Abstract
Daclatasvir (Daklinza™), a new oral direct-acting antiviral, is an inhibitor of hepatitis C virus NS5A protein and has recently been approved in the United States, Europe and Japan in chronic hepatitis C. It shows potent pangenotypic activity and moderately high genetic barrier to resistance improving the sustained virological response (SVR) rates. In COMMAND phase 2 trials, daclatasvir demonstrated high SVR rates in HCV genotype 1-4 chronically infected patients treated with peginterferon-a (pegIFNα) plus ribavirin (RBV). Furthermore, it produced even higher response rates in all-oral combination with sofosbuvir, an interferon-free regimen, with or without ribavirin, in patients with advanced liver disease, HCV/HIV coinfection, liver transplantation in ALLY studies and other real-world studies. This narrative review provides information on the pharmacological properties, role, efficacy and safety of daclatasvir-containing regimens in chronic hepatitis C patients. Daclatasvir administered once-daily in combination with sofosbuvir is an effective 12-week treatment in adult patients with chronic hepatitis C and is generally safe and well tolerated.
Keywords: Daclatasvir, chronic hepatitis C, treatment
Introduction
Hepatitis C: antiviral treatment history
Hepatitis C virus (HCV), member of the Flaviviridae family, is a single-stranded RNA virus [1] with high genetic diversity classifying it into seven major genotypes [2]. It is estimated that 46% of all HCV cases are genotype 1, 30% are genotype 3, 23% are genotypes 2, 4 and 6, and less than 1% of all HCV cases are genotype 5. HCV genotype 7 is very rare and so far it has been isolated only in one patient from Central Africa [3]. Genotype distribution of HCV shows geographic variations which are attributed to differences in mode of transmission and ethnic variability. Before the 1990s the main route of HCV transmission was through blood transfusion. However, the introduction of serological and virological tests, such as anti-HCV and serum HCV RNA [4], almost completely eliminated transmission of HCV parenterally. Today, the most significant risk factor for acquiring HCV is drug use when sharing of blood-contaminated needles and syringes occurs. However, in developing countries, unsafe medical procedures remain a major mode of acquiring HCV [5]. It has been estimated that almost 2 million people, or 2.8% of the human population, had anti-HCV antibodies. Of these people, 150 million were chronically infected and 15-30% of those were likely to develop cirrhosis within 20 years [6]. In Greece, it was estimated, using a telephone survey and modifying the prevalence rates for age and high-risk individuals, that for Greek adults the ‘true’ prevalence for anti-HCV is 1.87%, with only 20% of the patients tested for anti-HCV and almost 50% of the patients with chronic HCV infection ever been treated [7]. However, chronic hepatitis C is the only chronic viral disease that can be cured.
Treatment of chronic hepatitis C has evolved over the years. Up to 2011, combination of weekly peginterferon-α (pegIFNα) and daily doses of RBV in a 24- or 48-week course was the standard-of-care (SOC) treatment for chronic hepatitis C [8]. However, the SOC treatment was often discontinued due to several important adverse events (AEs) such as anemia, depression and nausea [9]. The goal of chronic hepatitis C treatment is to achieve SVR, defined by undetectable HCV-RNA at 24 weeks (SVR24) or recently at 12 weeks (SVR12) after the end of therapy. This is assessed by a sensitive molecular method with a lower limit of detection <15 IU/mL [10,11]. SVR rates are affected by the HCV genotypes; SVR rates are 70-90% for genotypes 2, 3, 5 and 6, but almost 50% for genotypes 1 and 4 [11]. Furthermore, a single nucleotide polymorphism of interleukin-28B (IL28B) affects HCV response to pegIFNα-based therapy [12,13]. Patients with the IL28B CC genotype, are 2-3 times more likely to respond to HCV clearance with pegIFNα plus RBV dual therapy than those with the CT or TT genotypes [12,13]. However in 2011, protease inhibitors (PIs), the first-generation direct acting agents (DAAs) have emerged as a third component of the SOC and have profoundly changed the landscape of HCV therapy and SVR rates.
DAAs target viral polyprotein processing a key step of viral replication. The PIs that were first indicated in HCV treatment were telaprevir (Incivek, Vertex) and boceprevir (Victrelis, Merck). Both agents act by forming a reversible but covalent complex with the HCV NS3/4A serine protease catalytic site [14]. This triple therapy, NS3/4A plus pegIFNα/RBV therapy increased SVR rates up to 75% in treatment-naïve patients and up to 64% for previous non-responders to SOC [8]. However, the unfavorable pharmacokinetic profile, the significant number of drug-drug interactions, AEs such as severe skin rashes/pruritus, anemia, and dysgeusia, the risk of fatal complications in patients with advanced liver disease and finally the restriction of this therapeutic option to patients with HCV genotype 1 chronic infection lead to a reduction in effectiveness and safety of both agents [15]. The second-generation PIs, improved pharmacokinetic profiles and safety and were recommended as a part of SOC therapy for chronic hepatitis C. In 2013, simeprevir (Olysio, Janssen), a second-generation PI, once daily, was approved in combination with SOC therapy increasing SVR rates up to 82-100% in treatment-naïve HCV genotype 1 infected patients. Moreover, there was no worsening of the known AEs associated with SOC therapy [16,17].
Later, in 2014, the first nucleotide NS5B polymerase inhibitor sofosbuvir (SOF, Sovaldi, Gilead Sciences) was approved [18]. SOF was considered safe and well-tolerated with pangenotypic activity and a high barrier to resistance. The combination of SOF with SOC therapy showed even better SVR rates up to 82-100% in naïve patients with HCV genotypes 1, 4, 5 or 6 treated for 12 weeks [19].
Recently, daclatasvir (DCV, Daklinza™; Bristol-Myers Squibb), an inhibitor of the HCV phosphoprotein NS5A directly acting in HCV replication, has been approved in the United States, Europe and Japan for use in the treatment of adult patients with chronic hepatitis C in combination with other drugs [20,21]. In particular, DCV is being used with SOF in various combinations with RBV with or without pegIFNα to treat chronic hepatitis C. However, emphasis is given on IFN-free, all-oral regimens [22].
Another inhibitor of the HCV NS5A protein is ledipasvir. The combination of ledipasvir and SOF in a single-tablet regimen (Harvoni, Gilead Sciences), once a day, achieved high SVR rates 93-99% of both naïve patients with HCV genotype 1 infection and of patients with a history of treatment failure to SOC therapy. So, this combination was approved for the treatment of chronically infected patients with HCV genotype 1 in October 2014 [23,24]. Furthermore, the so-called 3D combination regimens of the PI paritaprevir with ritonavir (ABT-450/r, AbbVie), the NS5A inhibitor ombitasvir (ABT-267, AbbVie) and the nonnucleoside polymerase inhibitor dasabuvir (ABT-333, AbbVie) also showed high SVR rates and was approved in 2015 [25]. Other combinations containing the regimens grazoprevir (MK-5172, Merck), a second-generation PI and the NS5A inhibitor elbasvir (MK-8742, Merck) in a single tablet and one dose daily [26-28] achieved high SVR rates and are expected for regulatory approval soon.
Finally, the combination of SOF and DCV once daily in patients with chronic HCV genotype 1, 2 or 3 infection achieved SVR rates up to 89-100% in treatment-experienced or -naïve patients [29] and has also been approved for marketing authorization. Finally, for the combination of DCV and the NS3/NS4A PI asunaprevir (Sunvepra, Bristol-Myers Squibb) high SVR rates (>90%) have also been revealed [30,31]. Clinical studies and real-world data in specific patient populations like decompensated cirrhosis, HIV coinfection and pre- and post-transplantation have also been presented.
In summary, the new class of DAAs has evolved the therapy of chronic hepatitis C by producing high SVR rates in almost all patient groups. This article provides information on the pharmacology, safety and efficacy of oral DCV in combination with other antiviral agents in the treatment of chronic HCV hepatitis in adults and special populations.
Pharmacodynamic properties
DCV is a highly selective nonstructural protein 5A (NS5A) replication complex inhibitor with high potency and pangenotypic coverage.
Mechanism of action
DCV is an HCV NS5A replication complex inhibitor. This NS5A viral protein has enzymatic activity that is essential for HCV RNA replication and assembly. In replication it appears to play a role in the formation of a membranous web along with viral protein NS4B, and this web provides a platform for replication.
The NS5A protein is composed of 3 domains; domains I and II are essential for HCV replication while the domain III seems to help the virion assembly [32,33]. In addition, NS5A contains phosphorylation sites, which act as regulators balancing RNA replication and downstream events [34]. The NS5A inhibitors have high antiviral potency but lower barrier to resistance development [35]. DCV acts as an NS5A inhibitor by binding within the first 100 amino acids of the amino terminus (i.e. domain 1) of the protein [36]. DCV results rapidly in serum HCV RNA drop by 2 logs within the first 6 h of administration, followed by a slower phase of decline, suggesting that it blocks virion assembly and release as well as viral RNA synthesis [37].
Antiviral activity
DCV showed potent antiviral activity against all HCV genotypes [20,21]. The half-maximal effective concentration (EC5O) values of DCV against HCV genotypes 1a, lb, 2a,3a, 4a and 5a were 50, 9, 71-103, 146, 12 and 33 pmol/L, respectively, in replicon assays [36]. Also, DCV showed synergistic antiviral activity in combination with SOC, NS3/4A inhibitors (danoprevir or asunaprevir) or NS5B inhibitors [EMS-791325 (beclabuvir) or NM-107] [36,38].
In cell culture, DCV inhibited JFH-l genotype 2a replication with an EC50 of 28 pmoUL [36]. DCV was specific for HCV as shown by the lower potency (EC50 of 9-12 nmol/L) against the related flavivirus bovine viral diarrhea virus [36].
Dosage and administration
In healthy adult subjects and in subjects with chronic hepatitis C infection the pharmacokinetic properties of DCV were studied [20]. Several oral doses of DCV, once a day, in combination with SOC were given in patients with chronic HCV hepatitis. The geometric mean (CV%) DCV Cmax was 1534 (58) ng/mL, AUC0-24h was 14122 (70) ng•h/mL, and Cmin was 232 (83) ng/mL.
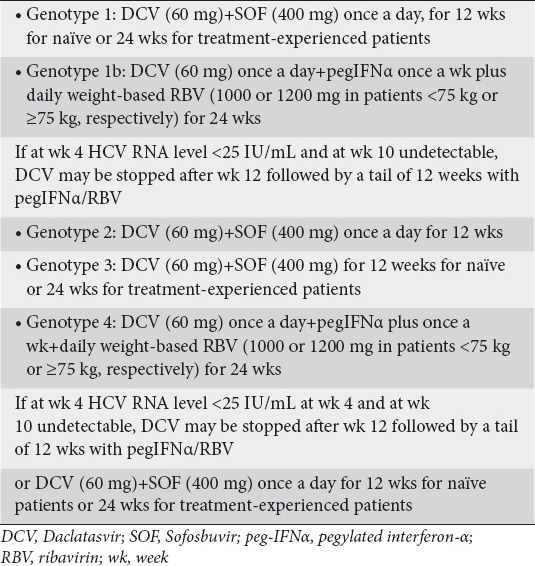
DCV is given as a 60 mg oral dose once per day with or without food. DCV should only be used in combination with other DAA regimens (Tables 1, 2). Recently, the European Association for the Study of the Liver published guidelines for the HCV treatment and included the use of DCV with SOF in patients with HCV genotypes 1-6 (EASL Management Guidelines), (Table 3) [22]. DCV with SOF is recommended for 12 weeks without RBV in non-cirrhotic patients with HCV genotypes 1-6, and with RBV for 12 weeks with compensated cirrhosis. DCV with SOF can also be taken without RBV in patients with genotypes 1-6 and compensated cirrhosis if treatment duration is extended to 24 weeks. In patients with HCV genotype 3 infection and compensated cirrhosis, the recommended regimen is DCV with SOF plus RBV for 24 weeks. DCV should be administered at 60 mg/day or at 30 mg/day when a reduced dose is warranted. Finally, dosage modification is not required in patients with liver failure Child-Pugh (CP) B or C, in the elderly or in patients with renal or hepatic impairment [20].
Table 1.
Daclatasvir: dosing and administration
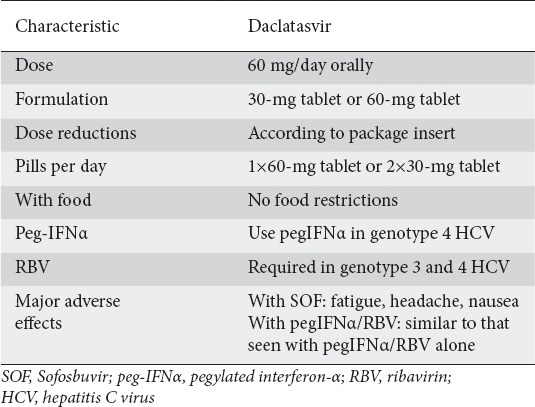
Table 2.
European medicines agency-approved regimens for daclatasvir in patients with hepatitis C virus infection
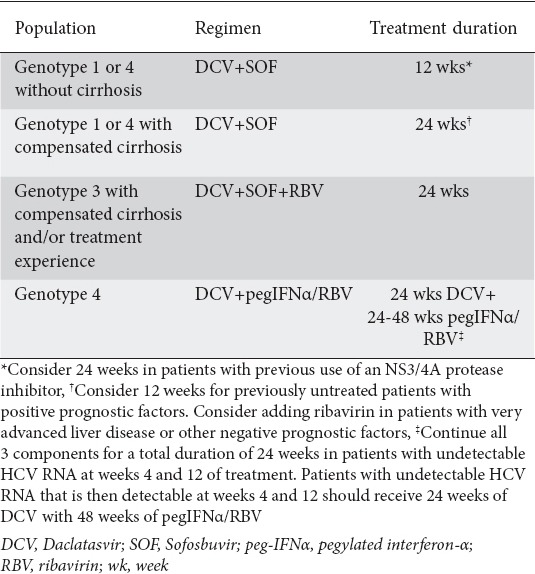
Table 3.
European association for the study of the liver (EASL) guidelines for DCV use in the treatment of HCV
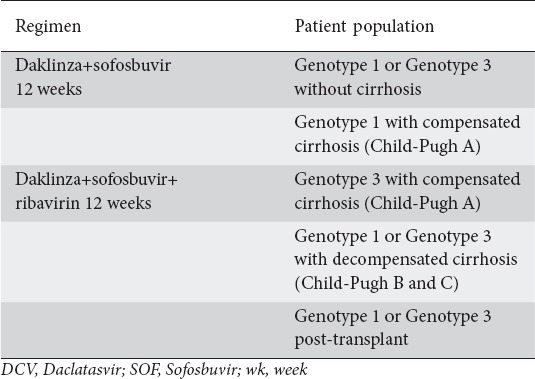
Regarding renal impairment, DCV requires no dose adjustment with any degree of renal impairment, but has been studied in subjects with a creatinine clearance (CrCl) ≥15 mL/min [20]. RBV does require dose adjustment for patients with renal failure [20]. The starting dose should be determined after assessing the CrCl and then adjusted appropriately according the anemia or potential complications. SOF is not currently recommended in severe renal impairment, thus a DCV+SOF combination should be avoided in this subset of patients [11]. Furthermore, DCV is also approved in Japan in combination with asunaprevir for patients infected with HCV genotype 1 who are ineligible for or have failed on IFN-based therapy. Finally, the US Food and Drug administration approved DCV for use with SOF to treat HCV genotype 1 and 3 infection (Table 4) [20].
Table 4.
Food and drug administration-approved regimens for DCV in patients with hepatitis C virus infection
Drug interactions
Since DCV is primarily metabolized through CYP3A metabolism it should not be given with strong inducers of the enzyme (Table 5) [20]. Thus, co-administration of DCV is not recommended with rifampin, phenytoin, carbamazepine, and St. John’s wort or with strong CYP3A inhibitors (such as ritonavir-boosted atazanavir, some azole agents, and clarithromycin). In this case, DCV reduction to 30 mg once a day is recommended [19,39]. However, when DCV is used with moderate CYP3A inducers (such as efavirenz, etravirine, dexamethasone, and nafcillin) a dose increase to 90 mg once daily is recommended.
Table 5.
Established daclatasvir interactions and dose recommendations with other drugs
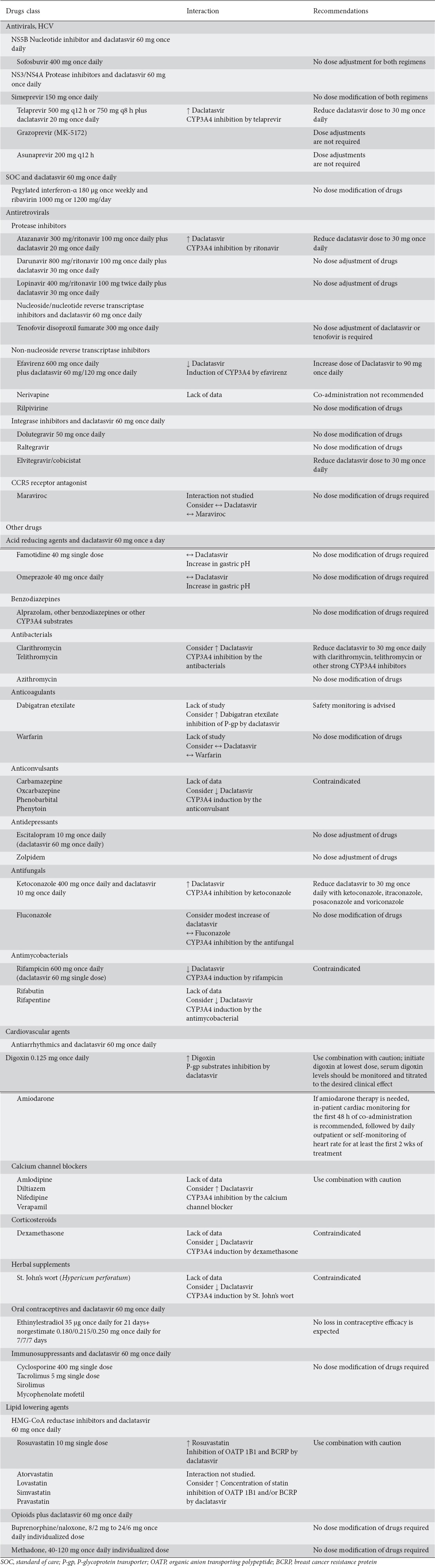
Furthermore, DCV is an inhibitor of P-glycoprotein transporter (P-gp), organic anion transporting polypeptide (OATP) 1B1 and 1B3, and breast cancer resistance protein (BCRP). Thus, dose adjustments of digoxin may be warranted when used with DCV [20]. Also, special caution is required when DCV is used together with drugs such as dabigatran etexilate, digoxin and HMG-CoA reductase inhibitors (statins) because they are substrates of these transporters (Table 5).
Interactions with SOF should also be considered when DCV is used in combination with SOF [40]. Moreover, DAA interactions in the HIV/HCV co-infected patients are largely extrapolated from drug-drug interaction studies when DAAs used with antiretrovirals. CYP450 enzyme induction or inhibition is the major cause for many of the pharmacokinetic interactions between HCV DAA and HIV antiretrovirals. However, there is an increase in the number of transporter-mediated interactions (Table 4). Physicians should always consider the recently reported drug-drug interactions from HCV DAA clinical trials [41]. If DCV and opioids co-administered, this combination was well tolerated suggesting no need for dose adjustments [42].
AEs and management strategies (tolerability)
The most commonly reported AEs were (≥5%): headache (14%), fatigue (14%), nausea (8%), and diarrhea (5%) [18]. From the DCV+ pegIFNα/RBV combination, the safety profile was similar to that of pegIFNα/RBV. AEs were most likely to arise from pegIFNα/RBV such as fatigue, headache, pruritus, insomnia, influenza-like illness, dry skin, nausea, decreased appetite, alopecia, rash, asthenia, irritability, myalgia, anemia, pyrexia, cough, neutropenia, diarrhea and arthralgia. In the largest, dose ranging, phase 2b command I study of DCV 20 mg or 60 mg +PEG-RBV vs. PEG-RBV (n=395), the rates of serious AEs recorded were 7.5% (12/159), 8.2% (13/158), and 7.7% (6/78), respectively. In COMMAND-1 study the AEs recorded were dry skin, influenza-like illness and nausea being >10% more common in the DCV+pegIFNα/RBV versus the pegIFNα/RBV group [43]. Furthermore, grade 3-4 laboratory abnormalities did not show any significant difference among the treatment groups [43]. Smaller phase 2 studies are also consistent with the above larger studies [44].
For the combination of DCV+SOF (n=211), the most frequently reported AEs were fatigue, headache and nausea [45]. The most common grade 3 or 4 laboratory abnormalities were low phosphorous and high glucose. Also, hemoglobin did not significantly decrease in patients not also receiving RBV [45]. In the pivotal Phase III open-label ALLY-3 there were no treatment-related serious AEs and no discontinuations due to AEs. The most common treatment-related AEs recorded, at a frequency of ≥5%, were headache (14%), fatigue (14%), nausea (8%), and diarrhea (5%) [46]. More recently, Landis et al presented an integrated safety analysis of DCV 60 mg + SOF 400 mg with or without RBV in a large number of patients from 4 studies. A total of 679 patients were analyzed including patients with advanced disease, post-transplantation and co-infection. The addition of RBV seems to increase the number of serious AEs (2.7 vs. 7.9%) and the rate of discontinuations (0.2 vs. 7.9%, respectively) mainly due to anemia. The presence of cirrhosis and the duration of treatment period did not show to have an effect on AEs [47].
Moreover, in a recent study by Shallow et al [48], the authors compared the efficacy and tolerability of 12 weeks of DCV + SOF versus both 24 weeks of SOF + RBV and 24 weeks of pegIFNα and RBV in HCV genotype 3 patients across balanced trial populations. Indeed, no patients in ALLY-3 discontinued due to AEs compared to 4.3% of patients in the pegIFNα-RBV trials and this difference became significant after weighing. However, there have been reported cases of symptomatic bradycardia and of pacemaker intervention when amiodarone is co-administered with SOF in combination with DCV [20]. Drug interactions of SOF and amiodarone are a risk of serious symptomatic bradycardia. A fatal cardiac arrest was reported also with ledipasvir/SOF. Subsequently, co-administration of amiodarone with DCV in combination with SOF is not recommended unless the patient undergoes cardiac monitoring [20]. Patients on β-blockers or those with underlying cardiac comorbidities and/or advanced liver disease may be at increased risk for developing symptomatic bradycardia with co-administration of amiodarone. Bradycardia will resolve after the end of HCV treatment.
Regarding toxicology studies, although dogs experienced bone marrow hypocellularity at 9-fold above the clinical AUC exposure these results have not been reported in humans. Also, mice or rats showed no mutagenesis at 8- or 4-fold above the clinical AUC exposure and no in vitro mutagenesis. Moreover, DCV does affect female fertility in rats up to 18-fold the clinical AUC exposure. However, DCV is embryotoxic and teratogenic at or above 4-fold (rat) and 16-fold (rabbit) the clinical AUC exposure [19]. Additional eight clinical studies were carried out and data emerged from 798 patients with chronic HCV infection treated with DCV 60 mg once a day and pegIFNα plus RBV combination or IFN-free regimens (e.g. SOF+DCV) have shown that DCV is safe and tolerable [20].
There is lack of data with DCV in pregnant women [20]. Animal studies have shown maternal and embryo-fetal toxicity if DCV exposure is above the recommended human dose. Moreover, although DCV was excreted into the milk of lactating rats it is unknown if DCV is excreted into human milk. Therefore, one should be cautious and weigh the benefits and risks of DCV to the pregnant woman, the mother and the infant when breastfeeding.
Drug resistance
Mutations associated with failure to DCV mono- or combination-therapy are often similar to those selected in the HCV replicon system or with the infectious clone [41]. In particular, NS5A amino acid substitutions that have been associated with resistance to DCV in cell-based replicon systems include M28T, Q30E/H/R, L31V, and Y93C/H/N for HCV genotype 1a and L31V and Y93H for HCV genotype 1b [49-51]. These amino acid substitutions most frequently map to the N-terminus of NS5A. In contrast to genotype 1b, genotype 1a appears to have lower levels of resistance [42]. Q30, L31 and Y93 mutations seem to confer the highest levels of resistance in both subtypes. Furthermore, L31V and Y93H mutations of genotype 1b individually show 24- and 28-fold resistance to DCV respectively. This increases to approximately 15,000-fold for the L31V-Y93H double mutation, a synergistic effect.
The above resistance pattern also leads to decreased susceptibility to DCV in the other HCV genotypes. In a recently published study, of 16 HCV genotype 3 infected patients who experienced post-treatment relapse [46], nine had treatment-emergent Y93H substitutions (another six had Y93H at baseline) and one had a treatment-emergent L31I substitution [48]. In addition, replicon assays have shown that HCV genotype 1b has the highest relative barrier to NS5A-DCV resistance and genotype 2a has the least, with the rank order being: 1b>4a >5a> 6a>1a>2a JFH>3a>2a >M31 [50]. However, resistance patterns from clinical samples may differ in complexity [49]. Adaptive and linked substitutions, which do not directly contribute to resistance, could co-emerge.
Resistance-associated variants (RAVs) are a major issue to consider in NS5A inhibitors. NS5A inhibitor RAVs tend to be fit, allowing them to persist long-term after failed NS5A inhibitor therapy. These RAVs occur spontaneously in 15-20% of treatment-naïve patients with HCV genotype 1 and at higher rates in patients infected with other genotypes [52]. NS5A inhibitors are almost part of all approved IFN-free regimens with the exception of the combination of SOF plus simeprevir regimen and probably all new regimens in development. Therefore, NS5A inhibitor resistance is important and is coming under scrutiny as more data emerge.
DCV shows a moderate high genetic barrier to resistance in clinical trials. Virological breakthrough reflects the development of resistant mutations and usually occurs in up to 10% of patients treated with DCV and pegIFNα/RBV [18]. Variants usually showed cross-resistance to the other NS5A inhibitors, but they remain fully sensitive to other DAA classes and host targeting antivirals [49,53-55]. However, when an NS5A inhibitor is combined with another DAA having a low barrier to resistance, baseline resistance seems to play a role. In particular, in the HALLMARK DUAL trial, patients with HCV genotype 1b were treated with DCV and asunaprevir. The SVR12 rate went down to just 40% in the 15% of patients who had baseline NS5A inhibitor RAVs compared to 90% in the overall population [56]. However, when an NS5A inhibitor is combined with other DAA having a high barrier to resistance such as SOF, the problem of NS5A inhibitor resistance can be eliminated. For instance, in the ALLY-3 trial of DCV in combination with SOF, resistance mutations did emerge in some HCV genotype 3 infected patients. Notably, in this study many of these patients were cirrhotic, traditionally harder to cure.
In selected phase 2/3 trials analysis by Christophe Sarrazin [57], in which cirrhotic patients have been treated with ledipasvir/SOF ± RBV, only 86% of the 13% of patients with HCV genotype 1a having baseline NS5A inhibitor RAVs achieved SVR12. In contrast, patients with no detectable RAVs achieved a higher SVR12 rate of 98%. The lower SVR12 rate among treatment-experienced was primarily due to the existence of high-level (>100-fold) NS5A RAVs. The SVR12 rate was only 67% in this subset of patients. In the C-EDGE TE trial [58], in which treatment-experienced patients received the second-generation PI/NS5A inhibitor combination of grazoprevir/elbasvir, SVR12 rates fell to 52% in those harboring baseline NS5A variants associated with >5-fold change in elbasvir susceptibility. However, with the combination of ledipasvir/SOF and grazoprevir/elbasvir, the effect of NS5A inhibitor resistance was largely overcome by extending therapy and/or adding RBV.
The clinical significance of RAVs is unclear, they have been found at baseline and posttreatment in clinical trials and baseline RAVs may persist for 96 weeks [59]. They have also been associated with reduced efficacy and DDA treatment failure. However, 90% of people with these baseline RAVs achieved SVR, although post treatment NS5A RAVs were found in most people who were not cured. Until the lifetime and significance of DAA drug resistance are well understood, avoiding treatment with DDAs from the same class is the preferred strategy. Data is needed for retreatment outcomes.
In conclusion, baseline or following treatment RAVs do not preclude SVR but may be an issue in a small number of difficult to treat patients such as those with cirrhosis. Strategies to overcome the problem of RAVs include a careful assessment of patients history, disease severity, viral genotype/subtype which helps in the selection of the more efficient drug combination and the optimal duration of treatment.
The role of DCV in the management of chronic hepatitis C infection
DCV has shown a favorable efficacy and safety profile while it has pangenotypic potential activity with a number of different combinations.
DCV in combination with PegIFNα and RBV
Two phase 3 trials assessing the efficacy of DCV in combination with pegIFNα/RBV have been published [60,61]. The COMMAND 3 trial [60] evaluated DCV with telaprevir, each in combination with pegIFNα/RBV, in HCV genotype 1 infected patients (eventually restricted to genotype 1b patients) a noninferiority study, and the placebo-controlled COMMAND-4 trial [61] evaluated DCV and pegIFNα/RBV with pegIFNα/RBV alone in HCV genotype 4 infected patients.
In COMMAND 3, a noninferiority trial, compared DCV plus pegIFNα/RBV with telaprevir plus pegIFNα/RBV in patients with HCV genotype lb infection. The SVR 12 difference was 4.3% (95% CI -3.3 to 11.9). Moreover, although noninferiority was not formally tested in HCV genotype 1a infected patients, these patients achieved undetectable HCV RNA at 4 and 12 weeks of treatment in 75% for DCV recipients and in 73% for telaprevir recipients. Finally, post-treatment relapse occurred in 5% of DCV-treated patients compared with 15% of telaprevir treated patients. Breakthrough infections occurred in 4% of DCV-treated patients [60].
COMMAND-4 study was a Phase 3 randomized placebo-controlled trial of DCV with pegIFNα/RBV in treatment-naïve patients with chronic HCV genotype 4. Patients were randomized to receive either DCV+pegIFNα/RBV (n=82) or placebo+ pegIFNα/RBV (n=42) for 24 weeks. SVR12 was achieved in 82% vs. 42% of patients on DCV+PEG-RBV vs. placebo+ pegIFNα/RBV [51]. In the DCV and placebo groups eRVR was achieved by 79% and 12% of patients, respectively. Post-treatment relapse occurred in 3% of patients receiving DCV plus pegIFNα/RBV compared with 30% of those receiving dual pegIFNα/RBV. Breakthrough HCV infection occurred in 7% of DCV recipients [61].
A phase 2b clinical trial (COMMAND I), randomized, double-blind, placebo-controlled trial studied for 24 weeks of DCV 20 mg or 60 mg daily and pegIFNα/RBV in patients with either genotype 1 or 4, which are generally less responsive to therapy than other genotypes. In the 20 mg and 60 mg groups, respectively, patients had a median age of 50 and 51 years; males comprised 67% and 65%; blacks were 9% and 13%; genotype 1a was found in 72% and 77%; and IL28B CT or TT genotypes were found in 63% and 65%. Of the 365 patients with HCV genotype 1 in the study, SVR12 rates for genotype 1a were 59%, 58% and 38% for the 20 mg, 60 mg and pegIFNα/RBV groups, respectively, and for genotype 1b 78%, 87% and 31% [43]. Of the 30 patients with HCV genotype 4 treated with either 20 mg or 60 mg of DCV or pegIFNα/RBV, SVR12 rates achieved were 67%, 100% and 50% respectively [43].
Furthermore, in COMMAND-2 study, 419 patients with HCV genotype 1 and a history of prior null or partial response to pegIFNα/RBV were treated for 24 weeks with 20 mg or 60 mg of DCV and pegIFNα/RBV. Of these patients, 17.4% had cirrhosis; and 92.6% had either IL28B CT or TT genotypes. The proportion of HCV genotype 1 patients with undetectable viral load at week 12 were 30% (DCV 20 mg) and 34% (DCV 60 mg) among null responders and 44% (DCV 20 mg), 57% (DCV 60 mg), among partial responders [40].
Finally, in the COMMAND GT2/3 trial (study AI444-031) 151 treatment-naïve chronic hepatitis C patients, 71 with genotype 2 and 80 with genotype 3 were treated either with DCV and pegIFNα/RBV for 12 or 16 weeks or standard 24 weeks of pegIFNα/RBV. Of these patients 23% of genotype 3 and 1.4% of genotype 2 patients were cirrhotic. SVR rates achieved at week 12 or 16 with pegIFNα/RBV tail and control group were 88%, 83% and 71%, respectively, for genotype 2 and 78%, 69% and 52%, respectively, for genotype 3 Relapse rates were higher for genotype 3 cirrhotic patients in the 12-week, but not 16-week groups [62]. However, IFN-free, all-oral regimens are likely to replace the IFN-based combinations as new, potent and highly effective DAA combinations have been developed.
DCV as an option of all-oral therapy for HCV infection
DCV in combination with other DAA: non-cirrhotic HCV genotype 1, 2 or 3 infected patients
Several studies have evaluated the all-oral DAAs combination of DCV and SOF so far. In a phase IIb study of non-cirrhotic patients, Sulkowski et al evaluated DCV 60 mg and SOF 400 mg orally once a day, with or without RBV to treat HCV genotypes 1, 2 and 3 for 12 or 24 weeks. SVR rates achieved were 100% for treatment-naïve patients following a 24-week course of therapy and 98% for 12 weeks. Moreover, the SVR rates achieved for treatment-experienced patients were 100% and 95% with treatment duration of 24 weeks without and with RBV, respectively [45]. In this study, naïve patients and those treated with pegIFNα/RBV plus either the PI telaprevir or boceprevir with virological breakthrough during treatment were included. High SVR rates were achieved, 98% of 126 naïve patients and 98% of 41 patients infected with HCV genotype 1 and treatment failure, respectively. In conclusion, it seems that SVR rates were not significantly affected by use of RBV or not, by the past history of treatment (treated-naïve or -experienced patients) or by the duration of treatment (12 or 24 weeks) [46]. Furthermore, Sulkowski et al also evaluated the combination DCV and SOF with or without RBV for 24 weeks in treatment-naïve patients with HCV genotype 2 or 3. Overall, 92% of 26 patients with HCV genotype 2 and 89% of 18 patients with HCV genotype 3 achieved SVR12 [45]. Asselah’s commentary on these spectacular results of phase IIb trial of non-cirrhotic patients underscored the high SVR rates achieved such as 100% for patients infected with HCV genotype 1, 2 or 3 for 12-week treatment duration with DCV+SOF, 88% for patients for 7-day lead-in of SOF and then 12 weeks treatment duration with DCV+SOF, and 86% for patients for 12 weeks treatment duration with SOF+DCV+RBV [63].
Considerations of DCV therapeutic efficacy for “hard-to-treat” patients
Although hepatitis C therapy has evolved in recent years toward more effective and better-tolerated treatments, we can now recognize that certain patient populations remain more difficult to treat and will require modified therapy or new treatment options to achieve the same efficacy as less difficult-to-treat individuals. Numerous patient characteristics such as cirrhosis status (compensated or decompensated), treatment experience, renal status, HCV genotype 3, HCV/HIV co-infection have been associated with greater difficulty in treating chronic hepatitis C. Clinical trials of DCV are evaluating the best anti-viral drug combination, treatment doses and duration for these special populations.
Decompensated cirrhotic patients infected with HCV genotype 1, 3, or 4
In 2014, DAAs combinations have completely modified the therapeutic approach of HCV patients with hepatic impairment. However new questions have been raised and need to be answered: who benefits, who deteriorates and is there a cut-off where hepatic function cannot be improved despite the virological response [64]. The SOC treatment in CP B patients shows low SVR rates ranging from 7-16% in genotypes 1 or 4 and 44-75% in genotypes 2 or 3. PegIFNα/RBV combination is contraindicated in patients with CP-B and CP-C due to the high risk of infection and death. Finally, very few data are available with triple therapy including first-generation PIs. A real life study showed unfavorable risk/benefit ratio in compensated cirrhotic patients with albumin <3.5 g/dL and platelets <100,000/mm3 [15]. Subsequently a small number of decompensated cirrhotic patients received treatment with first generation PIs triple therapy. In 2015, the DCV in combination with other DAAs, IFN-free DAAs combinations, has shown high SVR rates in decompensated cirrhotic patients.
In ALLY-1 phase III trial [65], 32 patients with Child-Pugh B and 16 with Child-Pugh C were evaluated for the treatment with SOF plus DCV and RBV for 12 weeks whatever genotypes. SVR achieved was 94% (30/32) among Child-Pugh B patients and 56% (9/16) in Child-Pugh C patients. Of those patients with advanced cirrhosis 11 discontinued treatment, mainly RBV (10/11), due to AEs. After 12 weeks of treatment, all patients with Child-Pugh B who respond either improve or did not modify their model for end-stage liver disease (MELD) score. Of course studies are required to define the appropriate CP-C patients for HCV therapy who will benefit from all-oral treatment.
A small real-world single center study [66], assessed the extent of liver function improvement in 80 cirrhotic patients infected with HCV genotypes 1-4, including 34 patients (43%) with CP-B/C cirrhosis and 42 patients (53%) with platelet counts of <90.000/μL), treated with novel IFN-free therapies (SOF/RBV, n=56; SOF/simeprevir ± RBV, n=15; and SOF/DCV ± RBV, n=9). At week 12, MELD scores improved in 44% of the patients but worsened in 15%. Also, 63% of the patients achieved SVR and serum HCV RNA re-appearance led to moderate ALT increases in 15/23 patients without hepatic decompensation. Therefore, IFN-free treatment of hepatitis C patients, especially those with advanced liver cirrhosis who urgently need treatment, seems to restore liver function in the majority of patients and subsequently to reduce the need for liver transplantation.
In the UK early access program [67], of 441 patients with decompensated cirrhosis, 223 infected HCV with genotype 1, 179 with HCV genotype 3 and 39 with other HCV genotypes received regimens containing SOF ± RBV and either DCV (172) or ledipasvir (252) for 12 weeks. In particular, 43 patients did not receive RBV. 309 patients were Child-Pugh B and 46 Child-Pugh C. SVR rates were 80% (36/45) with SOF + DCV and RBV and 60% without RBV among genotype 1 patients. In genotype 3 patients, SVR rates were 70% (80/114) with RBV. At follow-up week 4, 42% of patients showed MELD score improvement by 2 points but 10% showed MELD score worsening. Moreover, 14 patients died and serious AEs related either to liver disease or therapy were reported in 21.4% of the patients. RBV discontinuation occurred in 6.4% of the patients and dose reduction in 19.5%. Treatment seems to be less tolerable in patients over 65 years old and with albumin <35 g/L.
Moreover, among patients with HCV genotype 3 infection, SVR12 rates were significantly higher with the DCV-containing versus ledipasvir-containing regimens with SOF ± RBV in patients with decompensated cirrhosis [67]. In the EASL guidelines, the combination of ledipasvir/SOF is not recommended for patients infected with HCV genotype 3, whereas SOF and DCV are recommended for these patients [21]. Also, recently, the FDA approved DCV for HCV genotype 3 treatment. Regarding the response rates from the SOF plus DCV regimen, the SVR12 rate in these patients with decompensated cirrhosis and HCV genotype 3 is 71% with 12 weeks of treatment. This is probably better than what would have been expected with SOF/RBV alone, but the extending therapy for these patients, which is what the EASL guidelines recommend, would be preferable.
In this real-world study, the safety of these regimens was fairly good. The vast majority of patients (91%) received RBV, and only 6% discontinued it. Approximately one third of patients had anemia with hemoglobin levels falling <10 g/dL. Therefore, despite the high rates of anemia due to RBV use only a minority discontinued treatment. The main question remains whether longer therapy without RBV would improve the SVR rates. Moreover, fourteen deaths occurred during this study. Although this appears to be largely related to the underlying disease, a contribution of the study drugs to mortality cannot be precluded with certainty. Apart from the anemia the addition of RBV may increase the risk of infection, and therefore, needs to be evaluated closely.
In the French real-life HEPATHER cohort [68], 35 patients with HCV genotype 1 infection and decompensated cirrhosis evaluated for SOF plus DCV with RBV (n=6) and without RBV (n=29) for 12 or 24 weeks. Thirty (86%) achieved SVR. Furthermore, sensitive analysis recommends that these patients be treated either with RBV for 12 weeks or without RBV for 24 weeks. Finally, 22 patients, 6 of Child-Pugh B or C, were evaluated for the same combination and 21 patients achieved SVR with no serious AEs reported.
In the French early access program [69], 74 patients infected with HCV genotype 3 with decompensated cirrhosis (55 Child-Pugh B, 19 Child-Pugh C) were evaluated for SOF + DCV ± RBV for 12 or 24 weeks. High SVR rates were achieved, 76% for 12 weeks and 88% for 24 weeks, respectively. Discontinuation due to AEs events occurred in one patient but 2 patients died.
European interim findings from a compassionate use program of DCV plus SOF with and without RBV for patients with severe liver disease have been reported [70]. This multicenter program (AI444-237) registers adult, chronically HCV-infected patients with a high risk of hepatic decompensation or death within 12 months if no treatment received and without available therapeutic options. These patients were treated with DCV 60 mg + SOF 400 mg, once a day, for 24 weeks, with RBV added upon physician’s preference. At post-treatment week 12, SVR12 was achieved overall in 87% of the treated patients while the addition of RBV had no effect in efficacy [no-RBV vs. RBV group: 42/49 (86%) vs. 29/33 (88%)]. An improvement in liver disease parameters was observed while 6/102 patients discontinued treatment due to serious AEs or death.
An ongoing phase 2 trial (NCT02262728) is evaluating the efficacy of DCV 60 mg/day plus simeprevir 150 mg/day and SOF 400 mg/day for 12 weeks for decompensated cirrhotic patients infected with genotype 1 or 4. The 5-year follow up will be completed in 2020 [71].
In conclusion, in a real-life clinical setting, the all-oral combination of DCV + SOF with or without RBV achieved high response rates. Treatment was well tolerated in all patient groups including those with severe liver disease. Findings confirmed other results reported in clinical trials and real-life data. Liver transplantation could be avoided in many Child Pugh B patients. However, in Child Pugh C patients although the benefit remains high, manageable, safety issues should be considered. Unfortunately, in Child Pugh C patients who will not be listed for transplantation because of comorbidities, DAAs combinations are the only therapeutic option. One key question is when to treat, before or after transplant period. Data suggest that even if SVR achieved in decompensated cirrhotic patients it is unlikely to liver transplantation to be avoided. Although the MELD score may improve and subsequently delay transplantation there is still a risk of hepatocellular carcinoma occurrence. At the time there are no guidelines for the appropriate time of initiating treatment either pre- or post-transplant. Local transplantation rules may apply.
Cirrhotic or post-transplant HCV-infected patients
The ALLY-1 multicenter, open-label phase ΙΙΙ trial evaluated the DCV in combination with SOF and RBV for 12 weeks in patients with advanced cirrhosis or post-transplantation HCV recurrence. All patients received 12 weeks of DCV dosed at 60 mg once daily plus SOF 400 mg once daily plus RBV [65]. RBV was initially dosed at 600 mg/day and adjusted up to 1000 mg/day based on hemoglobin levels and creatinine clearence. The data from this study are quite encouraging. Overall, 83% of patients with advanced cirrhosis and 94% of transplanted patients achieved SVR12. When evaluated according to CP class, Child Pugh C patients had a poorer SVR12 rate than Child Pugh A or Child Pugh B patients (56% vs. 92% and 94%, respectively). This is consistent with other studies that have shown lower SVR12 rates for Child Pugh C patients treated with SOF plus an NS5A inhibitor [72].
It is important to highlight that 12-week regimens with SOF plus DCV with or without RBV are suboptimal in an advanced disease population. The only cirrhotic subpopulation that did well with this regimen were those patients with HCV genotype 1b, although there were small numbers in that population, and those patients are inherently easier to treat. Cirrhotic patients infected with HCV genotype 4 also appeared to do well on this regimen, although there were only 4 patients in that treatment group. When treating patients with advanced cirrhosis, to maximize opportunity for SVR a 24-week regimen in this population is recommended.
Two recent presentations with open label real-world data clearly showed that SOF plus DCV regimens with or without RBV achieved high SVR12 rates in patients with HCV recurrence after transplantation [73,74]. These data confirmed that SOF plus DCV is a very effective and safe strategy across all subpopulations of patients with recurrent HCV infection following transplantation, including patients with genotype 1a and 1b infection and patients with cirrhosis. The SVR12 rates seems higher among patients treated for 24 weeks. Importantly, this regimen has a favorable drug-drug interaction profile and, similar to ledipasvir/SOF, dose reductions of the tacrolimus and cyclosporine are not necessary when co-administered with DCV plus SOF and RBV.
In another prospective cohort study aimed to evaluate the efficacy and safety of SOF- and DCV-based regimens in 23 fibrosing cholestatic hepatitis (FCH) patients with recurrence of HCV infection after liver transplantation, the authors concluded that treatment of patients with recurrence of HCV infection and FCH after liver transplantation with SOF plus either DCV or RBV achieved high rates of SVR 12 (96%) and subsequently major clinical improvement almost in all of them [75].
Moving forward, clinicians will need to be aware of the issue of NS5A resistance, particularly among patients with previous exposure to NS5A inhibitors. In the post-transplantation population in ALLY-1, 3 patients relapsed, all of whom had developed NS5A resistance associated variants despite having none at baseline. However, neither approved options have been studied in post-transplantation patients with HCV genotypes 3 or 6.
Patients infected with HCV genotype 3
Patients with HCV genotype 3 infection have higher prevalence of liver steatosis and seem to have a higher risk for progression to cirrhosis and hepatocellular carcinoma [76]. The phase 3 ALLY-3 trial reported the efficacy of the all-oral combination of DCV 60 mg+SOF 400 mg daily in patients infected with HCV genotype 3. Noteworthy, DCV seems to have a better in vitro antiviral profile potency than ledipasvir in genotype 3. The ALLY-3 trial included 101 treatment-naïve and 51 treatment-experienced patients infected with genotype 3 treated with DCV+SOF combination without RBV for 12 weeks. SVR12 achieved was 90% and 86% for the treatment-naïve and -experienced patients, respectively [46].
Analyzing the cirrhotics separately, the ALLY-3 study showed suboptimal efficacy in patients with cirrhosis. Non-cirrhotic patients achieved SVR12 rates of 94% (32/34 treatment experienced) to 97% (73/75 treatment naïve) independently of previous treatment history. Among patients with cirrhosis (20/32), treatment-experienced patients had a 69% (9/13) SVR12, and the treatment-naïve patients had an efficacy of just 58% (11/19). The ALLY-3+ study investigated the combination of DCV+SOF+RBV for 12 or 16 weeks in patients with genotype 3 and advanced fibrosis or cirrhosis. A total of 50 patients were included 72% with compensated cirrhosis while 74% were treatment experienced. Overall, SVR12 was achieved in 45/50 (90%) patients; 88% of the 12-week regimen achieved SVR12, and 92% in those receiving the 16-week regimen respectively. SVR12 was achieved in all patients with advanced fibrosis and in 86% of those with cirrhosis [77]. Therefore, the addition of RBV in DCV+SOF combination seems to increase the SVR12 rates in patients with genotype 3 infection and cirrhosis.
Indeed, recent interim findings from a French multicenter compassionate use program of regimens containing DCV plus SOF, once a day, for 12 or 24 weeks with or without RBV upon physician’s preference for patients with HCV genotype 3 chronic infections [69]. The program enrolled 601 HCV genotype 3 patients with severe fibrosis (F3) or cirrhosis (F4), or HCV extrahepatic manifestations or post-liver transplant HCV recurrence or indication for liver or kidney transplantation. Most of the patient characteristics were male (75%) with single HCV infection (83%) and cirrhosis (77%) and treatment experienced (73%). This preliminary results are in agreement with previous results demonstrating high SVR4 rates after 12 weeks of DCV+SOF treatment of genotype 3, non-cirrhotic patients. Patients with cirrhosis seemed to be benefit from the longer duration of treatment up to 24 weeks.
HIV and HCV dually infected patients
Data on the efficacy of DCV in HIV and HCV co-infection emerges from several studies that are either recently completed or ongoing. Such studies are: NCT01866930, a phase 3 trial of DCV plus pegIFNα/RBV in dually infected patients with HIV and HCV genotype 1, 2, 3 or 4, NCT01471574, a phase 3 trial of DCV plus pegIFNα/RBV in dually infected patients with HIV and HCV genotype 1 and NCT02032888, a phase 3 trial of DCV plus SOF in dually infected patients with HIV and HCV genotypes 1-6.
The ALLY-2 trial examined the combination of SOF and DCV in HIV/HCV co-infected patients of any genotype. The design of ALLY-2 investigated not only a 12-week duration in treatment-experienced and treatment-naïve individuals, but also an 8-week regimen in treatment-naïve [79]. The efficacy results suggested that 8 weeks of this regimen was not sufficient, with only 76% of patients achieving SVR12. In addition, 10 of the 12 patients who relapsed following therapy were in the 8-week treatment arm. However, both treatment-experienced and treatment-naïve individuals did remarkably well with the 12-week regimen, with SVR12 rates of 96% in the naïve and 98% in the experienced genotype 1 populations. SVR was 100% in the naïve and 100% in the experienced non-genotype 1 populations. At the 12-week arms, SVR12 rates did not vary significantly by baseline factors. However, there were some numerical differences based on presence of cirrhosis. Treatment-naïve and treatment-experienced cirrhotic patients achieved SVR12 rates of 89% and 93%, respectively, compared with 98% to 100% in patients without cirrhosis. However, the lower response rates in the cirrhotic groups were driven by very small numbers; each arm had just 1 patient who failed to achieve SVR12.
Furthermore, in interim analysis of a French multicenter compassionate use program, a real-world trial where physicians were given the ability to choose either 12- or 24-week SOF plus DCV regimens with or without RBV to treat 733 HIV-HCV co-infected patients with severe fibrosis (F3) or cirrhosis (F4), or HCV extrahepatic manifestations or post-liver transplant HCV recurrence or indication for liver or kidney transplantation. RBV was added to DCV+SOF in 98 patients (13.6%) [78]. Treatment duration was 24 weeks in 573 (79.3%) and 12 weeks in 142 (19.6%) patients. The efficacy of DCV+SOF in HIV/HCV co-infected patients is comparable to that in the phase ALLY-2 study, with SVR12 rates ≥90% regardless of HCV genotype, the duration of treatment, or RBV use. The need for RBV and duration of treatment in cirrhotic patients remain to be determined in the complete database. The combination with DCV+SOF±RBV is a good therapeutic option in HIV-HCV co-infected patients in real-life clinical setting [79].
In summary, all-oral HCV therapy has significantly improved both tolerability and efficacy of HCV treatment, but challenging populations do remain. Cirrhosis can impact overall SVR rates and may require either the addition of RBV or extending the duration of therapy to achieve SVR rates comparable to those for non-cirrhotic patients, although these modifications will depend on patient characteristics and the regimen used. Patients with decompensated liver disease awaiting liver transplantation can also achieve high SVR rates; however, clinical improvement did not follow all cases with virological response and therefore the prediction of the group of patients who can achieve clinical and biological response after SVR is a question which has to be answered urgently.
Patients with HCV genotype 3 also remain a challenging population without an obvious way to augment existing treatment options, since extending treatment duration beyond 24 weeks may not increase efficacy. Multiple studies are under way with investigational strategies to better serve this population.
HIV co-infected individuals now see SVR rates comparable to mono-infected individuals, but drug-drug interactions can be challenging and need to be carefully considered before initiating therapy. These populations are likely to remain of interest but, as the use and duration of existing and new therapies evolve, the hope is that these patients will become as easy to treat as the general HCV population.
Concluding remarks
DCV is an NS5A inhibitor with high antiviral potency which has shown a clinical efficacy across all genotypes in phase I, II and III studies and in real-world data. DCV is well tolerated and has an excellent safety profile while it can be used in patients with renal and hepatic impairment and in the transplant setting. When it was used in combination with pegIFNα plus RBV in patients chronically infected with HCV genotypes 1-4 achieved SVR24 rates of 60-100% against HCV genotype 1, and 100% against genotype 4 which are higher compared to those of the dual pegIFNα+RBV combination. The all-oral combination of SOF (nucleoside polymerase inhibitor) with DCV (NS5A inhibitor) can achieve a SVR >90% of naïve patients or previously treated patients with genotype 1 infection. The DCV/SOF dual combination has shown an SVR rate of >90% in non-cirrhotic patients with genotype 3, while the triple DCV/SOF+RBV combination achieved SVR in 90% of genotype 3 with advanced fibrosis or cirrhosis. Finally, DCV + SOF combination has excellent efficacy in patients with decompensated liver disease and across all subpopulations post transplantation. In conclusion, DCV-containing regimens provide a highly effective with an excellent safety and tolerability profile option offering cure in a wide range of patients with HCV infection.
Biography
Hippocration General Hospital, Athens, Greece; Limassol General Hospital and University of Nicosia, Republic of Cyprus; University Hospital, Prince Sattam bin Abdulaziz University Al Kharj, KSA; Bristol-Myers Squibb, Athens, Greece
Footnotes
Conflict of Interest: Editorial/writing assistance was provided by MST International, and was funded by Bristol-Myers Squibb
References
- 1.Pfaender S, Cavalleri JM, Walter S, et al. Clinical course of infection and viral tissue tropism of hepatitis C virus-like nonprimate hepaciviruses in horses. Hepatology. 2015;61:447–459. doi: 10.1002/hep.27440. [DOI] [PubMed] [Google Scholar]
- 2.Smith DB, Bukh J, Kuiken C, et al. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes:updated criteria and genotype assignment web resource. Hepatology. 2014;59:318–327. doi: 10.1002/hep.26744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Messina JP, Humphreys I, Flaxman A, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo G, Choo QL, Alter HJ, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 5.Alter MJ. HCV routes of transmission:what goes around comes around. Semin Liver Dis. 2011;31:340–346. doi: 10.1055/s-0031-1297923. [DOI] [PubMed] [Google Scholar]
- 6.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection:new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 7.Papatheodoridis G, Sypsa V, Kantzanou M, Nikolakopoulos I, Hatzakis A. Estimating the treatment cascade of chronic hepatitis B and C in Greece using a telephone survey. J Viral Hepat. 2015;22:409–415. doi: 10.1111/jvh.12314. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C. 2014. J Hepatol. 2014;61:373–395. doi: 10.1016/j.jhep.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C:efficacy, side effects, and complications. Gut. 2006;55:1350–1359. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C:an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Association for the Study of the Liver. EASL clinical practice guidelines:management of hepatitis C virus infection. J Hepatol. 2014;60:392–420. doi: 10.1016/j.jhep.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 14.Ashfaq UA, Javed T, Rehman S, et al. An overview of HCV molecular biology, replication and immune responses. Virol J. 2011;8:161. doi: 10.1186/1743-422X-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hézode C, Fontaine H, Dorival C, et al. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434–441. doi: 10.1016/j.jhep.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1):a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2014;384:403–413. doi: 10.1016/S0140-6736(14)60494-3. [DOI] [PubMed] [Google Scholar]
- 17.Manns M, Marcellin P, Poordad F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2):a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2014;384:414–426. doi: 10.1016/S0140-6736(14)60538-9. [DOI] [PubMed] [Google Scholar]
- 18.Pawlotsky JM. Hepatitis C virus:standard-of-care treatment. Adv Pharmacol. 2013;67:169–215. doi: 10.1016/B978-0-12-405880-4.00005-6. [DOI] [PubMed] [Google Scholar]
- 19.Lawitz E, Mangia A, Wyles D, et al. SOF for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 20.Degasperi E, Aghemo A, Colombo M. Daclatasvir for the treatment of chronic hepatitis C. Expert Opin Pharmacother. 2015;16:2679–88. doi: 10.1517/14656566.2015.1109631. [DOI] [PubMed] [Google Scholar]
- 21.Majumdar A, Kitson MT, Roberts SK. Systematic review:current concepts and challenges for the direct-acting antiviral era in hepatitis C cirrhosis. Aliment Pharmacol Ther. 2016 Apr 18; doi: 10.1111/apt.13633. doi:10.1111/apt.13633. [DOI] [PubMed] [Google Scholar]
- 22.European Association for the Study of the Liver. EASL Recommendations on Treatment of Hepatitis C. 2015. J Hepatol. 2015;63:199–236. doi: 10.1016/j.jhep.2022.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 24.Raedler LA. Once-a-Day Harvoni (Ledipasvir plus Sofosbuvir), a new oral combination for the treament of patients with genotype 1 chronic hepatitis C infection. Am Health Drug Benefits. 2015;8:54–58. [PMC free article] [PubMed] [Google Scholar]
- 25.Deeks ED. Ombitasvir/Paritaprevir/Ritonavir Plus Dasabuvir:a review in chronic HCV genotype 1 infection. Drugs. 2015;75:1027–1038. doi: 10.1007/s40265-015-0412-z. [DOI] [PubMed] [Google Scholar]
- 26.Zeuzem S, Ghalib R, Reddy KR, et al. Grazoprevir-elbasvir combination therapy for treatment-naive cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4, or 6 infection:a randomized trial. Ann Intern Med. 2015;163:1–13. doi: 10.7326/M15-0785. [DOI] [PubMed] [Google Scholar]
- 27.Lawitz E, Gane E, Pearlman B, et al. Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-∦) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY):a randomised, open-label phase 2 trial. Lancet. 2015;385:1075–1086. doi: 10.1016/S0140-6736(14)61795-5. [DOI] [PubMed] [Google Scholar]
- 28.Sulkowski M, Hezode C, Gerstoft J, et al. Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-∦) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY):a randomised, open-label phase 2 trial. Lancet. 2015;385:1087–1097. doi: 10.1016/S0140-6736(14)61793-1. [DOI] [PubMed] [Google Scholar]
- 29.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 30.Chayama K, Suzuki F, Suzuki Y, et al. All-oral dual combination of daclatasvir plus asunaprevir compared with telaprevir plus peginterferon alfa/ribavirin in treatment-naive Japanese patients chronically infected with HCV genotype 1b:Results from a phase 3 study. Hepatology. 2014;60(4 Suppl):1135A. [Google Scholar]
- 31.Chayama K, Takahashi S, Toyota J, et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742–749. doi: 10.1002/hep.24724. [DOI] [PubMed] [Google Scholar]
- 32.Tellinghuisen TL, Foss KL, Treadaway J. Regulation of hepatitis C virion production via phosphorylation of the NS5A protein. PLoS Pathog. 2008;4:e1000032. doi: 10.1371/journal.ppat.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Appel N, Zayas M, Miller S, et al. Essential role of domain III of nonstructural protein 5A for hepatitis C virus infectious particle assembly. PLoS Pathog. 2008;4:e1000035. doi: 10.1371/journal.ppat.1000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neddermann P, Quintavalle M, Di Pietro C, et al. Reduction of hepatitis C virus NS5A hyperphosphorylation by selective inhibition of cellular kinases activates viral RNA replication in cell culture. J Virol. 2004;78:13306–13314. doi: 10.1128/JVI.78.23.13306-13314.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheel TK, Rice CM. Understanding the hepatitis C virus life cycle paves the way for highly effective therapies. Nat Med. 2013;19:837–849. doi: 10.1038/nm.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao M, Nettles RE, Belema M, et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature. 2010;465:96–100. doi: 10.1038/nature08960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guedj J, Dahari H, Rong L, et al. Modeling shows that the NS5A inhibitor daclatasvir has two modes of action and yields a shorter estimate of the hepatitis C virus half-life. Proc Natl Acad Sci USA. 2013;110:3991–3996. doi: 10.1073/pnas.1203110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelosi LA, Voss S, Liu M. Effect on hepatitis C virus replication of combinations of direct-acting antivirals, including NS5A inhibitor daclatasvir. Antimicrob Agents Chemother. 2012;56:5230–5239. doi: 10.1128/AAC.01209-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bifano M, Hwang C, Oosterhuis B, et al. Assessment of pharmacokinetic interactions of the HCV NS5A replication complex inhibitor daclatasvir with antiretroviral agents:ritonavir-boosted atazanavir, efavirenz and tenofovir. Antivir Ther. 2013;18:931–940. doi: 10.3851/IMP2674. [DOI] [PubMed] [Google Scholar]
- 40.Ratziu V, Gadano A, Pol S, et al. Triple therapy with daclatasvir (DCV;BMS-790052), peginterferon alfa-2a and ribavirin in HCV-infected prior null and partial responders:12-week results of phase 2b COMMAND-2 trial. J Hepatol. 2012;56(Suppl 2):S478–S479. [Google Scholar]
- 41.El-Sherif O, Back D. Drug interactions of hepatitis c direct-acting antivirals in the HIV-infected person. Curr HIV/AIDS Rep. 2015;12:336–343. doi: 10.1007/s11904-015-0277-5. [DOI] [PubMed] [Google Scholar]
- 42.Garimella T, Wang R, Luo WL, et al. Assessment of drug-drug interactions between daclatasvir and methadone or buprenorphine-naloxone. Antimicrob Agents Chemother. 2015;59:5503–5510. doi: 10.1128/AAC.00478-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hezode C, Hirschfield GM, Ghesquiere W, et al. Daclatasvir, an NS5A replication complex inhibitor, combined with peginterferon alfa-2a and ribavirin in treatment-naive HCV genotype 1 or 4 patients:phase 2b COMMAND-1 SVR 12 results. Hepatology. 2012;56:553A. [Google Scholar]
- 44.Pol S, Ghalib RH, Rustgi VK, et al. Daclatasvir for previously untreated chronic hepatitis C genotype-1 infection:a randomised, parallel-group, double-blind, placebo-controlled, dose-finding, phase 2a trial. Lancet Infect Dis. 2012;12:671–677. doi: 10.1016/S1473-3099(12)70138-X. [DOI] [PubMed] [Google Scholar]
- 45.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, et al. Daclatasvir plus SOF for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 46.Nelson DR, Cooper JN, Lalezari JP, et al. All-oral 12-week combination treatment with daclatasvir (DCV) and sofosbuvir (SOF) in patients infected with HCV genotype (GT) 3:ALLY-3 phase 3 study. Hepatology. 2015;61:1127–1135. doi: 10.1002/hep.27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landis C, Nelson DR, Sulkowski M, et al. An integrated safety analysis of SOF+DCV with or without ribavirin in patients with chronic HCV infection. Hepatology. 2015;62(Suppl 1):565A. [Google Scholar]
- 48.Swallow E, Song J, Yuan Y, et al. Daclatasvir +sofosbuvir versus standard of care for hepatitis C genotype 3 :a matching-adjusted indirect comparison. J Comp Eff Res. 2016;5:129–139. doi: 10.2217/cer.15.49. [DOI] [PubMed] [Google Scholar]
- 49.Fridell RA, Wang C, Sun JH, et al. Genotypic and phenotypic analysis of variants resistant to hepatitis c virus nonstructural protein 5a replication complex inhibitor bms-790052 in humans In vitro and in vivo correlations. Hepatology. 2011;54:1924–1935. doi: 10.1002/hep.24594. [DOI] [PubMed] [Google Scholar]
- 50.Wang C, Sun JH, O’Boyle DR, et al. Persistence of resistant variants in hepatitis c virus-infected patients treated with the NS5a replication complex inhibitor daclatasvir. Antimicrob Agents Chemother. 2013;57:2054–2065. doi: 10.1128/AAC.02494-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fridell RA, Qiu D, Wang C, Valera L, Gao M. Resistance analysis of the hepatitis c virus NS5a inhibitor bms-790052 in an in vitro replicon system. Antimicrob Agents Chemother. 2010;54:3641–3650. doi: 10.1128/AAC.00556-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poveda E, Wyles DL, Mena A, Pedreira JD, Iglesias A, Cachay E. Update on hepatitis C virus resistance to direct-acting antiviral agents. Antiviral Res. 2014;108:181–191. doi: 10.1016/j.antiviral.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 53.Lemm JA, O’Boyle D, Liu M, et al. Identification of hepatitis c virus NS5a inhibitors. J Virol. 2010;84:482–491. doi: 10.1128/JVI.01360-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kohler JJ, Nettles JH, Amblard F, et al. Approaches to hepatitis c treatment and cure using NS5a inhibitors. Infect Drug Resist. 2014;7:41–56. doi: 10.2147/IDR.S36247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lim PJ, Gallay PA. Hepatitis c NS5a protein:Two drug targets within the same protein with different mechanisms of resistance. Curr Opin Virol. 2014;8C:30–37. doi: 10.1016/j.coviro.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jensen DM, Kenneth E Sherman, Hezode C, et al. on behalf of the HALLMARK-QUAD Study Team. Daclatasvir and asunaprevir plus peginterferon alfa and ribavirin in HCV genotype 1 or 4 non-responders. J Hepatol. 2015;63:30–37. doi: 10.1016/j.jhep.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 57.Sarrazin C, Dvory-Sobol H, Svarovskaia ES, et al. The prevalence and the effect of HCV NS5A resistance-associated variants in patients with compensated cirrhosis treated with Ledipasvir/SOF ±RBV. J Hepatol. 2015;62(Suppl 2) Abstract PO773. [Google Scholar]
- 58.Kwo P, Gane E, Peng C-Y, et al. Efficacy and safety of grazoprevir/elbasvir +/− RBV for 12 weeks in patients with HCV G1 or G4 infection who previously failed peginterferon/RBV:C-EDGE treatment-experienced trial. J Hepatol. 2015;62(Suppl 2) Abstract P0886. [Google Scholar]
- 59.Drory Sobol H, Wyles D, Ouyang W, et al. Long term persistence of HCV NS5A variants after treatment with NS5A inhibitor ledipasvir. J Hepatol. 2015;62(Suppl 2) Abstract O059. [Google Scholar]
- 60.Jacobson I, Zeuzem S, Flisiak R. Daclatasvir vs telaprevir in combination with peginterferon alfa/ribavirin in treatment-naïve patients with HCV genotype 1:phase 3 COMMAND-3 results (abstract no. 0213] J Viral Hepat. 2014;21(Suppl S2):7–8. [Google Scholar]
- 61.Hezode C, Alric L, Brown A, et al. Randomized controlled trial of the NS5A inhibitor plus peginterferon and ribavirin for HCV genotype-4 (COMMAND-4) Antivir Ther. 2015;27 doi: 10.3851/IMP2985. doi:10.3851/IMP2985. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 62.Dore GJ, Lawitz E, Hezode C, et al. Daclatasvir plus peginterferon and ribavirin is noninferior to peginterferon and ribavirin alone, and reduces the duration of treatment for HCV genotype 2 or 3 infection. Gastroenterology. 2015;148:355–366. doi: 10.1053/j.gastro.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Asselah T. Daclatasvir plus SOF for HCV infection:an oral combination therapy with high antiviral efficacy. J Hepatol. 2014;61:435–438. doi: 10.1016/j.jhep.2014.04.042. [DOI] [PubMed] [Google Scholar]
- 64.Bourliere M. Treatment of patients with decompensated liver cirrhosis:who benefits, who deteriorates, where is the point of no return? J Viral Hepat. 2015;22(Suppl S3):O132. [Google Scholar]
- 65.Poordad F, Schiff ER, Vierling JM, et al. Phase III Daclatasvir, SOF, and Ribavirin combination for HCV patients with advanced cirrhosis or post-liver transplant recurrence (ALLY 1) J Hepatol. 2015;62(Suppl 2):S261–S262. Abstract L08. [Google Scholar]
- 66.Deterding K, Hner Zu, Siederdissen C, Port K, et al. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment Pharmacol Ther. 2015;42:889–901. doi: 10.1111/apt.13343. [DOI] [PubMed] [Google Scholar]
- 67.Foster GR, McLauchlan J, Irving W, et al. Treatment of decompensated HCV cirrhosis in patients with diverse genotypes:12 weeks SOF and NS5A inhibitors with/without ribavirin is effective in HCV genotypes 1 and 3. J Hepatol. 2015;62(Suppl 2):S190–S191. Abstract O002. [Google Scholar]
- 68.Pol S, Bourliere M, Lucier S, et al. Safety and efficacy of the combination daclatasvir-SOF in HCV genotype 1-mono-infected patients from the French observational cohort ANRS CO22 HEPATHER. J Hepatol. 2015;62(Suppl 2) Abstract LB3. [Google Scholar]
- 69.Hezode C, De Ledighen V, Fontaine H, et al. Daclatasvir plus SOF with or without ribavirin in patients with HCV Genotype 3 infection:interim analysis of a French multicenters compansionnate use program. J Hepatol. 2015;62(Suppl 2) Abstract LP05. [Google Scholar]
- 70.Welzer T, Petersen J, Ferenci P, et al. Efficacy and safety of Daclatasvir plus sofosbuvir with or without ribavirin for the treatment of HCV in patients with severe liver disease:interim results of a compassionate use program. Hepatology. 2015;62(Suppl 1):225A. Abstract 37. [Google Scholar]
- 71.US National Institutes of Health. [First received 6 Oct 2014];ClinicalTrials.gov 2015. https://clinicaltrials.gov/ct2/show/NCT02262728 . [Google Scholar]
- 72.Charlton M, Everson T, Flamm SL, et al. Ledipasvir and SOF plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 73.Coilly A, Fougerou C, De Ledinghen V, et al. The association of SOF and daclatasvir for treating severe recurrence of HCV infection after liver transplantation:results from a large French prospective multicentric ANRS CO23 cupilt cohort. J Hepatol. 2015;62(Suppl 2) Abstract G15. [Google Scholar]
- 74.Herzer K, Welzel T, Ference P, et al. Daclatasvir in combination with sofosbuvir with or without ribavirin is safe and efficacious in liver transplant recipients with HVB recurrence:interim results of a multicenter compassionate use program. Hepatology. 2015;62(Suppl 1):341A. [Google Scholar]
- 75.Leroy V, Dumortier J, Coilly A, et al. Efficacy of SOF and daclatasvir in patients with fibrosing cholestatic hepatitis C after liver transplantation. Clin Gastroenterol Hepatol. 2015;13:1993–2001. doi: 10.1016/j.cgh.2015.05.030. [DOI] [PubMed] [Google Scholar]
- 76.Probst A, Dang T, Bochud M, Egger M, Negro F, Bochud PY. Role of hepatitis C virus genotype 3 in liver fibrosis progression-a systematic review and meta-analysis. J Viral Hepat. 2011;18:745–759. doi: 10.1111/j.1365-2893.2011.01481.x. [DOI] [PubMed] [Google Scholar]
- 77.Leroy V, Augus PW, Bronowicki T-P, et al. All-oral treatment with Daclatasvir plus Sofosbuvir plus Ribavirin for 12 or 16 weeks in HCV genotype 3-infected patients with advanced fibrosis or cirrhosis:the ALLY-3+phase 3 study. Hepatology. 2015;62(Suppl 1) Abstract LB-3. [Google Scholar]
- 78.Fontaine H, Lacombe K, Dhiver C, et al. Daclatasvir plus SOF with or without ribavirin in patients with HIV-HCV coinfection:interim analysis of a french multicenter compassionate use program. J Hepatol. 2015;62(Suppl 2) Abstract LP23. [Google Scholar]
- 79.Wyles DL, Ruane P, Sulkowski MS, et al. Daclatsvir plus Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015;373:714–725. doi: 10.1056/NEJMoa1503153. [DOI] [PubMed] [Google Scholar]


