Abstract
Good Clinical Practice (GCP) is an international standard for the design, conduct, performance, monitoring, auditing, recording, analyses, and reporting of clinical trials. The goal of GCP is to ensure the protection of the rights, integrity, and confidentiality of clinical trial participants and to ensure the credibility and accuracy of data and reported results. In the United States, trial sponsors generally require investigators to complete GCP training prior to participating in each clinical trial to foster GCP and as a method to meet regulatory expectations (ie, sponsor’s responsibility to select qualified investigators per 21 CFR 312.50 and 312.53(a) for drugs and biologics and 21 CFR 812.40 and 812.43(a) for medical devices). This training requirement is often extended to investigative site staff, as deemed relevant by the sponsor, institution, or investigator. Those who participate in multiple clinical trials are often required by sponsors to complete repeated GCP training, which is unnecessarily burdensome. The Clinical Trials Transformation Initiative convened a multidisciplinary project team involving partners from academia, industry, other researchers and research staff, and government to develop recommendations for streamlining current GCP training practices. Recommendations drafted by the project team, including the minimum key training elements, frequency, format, and evidence of training completion, were presented to a broad group of experts to foster discussion of the current issues and to seek consensus on proposed solutions.
Keywords: Good Clinical Practice, GCP, clinical trials, clinical research training
Introduction
Good Clinical Practice (GCP) is the ethical and scientific standard for the conduct and reporting of clinical trials involving human participants. GCP is focused on the protection of the rights, integrity, and confidentiality of clinical trial participants and the accuracy and scientific integrity of the data collected during clinical trials and reported in the results. To ensure that results from clinical trials can support authorization of investigational products internationally, the principles of GCP have been adopted by regulatory authorities in Japan, the United States, and the European Union through the International Conference on Harmonisation (ICH) and implemented through the ICH GCP guideline E6.1,i These principles have also been adopted by the International Standards Organization (ISO), an independent, nongovernmental membership organization made up of 163 member countries, through ISO-14155:201. Several regulatory agencies, including the United States Food and Drug Administration (FDA), expect clinical research personnel to have an understanding of GCP prior to engaging in clinical research.
To foster GCP principles and provide a method to meet regulatory expectations (ie, sponsors’ responsibility to select qualified investigators per 21 CFR 312.50 and 312.53(a) for drugs and biologics and 21 CFR 812.40 and 812.43(a) for medical devices), commercial sponsors and some government sponsors of clinical trials generally require that all investigators involved in these trials, regardless of previous experience and training, complete GCP training prior to participating in each clinical trial. Investigative site staff is sometimes required to receive GCP training as well. Funding agencies, such as the National Institutes of Health and the Department of Veterans Affairs, require investigators and personnel participating in funded research to provide documentation of current training certification (training is recommended to be repeated or updated every 3 years).2–4 Thus, investigators and site staff may receive GCP training multiple times each year, an inefficient and burdensome process. Note that GCP training is in addition to protocol-specific training that investigators and site staff receive.
In order to reduce these redundancies, the Clinical Trials Transformation Initiative (CTTI) aimed to develop recommendations regarding the key elements of GCP training as well as the optimal frequency for refresher training, format of training, and evidence of completion—which, if implemented, would conserve resources currently used to conduct repeated GCP training.5,6 Further, this would enable investigators and site staff to focus their attention on the safety of patients and the credibility and accuracy of data and reported results.
Methods
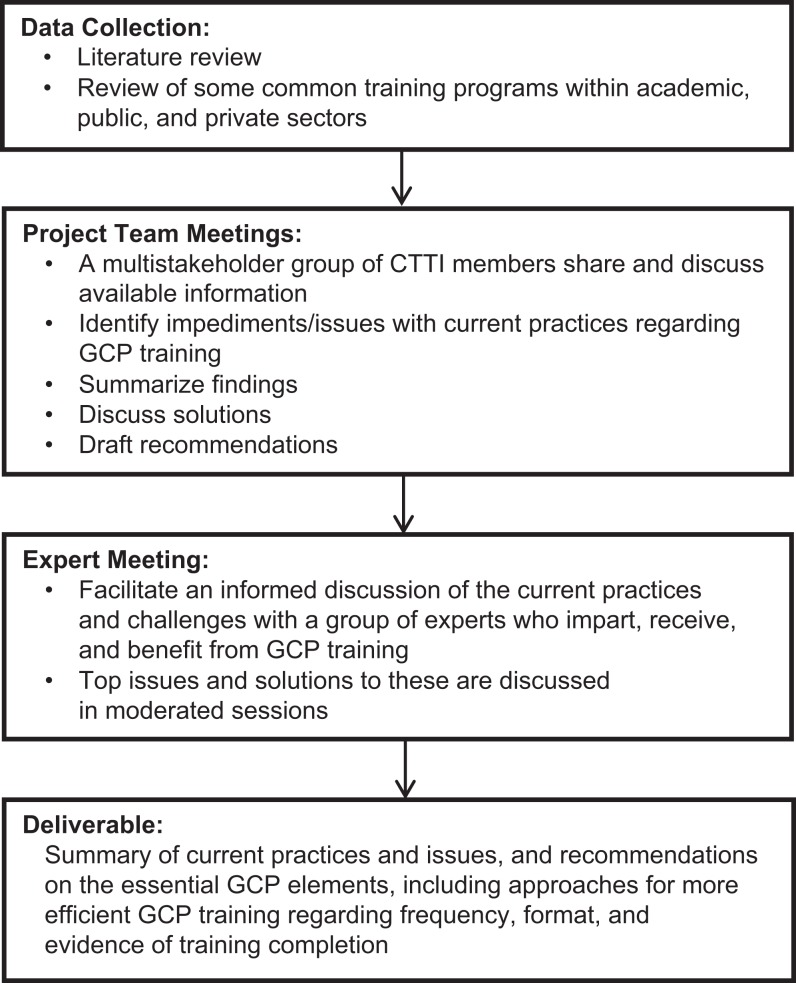
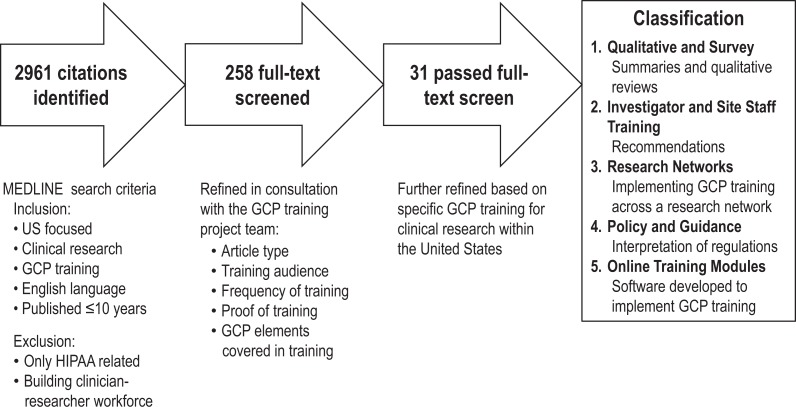
The objective of our project was to summarize information regarding the scope and content of existing GCP training practices. We gathered this information through a literature review7 and a review of several available GCP training programs. Figure 1 outlines the overall process used to develop the recommendations. Figure 2 shows the literature review process during the data collection phase.
Figure 1.
Overall process used to develop the recommendations.
Figure 2.
Literature review process.
Results
Current Landscape of GCP Training Programs
Literature review
We identified 31 articles related to GCP training and classified them into the following 5 categories: qualitative and survey,8–17 investigator and site staff training,18–24 research networks,25–30 policy and guidance,31–34 and online training modules.35–38 GCP training programs referenced in the literature range from departmental-based training for researchers to centralized GCP training programs offered at the university or organizational level (eg, training offered by government agencies).
Table 1 summarizes the findings from the literature review. As described in the literature, most GCP training programs cover the following topics: institutional review board or independent ethics committee oversight, investigator responsibilities, staff training and delegation of responsibilities, protocol adherence, data management, informed consent, vulnerable populations, adverse event reporting, and site monitoring. These papers generally did not address the frequency of GCP training, the format of training, or evidence of training completion.
Table 1.
Summary of Current Landscape of GCP Training Programs.
| Literature Review | ||
|---|---|---|
Findings
| ||
| Sample Training Programs | ||
Content
| ||
| Frequency | Format | Evidence of completion |
| Variable, typically ranging from 1 to 3 years |
|
End-of-course quiz with passing grade, certification or transcript, or qualification test to opt out of training |
Abbreviations: GCP, Good Clinical Practice; ICH, International Conference on Harmonisation.
Sampling of GCP training programs
The project team reviewed a sample of five programs designed to train investigators and site staff on the principles of GCP.39–43 On the basis of team members’ familiarity and experience, we chose a few programs that appeared representative of academic, public, and private sectors. Programs ranged from multiday onsite training to web-based modules completed at the trainee’s convenience. Some programs were tiered or differentiated based on job function. Courses also varied in regard to evidence of completion (Table 1).
Expert Meeting Discussions: GCP Training Is Floor, Not Ceiling
In January 2014, the CTTI project team convened an expert meeting of representatives from academia, industry (including pharmaceutical, medical device, and contract research organizations), government agencies, health systems, independent consulting companies, investigative sites, and patient advocacy groups.5 The recommendations drafted by the project team, informed in part by the literature review and sample GCP training programs reviewed, were shared with attendees to obtain feedback to help refine the recommendations. The experts agreed that the 13 elements in the investigator section of ICH E61 are only the minimal requirements, or “floor,” of GCP training; knowledge of the key GCP elements is only part of the information that investigators need to conduct a trial (Table 2).
Table 2.
GCP Elements Listed in Investigator Section of ICH E6.1
| GCP Element | |
|---|---|
| 1 | Investigator’s qualifications and agreements |
| 2 | Adequate resources |
| 3 | Medical care of trial subjects |
| 4 | Communication with IRB/IEC |
| 5 | Compliance with protocol |
| 6 | Investigational product(s) |
| 7 | Randomization procedures and unblinding |
| 8 | Informed consent of trial subjects |
| 9 | Records and reports |
| 10 | Progress reports |
| 11 | Safety reporting |
| 12 | Premature termination or suspension of a trial |
| 13 | Final report(s) by investigator/institution |
Abbreviations: GCP, Good Clinical Practice; IRB/IEC, institutional review board / institutional ethics committee.
The experts also discussed the value of differential or role-based GCP training (ie, training customized for research personnel performing different functions) and of evaluating GCP knowledge as it pertains to each function. There was further discussion of the benefits of interactive learning and mentorship for new investigators, how to incorporate GCP principles into everyday practice, and optimal formats for conducting training and evaluating its impact.
Recommendations for GCP Training
After careful consideration of the expert feedback, the project team developed a final set of recommendations for GCP training.6 Table 3 summarizes our recommendations for the minimum content, minimum frequency, format, and evidence of completion for investigators and for site staff undergoing GCP training.
Table 3.
Recommendations for GCP Training From the Clinical Trials Transformation Initiative.
| Component | Recommendation |
|---|---|
| Recommended minimum essential elements for a GCP training program and other aspects of training content |
|
| Frequency of training |
|
| Format of training |
|
| Evidence of completion | Satisfactory completion of a training program—such as certificate, test score, or other formal confirmation of training received—should be documented. |
Abbreviations: GCP, Good Clinical Practice; ICH, International Conference on Harmonisation.
Discussion
GCP training is only one part of the preparation needed for conducting a clinical trial and for laying the foundation for achieving clinical research competence. Protocol-specific training and opportunities to gain experience in clinical research, possibly through mentorship for new investigators, are also needed to develop qualified investigators.
While it is essential that investigators be familiar with the 13 key GCP elements, developers of GCP training programs should understand that the basic messages within the key elements require deeper focus depending on the individual’s role. For example, the element “Investigator’s Qualifications and Agreements”1 specifies that the investigator be able to identify what it means to be a qualified investigator with a qualified team, how to supervise the team and delegate tasks, and know what is required in the Form FDA 1572 (“Statement of Investigator”),44 while in-depth training on this aspect may not be required for a study coordinator. It is also important that the stated rationale and scope for the GCP training course be based on the purpose of the training and the roles of the participants. A GCP course for investigators involved in device trials would have a different focus from a GCP course for support personnel involved in drug trials. Similarly, a GCP course for support personnel at a site should focus on those elements related to site support duties such as data management and trial records. It would be desirable for the training module to include a written explanation of how the course content relates to the job function (ie, course curriculum, course topics, and objectives).
We recommend that GCP training programs emphasize how research conduct may differ from medical practice by including topics that are outside the scope of medical practice, such as safety reporting, IRB review, and research informed consent. Also beneficial would be a focus on any recurring noncompliance and criteria for retraining.
The project team recommends that GCP training be completed at a minimum of every 3 years. We also recommend that GCP training completion be mutually accepted across organizations so that trainees qualify for a 3-year period without requiring retraining for each new clinical trial or sponsor during that period.
As indicated in the recommendations, the frequency of refresher training should be flexible to accommodate, for example, the experience level of the trainee. Refresher training can serve to selectively train on new regulations, if any, to cover gaps in knowledge or training, or to cover any areas of recurring noncompliance. Currently several formats are available for GCP training (eg, online, face-to-face), allowing flexibility of access balanced with cost and resources. While online training is attractive because of flexibility of access, its challenges should be recognized; some individuals are concerned that online learning may not be retained as well as in-person approaches.45 Real-world training that integrates adult learning methods, such as interactive feedback, case studies, and group exercises,46 may provide a higher degree of knowledge retention; however, this format would be challenging to administer to large, diverse audiences. Another consideration is that different training formats may be more appropriate for different job functions. Also, consideration should be given to whether training by active involvement, serving as a principal investigator (PI) in new studies, or authoring publications on GCP should also qualify as acceptable training. Last, the project team recommends that evidence of completion be provided to document that training was received.
Integrating GCP Principles in Clinical Research Conduct
In addition to trainee comprehension and retention of GCP knowledge, an important objective of GCP training is the incorporation of learning into the daily activities of clinical research. To achieve applied learning, training programs that go beyond a basic review of key elements may be desirable.47 Applied courses could be developed using different approaches such as presentation and discussion of case studies or hypothetical scenarios, role-playing, and small-group interactive sessions to get learners to think critically about issues that make up the real world of clinical research. For example, a course module could involve a critique of an informed consent document, the GCP elements related to human subject protection. Providing both good and bad examples may support learning the required elements. Such applied courses could be developed as face-to-face workshops, interactive web-based modules, or a blend of both. An applied approach can also be used to impart targeted training that focuses on common errors.
Another approach is to foster mentoring programs that instill the culture of GCP. In these programs, new investigators are paired with more experienced mentors who provide guidance on interpreting and building on the fundamentals of GCP. This model has been successfully implemented in some trades and professions and may be a valuable enhancement of training when mentors can be co-located with trainees.
Conclusion
In this era of increasingly complex and often global clinical trials, the need for an understanding of GCP principles is greater than ever. Implementing an integrated approach to GCP training that would be widely accepted by research sponsors could provide a path forward in sustaining a workforce of well-trained researchers who conduct clinical trials successfully while reducing the burden of repeated training.
Acknowledgment
The authors thank Amy Kendrick, MSN, and Megan Chobot, MSLS, for conducting the literature review; Liz Wing, MA, for manuscript development and editorial assistance; and Gerrit Hamre, MA, for technical review. All were employees of the Duke Clinical Research Institute, Durham, NC, and did not receive any compensation for their work other than their usual salary.
Note
The work of the CTTI project described in this article preceded the publication of the ICH E6(R2) draft addendum in September 2015 (Integrated Addendum: Good Clinical Practice (E6[R2]). http://www.ich.org/products/guidelines/efficacy/efficacy-single/article/addendum-good-clinical-practice.html. Accessed November 23, 2015).
Footnotes
Author Note: This study was presented as a poster presentation at the DIA Annual Meeting, San Diego, June 15-19, 2014.
The views expressed in this article represent those of the authors and not necessarily the views or practices of the authors’ employer or any other party.
Declaration of Conflicting Interests: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this manuscript was made possible, in part, by the Food and Drug Administration through grant R18FD005292 and cooperative agreement U19FD003800. Views expressed in written materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government. Partial funding was also provided by pooled membership fees from CTTI’s member organizations.
References
- 1. US Department of Health and Human Services. Guidance for Industry. E6 Good Clinical Practice: Consolidated Guidance. International Conference on Harmonisation http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073122.pdf. Published April 1996. Accessed March 11, 2014. [Google Scholar]
- 2. US Department of Veterans Affairs. Options for fulfilling Office of Research and Development (ORD) training requirements. http://www.research.va.gov/pride/training/options.cfm. Accessed September 16, 2015.
- 3. National Institute of Allergy and Infectious Diseases (NIAID). Good Clinical Practice training for NIAID and awardee clinical research staff. https://www.niaid.nih.gov/LabsAndResources/resources/toolkit/Documents/NIAIDGCPTrainingPolicy.pdf. Accessed September 16, 2015.
- 4. Division of Acquired Immunodeficiency Syndrome (DAIDS). Human Subjects Protection (HSP) and Good Clinical Practice (GCP) training requirements. https://www.niaid.nih.gov/LabsAndResources/resources/toolkit/Documents/gcp_hsp_sitetrain_policy.pdf. Accessed September 16, 2015.
- 5. Clinical Trials Transformation Initiative. GCP training expert meeting. http://www.ctti-clinicaltrials.org/what-we-do/ctti-projects/gcp-training/expert-meeting. Accessed November 23, 2015.
- 6. Clinical Trials Transformation Initiative. GCP training recommendations. http://www.ctti-clinicaltrials.org/what-we-do/ctti-projects/gcp-training/products. Accessed November 30, 2015.
- 7. Clinical Trials Transformation Initiative. Literature review. http://www.ctti-clinicaltrials.org/what-we-do/ctti-projects/gcp-training/products. Accessed November 30, 2015.
- 8. Anderson MS, Horn AS, Risbey KR, Ronning EA, De Vries R, Martinson BC. What do mentoring and training in the responsible conduct of research have to do with scientists’ misbehavior? Findings from a National Survey of NIH-funded scientists. Acad Med. 2007;82:853–860. [DOI] [PubMed] [Google Scholar]
- 9. DeBruin DA, Scholder SL, Kahn J, et al. Educational approaches to the responsible conduct of clinical research: an exploratory study. Acad Med. 2007;82:32–39. [DOI] [PubMed] [Google Scholar]
- 10. DuBois JM, Schilling DA, Heitman E, Steneck NH, Kon AA. Instruction in the responsible conduct of research: an inventory of programs and materials within CTSAs. Clin Transl Sci. 2010;3:109–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Friedman JY, Sugarman J, Dhillon JK, et al. Perspectives of clinical research coordinators on disclosing financial conflicts of interest to potential research participants. Clin Trials. 2007;4:272–278. [DOI] [PubMed] [Google Scholar]
- 12. Heitman E, Olsen CH, Anestidou L, Bulger RE. New graduate students’ baseline knowledge of the responsible conduct of research. Acad Med. 2007;82:838–845. [DOI] [PubMed] [Google Scholar]
- 13. Kalichman MW, Plemmons DK. Reported goals for responsible conduct of research courses. Acad Med. 2007;82:846–852. [DOI] [PubMed] [Google Scholar]
- 14. Kotzer AM, Milton J. An education initiative to increase staff knowledge of Institutional Review Board guidelines in the USA. Nurs Health Sci. 2007;9:103–106. [DOI] [PubMed] [Google Scholar]
- 15. Redman BK, Templin TN, Merz JF. Research misconduct among clinical trial staff. Sci Eng Ethics. 2006;12:481–489. [DOI] [PubMed] [Google Scholar]
- 16. Resnik DB, Dinse GE. Do U.S. research institutions meet or exceed federal mandates for instruction in responsible conduct of research? A national survey. Acad Med. 2012;87:1237–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steneck NH, Bulger RE. The history, purpose, and future of instruction in the responsible conduct of research. Acad Med. 2007;82:829–834. [DOI] [PubMed] [Google Scholar]
- 18. Beresin EV, Baldessarini RJ, Alpert J, Rosenbaum J. Teaching ethics of psychopharmacology research in psychiatric residency training programs. Psychopharmacology (Berl). 2003;171:105–111. [DOI] [PubMed] [Google Scholar]
- 19. Chen DT. Curricular approaches to research ethics training for psychiatric investigators. Psychopharmacology (Berl). 2003;171:112–119. [DOI] [PubMed] [Google Scholar]
- 20. Hamrell MR. Raising suspicions with the Food and Drug Administration: detecting misconduct. Sci Eng Ethics. 2010;16:697–704. [DOI] [PubMed] [Google Scholar]
- 21. Jha S. The nurturing of the clinical research coordinator. Acad Radiol. 2010;17:1325. [DOI] [PubMed] [Google Scholar]
- 22. Larson EL, Cohn EG, Meyer DD, Boden-Albala B. Consent administrator training to reduce disparities in research participation. J Nurs Scholarsh. 2009;41:95–103. [DOI] [PubMed] [Google Scholar]
- 23. Trembath L, Opanowski A. Clinical trials in molecular imaging: the importance of following the protocol. J Nucl Med Technol. 2011;39:63–69. [DOI] [PubMed] [Google Scholar]
- 24. Vulcano DM. CPI certification as predictor of clinical investigators’ regulatory compliance. Drug Inf J. 2012;46:84–87. [Google Scholar]
- 25. Dolor RJ, Smith PC, Neale AV. Institutional review board training for community practices: advice from the Agency for Health Care Research and Quality Practice-Based Research Network listserv. J Am Board Fam Med. 2008;21:345–352. [DOI] [PubMed] [Google Scholar]
- 26. Graham DG, Spano MS, Manning B. The IRB challenge for practice-based research: strategies of the American Academy of Family Physicians National Research Network (AAFP NRN). J Am Board Fam Med. 2007;20:181–187. [DOI] [PubMed] [Google Scholar]
- 27. Graham DG, Spano MS, Stewart TV, Staton EW, Meers A, Pace WD. Strategies for planning and launching PBRN research studies: a project of the Academy of Family Physicians National Research Network (AAFP NRN). J Am Board Fam Med. 2007;20:220–228. [DOI] [PubMed] [Google Scholar]
- 28. Hu X, Graesser AC. Human use regulatory affairs advisor (HURAA): learning about research ethics with intelligent learning modules. Behav Res Methods Instrum Comput. 2004;36:241–249. [DOI] [PubMed] [Google Scholar]
- 29. Rosa C, Campbell A, Kleppinger C, Sampson R, Tyson C, Mamay-Gentilin S. Quality assurance of research protocols conducted in the community: the National Institute on Drug Abuse Clinical Trials Network experience. Clin Trials. 2009;6:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yawn BP, Graham DG, Bertram SL, et al. Practice-based research network studies and institutional review boards: two new issues. J Am Board Fam Med. 2009;22:453–460. [DOI] [PubMed] [Google Scholar]
- 31. Anderson C, Young PA, Berenbaum A. Food and Drug Administration guidance: supervisory responsibilities of investigators. J Diabetes Sci Technol. 2011;5:433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), Center for Devices and Radiological Health (CDRH). Investigator responsibilities—protecting the rights, safety and welfare of study subjects. 2009.
- 33. Koren M, Koski G, Reed D, et al. APCR physician investigator competence statement. The Monitor. 2011;25:79–82. [Google Scholar]
- 34. Schwetz BA. Protecting subjects without hampering research progress: guidance from the Office for Human Research Protections. Cleve Clin J Med. 2007;74(suppl 2):S60–S62; discussion S68-S69. [DOI] [PubMed] [Google Scholar]
- 35. Barnes BE, Friedman CP, Rosenberg JL, Russell J, Beedle A, Levine AS. Creating an infrastructure for training in the responsible conduct of research: the University of Pittsburgh’s experience. Acad Med. 2006;81:119–127. [DOI] [PubMed] [Google Scholar]
- 36. Braunschweiger P, Goodman KW. The CITI program: an international online resource for education in human subjects protection and the responsible conduct of research. Acad Med. 2007;82:861–864. [DOI] [PubMed] [Google Scholar]
- 37. Merritt MW, Labrique AB, Katz J, Rashid M, West KP, Jr, Pettit J. A field training guide for human subjects research ethics. PLoS Med. 2010;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rivera R, Borasky D, Rice R, Carayon F. Many worlds, one ethic: design and development of a global research ethics training curriculum. Dev World Bioeth. 2005;5:169–175. [DOI] [PubMed] [Google Scholar]
- 39. Collaborative Institutional Training Initiative (CITI). www.citiprogram.org. Accessed November 5, 2015.
- 40. Academy of Physicians in Clinical Research (APCR). www.apcrnet.org/default.aspx. Accessed November 5, 2015.
- 41. National Institute of Allergy and Infectious Diseases (NIAID). www.niaid.nih.gov/Pages/default.aspx. Accessed November 5, 2015.
- 42. TransCelerate. www.transceleratebiopharmainc.com/. Accessed November 5, 2015.
- 43. US Food and Drug Administration (FDA). Clinical Investigator Training Course. www.fda.gov/Training/ClinicalInvestigatorTrainingCourse/default.htm. Accessed November 5, 2015.
- 44. US Department of Health and Human Services. Food and Drug Administration. Statement of Investigator. Title 21, Code of Federal Regulations Part 312 (21CFR312.53). http://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM074728.pdf. Accessed October 30, 2014.
- 45. Boston WE, Ice P, Gibson AM. Comprehensive assessment of student retention in online learning environments. Spring 2011. University of West Georgia, Distance Education Center; http://www.westga.edu/∼distance/ojdla/spring141/boston_ice_gibson141.html. Accessed October 27, 2014. [Google Scholar]
- 46. Gorrindo T, Chevalier L, Goldfarb E, Hoeppner BB, Birnbaum RJ. Autonomic arousal and learning in web-based simulation: a feasibility study. J Contin Educ Health Prof. 2014;34(suppl 1):S17–S22. [DOI] [PubMed] [Google Scholar]
- 47. Schmidt KL, Yasko L, Green M, Alexander J, Ryan C. Evolution of an innovative approach to the delivery of in-person training in the responsible conduct of research. Clin Transl Sci. 2014;7:512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]