ol | — | |||
| 3-Phenylpropan-1-ol | — | |||
| 2-Phenylbutan-1-ol | — | |||
| Methanol | — | |||
| Ethanol | — | |||
| Propanol | — | |||
| Butanol | — | |||
| 1-Hexanol | FAO1/FAO2a | |||
| 1-Octanol | FAO1/FAO2a | |||
| 1-Decanol | FAO1/FAO2a | |||
| 1-Dodecanol | FAO1/FAO2a | |||
| 1-Tetradecanol | FAO1/FAO2a | |||
| 1-Hexadecanol | FAO1/FAO2a | |||
| 4-Cyclohexyl-1-butanol | — | |||
| 3-Cyclohexyl-1-propanol | — | |||
| 2-Cyclohexylethanol | — | |||
| Cyclohexylmethanol | — |
Activity was detected with the substrate shown with either FAO1 or FAO2a (C. tropicalis ATCC 20336).
—, no activity.
FIG. 2.
Relative activities of FAO1, FAO2a, and FAO2a′ on 1-alkanols (A), 2-alkanols (B), and other alcohols (C). The relative enzyme activities were determined as described in Materials and Methods. Ten microliters of each substrate (20 mM dissolved in acetone) was added to a 1-ml reaction mixture as described in Materials and Methods. The HEPES buffer used contained 0.5% (wt/vol) Triton X-100. Duplicate reactions were averaged to determine each of the data-points. 1,2-DDD, 1,2-dodecanediol; 1,10-DD, 1,10-decanediol; 1,16-HDD, 1,16-hexadecanediol; 10-OH-DA, 10-OH-decanoic acid; 12-OH-DDA, 12-OH-dodecanoic acid; 16-OH-HDA, 16-OH-hexadecanoic acid.
The activity of the three enzymes on 2-alkanols is shown in Fig. 2B. FAO2a and FAO2a′ both oxidize 2-alkanols, while FAO1 exhibited no oxidation of 2-alkanols. In contrast, FAO1 oxidizes ω-hydroxy fatty acids, but FAO2a and FAO2a′ do not oxidize ω-hydroxy fatty acids (Fig. 2C). These results indicate that FAO1 and FAO2a appear to be very different enzymes, with significant differences in substrate specificity. Only FAO2a and FAO2a′ oxidized 1,2-alkane-diols, although the activity with 1,2-dodecanediol was low relative to that of either 1-dodecanol or 2-dodecanol. Interestingly FAO2a and FAO2a′ oxidize 10-undecen-1-ol much better than FAO1, whereas FAO1 oxidizes 1,10-decanediol much faster than FAO2a and FAO2a′. These data show that the substrate specificity of FAO2a and FAO2a′ are essentially the same, indicating that having a serine or a leucine at amino acid position 177 has little if any effect on the substrate specificity of the enzyme. Although the substrate specificity of FAO2b was not performed, due to the close homology with FAO2a (five amino acids are different), its substrate specificity is anticipated to be very similar.
Microsomal preparations made from C. tropicalis fermentor samples induced with HOSFFA show significant activity with 16-hydroxyhexadecanoic acid and 12-hydroxydodecanoic acid (Table 4), but undetectable activity with 2-dodecanol. In addition, the percentage of activity with these substrates, relative to 1-dodecanol activity, closely followed that for the recombinant FAO1. Hence, it appears that in C. tropicalis (ATCC 20336), FAO1, at least in HOSFFA fermentations, is induced to a much greater extent than FAO2a and appears to be the predominant FAO.
TABLE 4.
Comparison of FAO activity in oleic acid fermentation to FAO1 and FAO2a activities expressed in E. coli
| Substratea | Fermentor sampleb
|
FAO1
|
FAO2a
|
|||
|---|---|---|---|---|---|---|
| Activity (U) | % of controlc | Activity (U) | % of controlc | Activity (U) | % of controlc | |
| 1-DD | 5.79 ± 0.06 | 100.0 | 9.61 ± 0.1 | 100.0 | 10.13 ± 0.01 | 100.0 |
| HHA | 1.10 ± 0.08 | 18.9 | 1.83 ± 0.05 | 19.1 | 0.0 | 0.0 |
| HDA | 0.48 ± 0.08 | 8.2 | 0.80 ± 0.01 | 8.3 | 0.0 | 0.0 |
| 2-DD | 0.0 | 0.0 | 0.0 | 0.0 | 0.73 ± 0.03 | 7.3 |
Ten microliters of each substrate (5 mM dissolved in acetone) was added to a 1-ml reaction mixture as described in Materials and Methods. The HEPES buffer used contained 0.5% (wt/vol) Triton X-100. 1-DD, 1-dodecanol; HHA, 16-hydroxyhexadecanoic acid; HDA, 12-hydroxydodecanoic acid; 2-DD, 2-dodecanol.
Sample was taken at 22.5 h after addition of oleic acid substrate to the fermentor. Microsomes were prepared as described in Materials and Methods.
Control is the activity using 1-dodecanol as substrate.
Km determinations.
Km values for most of the alcohols that demonstrated activity with FAO1, FAO2a, and FAO2a′ were determined. Most of the Km values were determined at pH 7.6 by using the HEPES-Triton X-100 buffer. For FAO2a′, the Km values were determined in 0.1 M HEPES, pH 7.6, with the substrate dissolved in acetone. The results are shown in Table 5. For most of the alcohols tested, FAO1 yielded Km values between 10 and 20 μM. The lowest Km value was found with 16-hydroxy-hexadecanoic acid. Using either FAO2a or FAO2a′ as the enzyme, the Km values for the various alcohols were very similar, again demonstrating that having a serine or leucine at amino acid position 177 has little effect on the affinity of the enzyme for a particular substrate. With FAO2a, the Km values were generally much more variable and ranged from 2 μM to >1 mM. The substrates with the lowest Km values were 1-dodecanol and 1-tetradecanol.
TABLE 5.
Km values for FAO1, FAO2a, and FAO2a′
| Substrate |
Km (range [μM])a
|
||
|---|---|---|---|
| FAO1 | FAO2a | FAO2a′ | |
| Octanol | 41.0 (35.0-49.3) | 503 (329.3-1069.2) | 654 (323.5-30,607.9) |
| Decanol | 15.1 (11.8-21.3) | 74 (64.3-85.9) | 69.6 (65.1-74.8) |
| Dodecanol | 14.9 (11.9-19.9) | 2.5 (2.4-2.5) | 4.4 (3.9-4.4) |
| Tetradecanol | 28.1 (20.8-43.3) | 4.6 (4.5-4.8) | 2.4 (2.2-2.5) |
| Hexadecanol | 11.9 (11.1-11.8) | 56 (51.2-61.7) | 85 (78.4-93.5) |
| 2-Decanol | NRb | 934 (823.2-1078.5) | 1,090 (567.3-13,696.9) |
| 2-Undecanol | NR | 141 (125.9-159.0) | 162 (150.9-174.5) |
| 2-Dodecanol | NR | 41.3 (39.60-43.06) | 75.3 (67.9-84.5) |
| 2-Hexadecanol | NR | 350 (332.5-370.0) | 720 (628.1-844.2) |
| 1,2-Dodecanediol | NR | 998 (720.2-1,622.7) | 928 (742.6-1,237.3) |
| 1,10-Decanediol | 67.0 (58.9-77.6) | 425 (394.4-462.3) | 1,607 (1,377.2-1,927.5) |
| 1,16-Hexadecanediol | 10.3 (9.3-11.6) | 37.3 (33.9-41.5) | 16.3 (15.7-16.9) |
| 10-Undecene-1-ol | 9.9 (8.8-11.3) | 45 (40.7-50.1) | 36.1 (32.7-40.4) |
| 12-Hydroxydodecanoic | 99.0 (78.2-134.4) | NR | NR |
| 16-Hydroxyhexadecanoic | 7.4 (5.9-9.9) | NR | NR |
Km values were derived from a double-reciprocal plot of substrate concentration and enzyme activity. Correlation coefficient values were always greater than 0.990.
NR, no reaction with this substrate.
DISCUSSION
A small number of FAOs have been described in the scientific literature for various yeasts, examples of which are C. tropicalis (4, 10, 11, 12, 19), Candida maltosa (2, 13), Candida cloacae (19), Torulopsis candida (9), Candida (Torulopis) bombicola (8), and Candida (Torulopsis) apicola (7). FAO has been purified from the hexadecane-grown yeast T. candida (9) and was described as a tetramer with a subunit molecular weight (MW) of 75,000 (MW of 290,000 for the tetramer). It had a pH optimum of 7.6 and could oxidize higher alcohols with a carbon chain length of C4 to C16. Hexadecane-grown C. bombicola (8) apparently has two different alcohol oxidase activities, one with an optimal specificity for 10-carbon n-alcohols and the other with an optimal specificity for 14-carbon n-alcohols. The FAO from C. maltosa (13) catalyzed the oxidation of 1-alkanols (C4 to C22) with highest activity utilizing 1-octanol. It also oxidized 2-alkanols (C8 to C16). α,ω-Alkanediols, ω-hydroxypalmitic acid, phenylalkanols, and terpene alcohols were all found to be substrates for the FAO, but at fairly low rates of oxidation. The oxidation of 2-alkanols was stereoselective for the R(−) enantiomers only.
FAO activity from C. tropicalis (ATCC 20336) grown on hexadecane was first described by Kemp et al. in 1988 (12). Kemp et al. observed oxidation of 1-alkanols from C4 to C18, with maximal activity with 1-dodecanol. Oxidation with 16-hydroxypalmitate but not 12-hydroxylaurate was also observed. An FAO was later purified (4) and was shown to be a dimer (MW = 145,000) with a subunit MW of 68,000 to 72,000. The purified enzyme showed a substrate specificity similar to that determined previously, but demonstrated additional activity with 12-hydroxylaurate and 2-dodecanol. The enzyme was found to be a flavoprotein, but the identity of the flavin was not known. Due to the presence of the flavin, the enzyme was found to be light sensitive (11).
Recently two FAO genes from C. cloacae (FERM O-736) and a single FAOT gene from C. tropicalis (NCYC 470) were cloned, and the DNA sequence was determined (19). The ORFs of FAO1 and FAO2 from C. cloacae were 2,094 and 2091 bp, respectively. The ORF of FAOT from C. tropicalis (NCYC 470) was 2,112 bp. FAOT shared 60.6 and 61.7% nucleotide identities and 74.8 and 76.2% amino acid sequence similarities with C. cloacae FAO1 and FAO2, respectively. The FAO1 gene but not the FAO2 gene was successfully cloned and expressed in E. coli. An international patent application utilizing this information has been filed (A. R. Slabas, K. Elborough, S. Vanhanen, M. West, Q. Cheng, N. Lindner, J. Casey, and D. Sanglard, 23 September 1999. International patent application WO 99/47685).
Although previously published research with FAO of C. tropicalis (NCYC 470) found no evidence for more than one FAO gene (19), our research demonstrates that there are two distinct genes in C. tropicalis strain ATCC 20336. Not only are the DNA sequences (and deduced amino acid sequences) of the FAO1 and FAO2 genes quite different, but the substrate specificity data for recombinant FAO enzymes expressed in E. coli indicate a difference in activity as well. It is interesting to note that the FAOT gene sequence is no more similar to the FAO1 or FAO2 genes from C. tropicalis (ATCC 20336) than these genes are to each other. This suggests that FAOT codes for a unique gene product, whose substrate specificity is likely to be different from either FAO1 or FAO2 from C. tropicalis (ATCC 20336).
It is also interesting that FAO1 utilizes ω-hydroxy fatty acids as substrate but not 2-alkanols. FAO2 utilizes 2-alkanols but not ω-hydroxy fatty acids. In microsomal preparations of C. tropicalis grown on oleic acid (HOSFFA), FAO activity was detected only with ω-hydroxy fatty acids as substrates and not with 2-alkanols, indicating that FAO1 is predominantly expressed in fatty acid-oxidizing fermentations. Curiously, Dickinson and Wadforth (4) showed that the purified alcohol oxidase from the same parent strain of C. tropicalis (ATCC 20336) grown on hexadecane had activity with both ω-hydroxy fatty acids and with 2-alkanols. Based on the results presented in this report, it appears that both FAO1 and FAO2 were both induced by growth on hexadecane and probably copurified.
The FAO from C. maltosa also demonstrated activity with both ω-hydroxy fatty acids and with 2-alkanols when grown on alkane substrates (13). Interestingly, they determined significant activity with 3-phenylpropan-1-ol, whereas activity with this substrate was not detected with either FAO1 or FAO2a.
The FAO1 gene from C. tropicalis (ATCC 20336) has no CTG codons while the FAO2a and FAO2b genes both have one CTG codon centered at 530 bp from the start of the ORF. The sequence for the FAOT ORF has three CTG codons: one centered at 239 bp, one at 530 bp, and a third at 566 bp. The CTG codons, which are translated as serine in C. tropicalis, would be translated as leucine in E. coli. One might expect, therefore, that FAOT, if expressed in E. coli without codon alterations, might yield a less active protein than the enzyme expressed in C. tropicalis by using the identical gene sequence.
In their patent application, Slabas et al. identified seven peptide sequences, called signature peptides, that were indicative of an FAO. A comparison of the corresponding peptide sequences for FAO1, FAO2, FAOT and the seven signature peptides is shown in Table 6. This comparison shows that all seven FAOT peptides agree with the signature peptides. Six of the seven FAO1 peptides but only four of the seven FAO2 peptides agree with the signature peptides. It is interesting to note that, even though FAO2 is closest to FAOT in deduced amino acid sequence identity and similarity (Table 2), FAO1 is most similar to FAOT when the seven signature peptides are compared. Although similarity in deduced amino acid sequence or signature peptides may give an indication of the genetic relatedness of proteins, this information provides no direct information about the physiological properties of an enzyme, such as substrate specificity or kinetic properties. This type of information can only be obtained by performing enzyme assays on proteins expressed in a host free of background FAO activity.
TABLE 6.
Comparison of signature peptides between Cognis' FAO1, FAO2a, FAO2b, C. albicans FAO, and C. tropicalis FAOT
| Peptide | Sequencea
|
||||
|---|---|---|---|---|---|
| Signature peptideb | FAOT | FAO1 | FAO2a and FAO2b | FAO-CAc | |
| 1 | IIGSG(X)GAGVVA | IIGSGAGAGVVA | IIGSGAGAGVVA | IIGSGAGAGVMA | IIGSGAGSGVVA |
| 2 | AGSTFGGG | AGSTFGGG | AGSTFGGG | AGSTLGGG | AGSTFGGG |
| 3 | NWSACLKTP | NWSACLKTP | NWSACLKTP | NWSACLKTP | NWSACIKTP |
| 4 | CG(X)CHLGC | CGFCHLGC | CGFCYLGC | CGFCYLGC | CGFCHLGC |
| 5 | IG(X)NL(X)LHPVS | IGKNLTLHPVS | IGKNLTLHPVS | IGKNLTLHPVS | IGANLTLHPVT |
| 6 | SAHQMS(X)CRMSG | SAHQMSTCRMSG | SAHQMSTCRMSG | SAHQMSTCRMSG | SAHQMSSCRMSG |
| 7 | PTASG(X)NPM | PTASGANPM | PTASGANPM | PTASGANPM | PTASGANPM |
Amino acid in bold indicate amino acid differences from signature peptide. (X) indicates that any amino acid may be at this position.
A. R. Slabas, K. Elborough, S. Vanhanen, M. West, Q. Cheng, N. Lindner, J. Casey, and D. Sanglard, 23 September 1999. International patent application WO 99/47685.
Putative FAO from C. albicans.
When this was done for the two genes from C. tropicalis ATCC 20336, there was an obvious difference between the FAO enzymes encoded by these genes. With substrates that were oxidized by both enzymes, such as the 1-alkanols, there were differences observed in chain-length preference and in Km values for the substrates. However, there were also distinct substrate specificities between the two FAOs, and these specificities allowed us to determine which genes were induced during production of diacids. FAO1 and FAO2 could be easily distinguished because FAO1 uses ω-hydroxy fatty acids but not 2-alkanols as substrates, whereas FAO2 utilizes 2-alkanols but not ω-hydroxy fatty acids. Because FAO1 oxidizes ω-hydroxy fatty acids, it would be expected to be strongly induced during the conversion of fatty acids to diacids, since ω-hydroxy fatty acids are intermediates in the ω-oxidation of fatty acids to diacids. In agreement with this concept, it was determined that microsomal preparations of a β-oxidation-blocked strain of C. tropicalis (ATCC 20336) obtained from oleic acid fermentations during diacid production contained high activity toward ω-hydroxy fatty acids, but no detectable activity toward 2-alkanols. Therefore, FAO1 appears to be highly induced under these conditions, and FAO2 appears to be only weakly induced, if at all. For future metabolic engineering of C. tropicalis to improve diacid productivity from fatty acids, FAO1 is clearly the gene of choice for amplification. Amplification of FAO2 would be needed if oxidation of the alcohol group of 2-alkanols to the ketone were desired. Unfortunately, the conditions for induction of FAO2 are unknown, and additional work would need to be done to understand FAO2 induction and to successfully amplify this gene in C. tropicalis ATCC 20336.
Acknowledgments
This work was supported by the United States Department of Energy, project no. DE-FC36-95GO10099, and the National Institute of Standards and Technology, Advanced Technology Program 70NANB8H4033, and under interagency agreement through U.S. Department of Energy contract W-31-109-Eng-37. Sequencing of Candida albicans was accomplished with the support of the NIDR and the Burroughs Wellcome Fund.
We thank Ronald W. Davis, Stanford Genome Technology Center, for permission to use the sequence data for Candida albicans, which was obtained from the Stanford Genome Technology Center website at http://www-sequence.stanford.edu/group/candida.
REFERENCES
- 1.Anderson, K. W., and J. D. Wenzel. May2003. Fermentation process. U.S. patent 6,569,670.
- 2.Blasig, R., S. Mauersberger, R. Riege, W. H. Schunck, W. Jockisch, and P. Franke. 1988. Degradation of long-chain n-alkanes by the yeast Candida maltosa. II. Oxidation of n-alkanes and intermediates using microsomal membrane fractions. Appl. Microbiol. Biotechnol. 28:589-597. [Google Scholar]
- 3.Craft, D. L., K. M. Madduri, M. Eshoo, and C. R. Wilson. 2003. Identification and characterization of the CYP52 family of Candida tropicalis ATCC 20336 important for the conversion of fatty acids and alkanes to α,ω-dicarboxylic acids. Appl. Environ. Microbiol. 69:5983-5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson, F. M., and C. Wadforth. 1992. Purification and some properties of alcohol oxidase from alkane-grown Candida tropicalis. Biochem. J. 282:325-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eschenfeldt, W. H., Y. Zhang, H. Samaha, L. Stols, L. D. Eirich, C. R. Wilson, and M. I. Donnelly. 2003. Transformations of fatty acids catalyzed by cytochrome P450 monooxygenase enzymes of Candida tropicalis. Appl. Environ. Microbiol. 69:5992-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fürste, J. P., W. Pansegrau, R. Frank, H. Blöcker, P. Scholz, M. Bagdasarian, and E. Lanka. 1986. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene 48:119-131. [DOI] [PubMed] [Google Scholar]
- 7.Hommel, R., D. Lassner, J. Weiss, and H. P. Kleber. 1994. The inducible microsomal fatty alcohol oxidase of Candida (Torulopsis) apicola. Appl. Microbiol. Biotechnol. 40:729-734. [Google Scholar]
- 8.Hommel, R., and C. Ratledge. 1990. Evidence for two fatty alcohol oxidases in the biosurfactant-producing yeast Candida (Torulopsis) bombicola. FEMS Microbiol. Lett. 70:183-186. [DOI] [PubMed] [Google Scholar]
- 9.Ilchenko, A. P., and I. M. Tsfasman. 1988. Isolation and characterization of alcohol oxidase from higher alcohols of the yeast Torulopsis candida grown on hexadecane. Biokhimiya 53:263-271. [Google Scholar]
- 10.Kemp, G. D., F. M. Dickinson, and C. Ratledge. 1991. Activity and substrate specificity of the fatty alcohol oxidase of Candida tropicalis in organic solvents. Appl. Microbiol. Biotechnol. 34:441-445. [Google Scholar]
- 11.Kemp, G. D., F. M. Dickinson, and C. Ratledge. 1990. Light sensitivity of the n-alkane-induced fatty alcohol oxidase from Candida tropicalis and Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 32:461-464. [Google Scholar]
- 12.Kemp, G. D., F. M. Dickinson, and C. Ratledge. 1988. Inducible long chain alcohol oxidase from alkane-grown Candida tropicalis. Appl. Microbiol. Biotechnol. 29:370-374. [Google Scholar]
- 13.Mauersberger, S., H. Drechsler, G. Oehme, and H. G. Müller. 1992. Substrate specificity and stereoselectivity of fatty alcohol oxidase from the yeast Candida maltosa. Appl. Microbiol. Biotechnol. 37:66-73. [Google Scholar]
- 14.Ohama, T., T. Suzuki, M. Mori, S. Osawa, T. Ueda, K. Watanabe, and T. Nakase. 1993. Non-universal decoding of the leucine codon CUG in several Candida species. Nucleic Acids Res. 21:4039-4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Picataggio, S., K. Deanda, and J. Mielenz. 1991. Determination of Candida tropicalis acyl coenzyme A oxidase isozyme function by sequential gene disruption. Mol. Cell. Biol. 11:4333-4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picataggio, S., T. Rohrer, K. Deanda, D. Lanning, R. Reynolds, J. Mielenz, and L. D. Eirich. 1992. Metabolic engineering of Candida tropicalis for the production of long-chain dicarboxylic acids. Biotechnology 10:894-898. [DOI] [PubMed] [Google Scholar]
- 17.Sugita, T., and T. Nakase. 1999. Non-universal usage of the leucine CUG codon and the molecular phylogeny of the genus Candida. Syst. Appl. Microbiol. 22:79-86. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki, T., T. Ueda, and K. Watanabe. 1997. The ‘polysemous' codon—a codon with multiple amino acid assignment caused by dual specificity of tRNA identity. EMBO J. 16:1122-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vanhanen, S., M. West, J. T. M. Kroon, N. Lindner, J. Casey, Q. Cheng, K. M. Elborough, and A. R. Slabas. 2000. A consensus sequence for long-chain fatty-acid alcohol oxidases from Candida identifies a family of genes involved in lipid-oxidation in yeast with homologues in plants and bacteria. J. Biol. Chem. 275:4445-4452. [DOI] [PubMed] [Google Scholar]