Abstract
The use of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) appears to be a safe and feasible means of confirming or excluding malignancy in the adrenal glands. EUS-FNA with biopsy of suspicious masses in either adrenal gland allows for assessment of these lesions while keeping complications relatively rare. The main advantages of EUS-FNA are that it can be done as an outpatient procedure, with good results, minimal morbidity, and a short hospital stay. Nevertheless, EUS-FNA of adrenal masses should be indicated only in selected cases, in which there is potential to either decrease unnecessary treatment or guide therapy in cancer patients by aiding in either staging of malignancy or treatment of recurrence.v
Keywords: Adrenal gland, endoscopic ultrasound, fine-needle aspiration, puncture
Introduction
Ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), and the use of other advanced imaging techniques has led to increased detection of adrenal masses [1-3]. About 5% of patients undergoing CT of the abdomen are found to have an adrenal lesion [4] and the incidence of an adrenal incidentaloma (detection of an otherwise unsuspected adrenal mass on imaging), ranges from 0.2 to 7% [5-7]. Most of these incidentally found lesions are non-functioning adenomas, but 2% are metastatic lesions.
About 75% [1] of adrenal masses identified during staging of patients with cancer are metastatic lesions that could be metastases from malignancy involving mostly the lung, breast, stomach, kidney, skin, or lymphatic system [8-10]. The imaging techniques currently offered to differentiate a benign mass from a malignant one are not sensitive or specific enough. For this reason, patients with a high index of suspicion for malignancy are often referred for percutaneous biopsy [11,12].
Image-guided fine-needle aspiration (FNA) performed percutaneously using either US or CT has traditionally been the modality of choice for sampling of the adrenal glands. This technique, however, yields non-diagnostic samples in up to 14% of patients and is associated with adverse events in 0.4-12% [13-16]. Endoscopic ultrasound (EUS)-guided FNA of either adrenal gland offers a less invasive and more accurate method for sampling the adrenals with a few side effects and complications [17]. The use of EUS-FNA is infrequently performed for the evaluation of adrenal lesions and there are few reported studies addressing the safety and feasibility of this technique, as shown in Tables 1 and 2. Few authors have recently summarized the use of EUS-FNA in the diagnosis of adrenal lesions. In the present review the indications, techniques, success rates, and complications reported with EUS-FNA of the adrenals are described.
Table 1.
Patient characteristics in endoscopic ultrasound-guided adrenal fine-needle aspiration cases
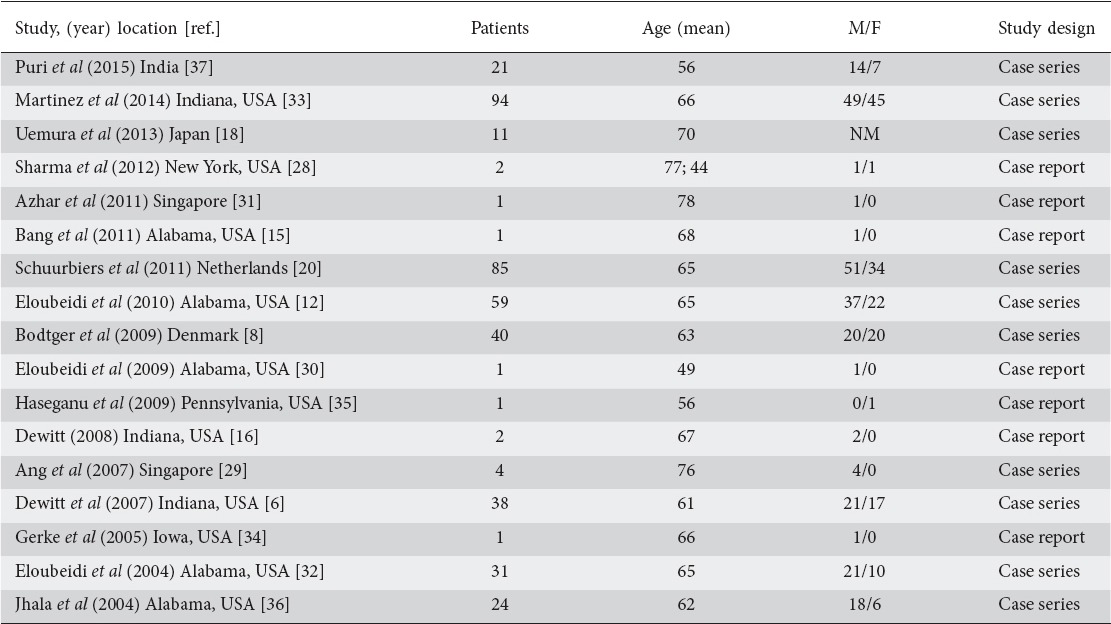
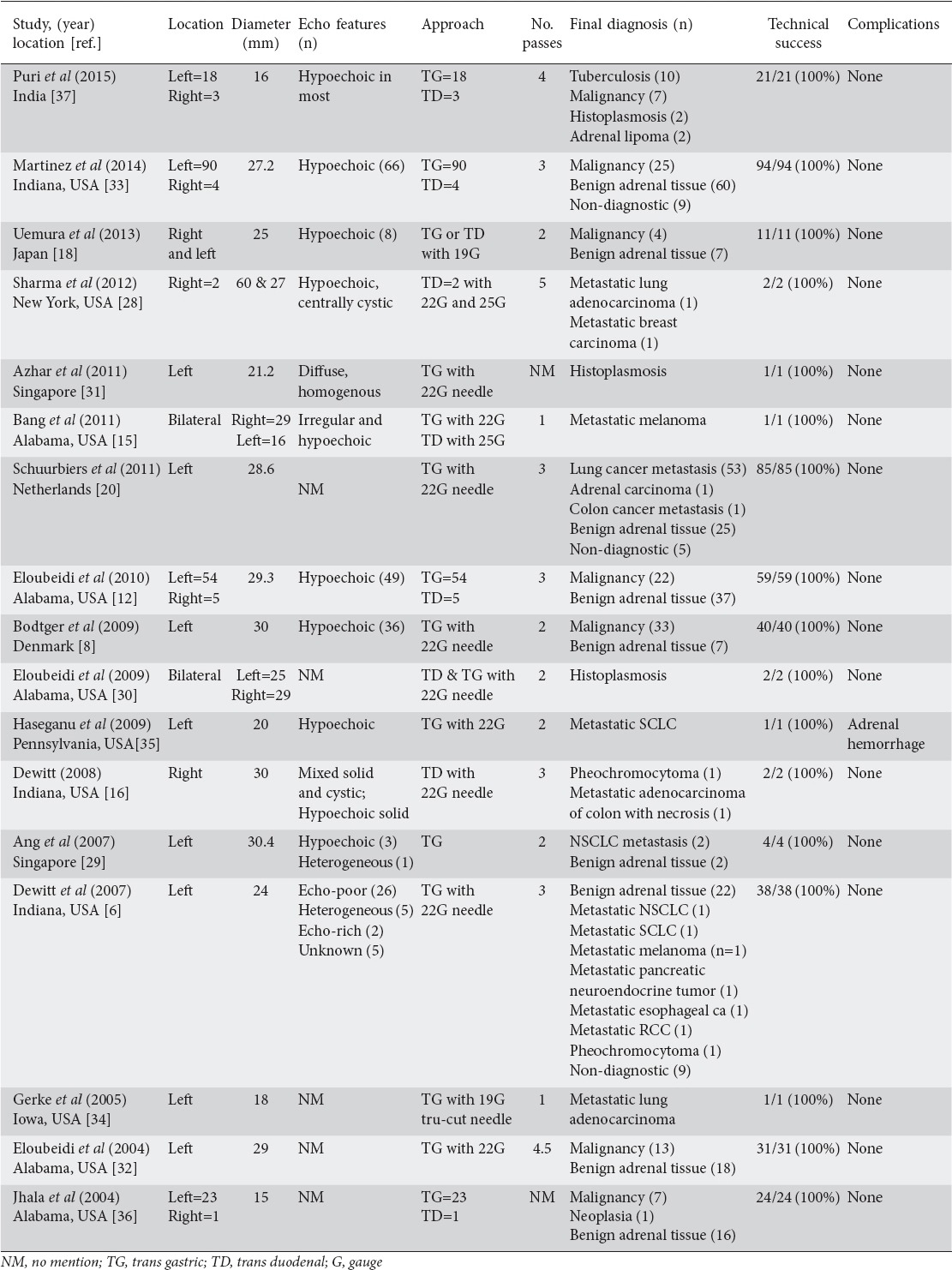
Table 2.
Endoscopic ultrasound (EUS) and clinical features of adrenal masses undergoing attempted EUS-guided fine-needle aspiration
Materials and methods
An extensive English language literature search was conducted using PubMed, Medline, Embase, and Google to identify peer-reviewed original and review articles using the keywords ‘endoscopic ultrasound’, ‘fine-needle aspiration’, ‘adrenal’, ‘puncture’, and ‘EUS-FNA’. Only articles in humans were selected. The references of pertinent studies were manually searched to identify additional relevant studies. The procedural details, technical success rates, and complications were considered as part of the inclusion criteria. Search results yielded mostly small sample sized prospective or retrospective studies including case reports and case series.
Results
Seventeen original articles published were considered appropriate to be included in the review article. Of these, seven were case reports while the other ten were case series. The total number of patients included across all studies was 416. All cases have been summarized in Tables 1 and 2.
Demographics
A total of 416 patients (mean age 65 years; 242 male) were included across all studies. Gender was not specified for the 11 patients included in the study by Uemura et al [18].
Technique and technical success rate
Linear EUS was performed in most patients. The left adrenal gland was visualized by transgastric imaging and transduodenal imaging was used for the right adrenal gland. To identify the left adrenal gland with the echoendoscope, the descending aorta was traced to the celiac trunk and gentle clockwise torque of the echoendoscope was applied to identify the left adrenal gland which appeared as a seagull-looking structure [12]. The right adrenal gland was identified from the second part of the duodenum through gentle withdrawal of the echoendoscope anterior to the right kidney while keeping counter-clockwise torque on the instrument. This would ultimately lead to visualization of the right adrenal gland in most cases. Though sometimes inaccessible and more difficult to visualize, the right adrenal gland could be seen with this gentle maneuvering of the echoendoscope and slight torque. A safe window often showed the liver on the right of the screen, the inferior vena cava on the left, and the right adrenal gland between them [14].
Once a right or left adrenal gland mass was identified, EUS-FNA was performed by using a linear array echoendoscope. The size of the adrenal gland recorded in most studies was the maximal cross-sectional diameter of the gland. Average size of adrenal lesions across all studies was 25.9 mm. EUS-FNA was performed using a 19-, 22-, or 25-gauge needle. Doppler imaging was used to confirm that there were no vascular structures along the path of the needle. After needle puncture of the adrenal gland, the stylet was removed. Depending on the operator, suction was applied or not applied using a vacuum-containing syringe and if excess blood was present in the initial specimen, the operator attempted more passes with the same needle but without suction. This approach to sampling was uniform across all studies, and there was no maximum number of biopsy attempts allowed. Biopsy attempts were performed at the discretion of the gastroenterologist. The average number of biopsy attempts across all studies in our review was two. A transgastric approach was documented as performed in 359/416 (86%) patients while a transduodenal approach was documented as performed in 25/416 (6%) cases. In 32 patients, the respective study did not document which approach was used. Further details regarding techniques of EUS-FNA of the adrenal gland are included in Table 2. The combined technical success rate was 100% in all case reports and series.
EUS features of adrenal lesions
Eleven of the seventeen studies included in this review reported that the majority of adrenal lesions were hypoechoic in nature as seen through echoendoscopic visualization. Although these lesions were more suspicious for malignancy based on this feature, the cytologic outcome did not support that the majority of lesions were malignant. For example, in Martinez et al [33] 66/94 (70%) patients had an adrenal lesion with hypoechoic features, however 60% of cytology showed benign adrenal tissue.
Complications
No major complications were reported after EUS-FNA of the adrenal gland. Haseganu et al [30] reported a case of adrenal hemorrhage that occurred immediately after the procedure. The patient was admitted to the hospital and treated symptomatically, but remained hemodynamically stable.
Discussion
EUS-FNA is a novel method for diagnosing adrenal lesions. When compared with the traditional diagnostic modalities, it is not only more accurate than imaging studies such as CT or MRI, but also a very safe procedure with fewer complications than percutaneous biopsies [18-21]. When an adrenal incidentaloma is identified, the major concern is malignancy. According to guidelines from the National Institute of Health in 2002, malignancy is more likely in lesions >4 cm in size [22]. In a recent study, a lesion with a diameter greater than 4 cm was shown to have 90% sensitivity for the detection of adrenocortical carcinoma [23]. For adrenal masses larger than 6 cm in diameter, most experts recommend resection [24].
The primary role of FNA biopsy is to differentiate between adrenal tissue and non-adrenal tissues (e.g. metastases or infection). Though no major complications were noted in our review of EUS-FNA, some potential complications from image or EUS-FNA include adrenal hematoma, abdominal pain, formation of an adrenal abscess, and tumor recurrence along the needle track [25]. Furthermore, pheochromocytoma needs to be ruled out prior to FNA of an incidental adrenal lesion to prevent the possibility of hemorrhage and hypertensive crisis [26]. Overall, the utility and indications for EUS-FNA of adrenal incidentalomas of small size is rare, given the unclear benefit and potential complications. For patients who do undergo the procedure, EUS-FNA of the left adrenal gland is a relatively simple procedure while EUS-FNA of the right adrenal gland is more challenging because the echoendoscope must be maneuvered carefully along vascular structure to visualize the gland. When compared with the 22-gauge needle, 25-gauge needles are relatively more flexible and are preferred for performing trans-duodenal FNAs [27-31].
In summary, if EUS-FNA of the adrenal glands is available and there is a gastroenterologist on staff to perform this advanced procedure, it is a viable and minimally invasive alternative to adrenalectomy or percutaneous image-guided biopsy of the adrenal glands and may be considered by practitioners as it also has excellent yield for an accurate tissue diagnosis. As shown in our review, EUS-FNA of the adrenal gland had 100% technical success with no major complications and only one case of minor adrenal hemorrhage. This procedure can be done as an outpatient as is well-tolerated by patients. Likewise, results of tissue biopsy greatly affect patient management and direct further therapy. Results from FNA cytology for many patients in multiple studies included in our review showed the impact of EUS-FNA of the adrenal gland on final diagnosis of metastatic malignancy. For example, in patients with lung cancer, few studies included in this review evaluated the efficacy of EUS and EUS-FNA for the diagnosis of adrenal metastasis in patients with potentially resectable lung cancer [18]. As a result, EUS-FNA correctly diagnosed a patient with metastasis who was negative on both CT and positron emission tomography-CT. Whether a patient has a first-time malignancy diagnosis or recurrence, EUS-FNA can help guide therapy by providing information for staging and possibly avoiding further invasive procedures in palliative cases. EUS-FNA of the adrenal gland has been shown to be particularly useful in this regard in patients with lung cancer [32-37]. Future prospective, randomized, controlled studies that compare EUS with percutaneous US and/or CT-guided FNA of the adrenal glands would be helpful to further delineate the indications and limitations of each technique.
Concluding remarks
EUS-FNA of the adrenal gland appears to be a safe and feasible procedure with good results, minimal morbidity and a short hospital stay in the cases reported in the literature. We recommend that EUS-FNA of adrenal masses should be indicated only in selected cases, in which the procedure may alter clinical management by either avoiding unnecessary treatment or helping with staging of malignancy or its recurrence. Further research should compare the benefits of percutaneous and echo-endoscopic approaches to adrenal FNA.
Biography
Mount Sinai Health Systems, New York, New York; The Brooklyn Hospital Center, Academic Affiliate of The Icahn School of Medicine at Mount Sinai, Clinical Affiliate of The Mount Sinai Hospital, Brooklyn, New York; MD Anderson Cancer Center, Academic and Clinical Affiliate of the University of Texas, Houston, Texas; Cedars-Sinai Medical Center, Los Angeles, CA, USA
Footnotes
Conflict of Interest: None
References
- 1.Lee MJ, Hahn PF, Papanicolaou N, et al. Benign and malignant adrenal masses:CT distinction with attenuation coefficients, size, and observer analysis. Radiology. 1991;179:415–418. doi: 10.1148/radiology.179.2.2014283. [DOI] [PubMed] [Google Scholar]
- 2.Abrams HL, Siegelman SS, Adams DR, et al. Computed tomography versus ultrasound of the adrenal glad:a prospective study. Radiology. 1982;143:121–128. doi: 10.1148/radiology.143.1.7063713. [DOI] [PubMed] [Google Scholar]
- 3.Krebs TL, Wagner BJ. MR imaging of the adrenal glad:radiologic-pathologic correlation. RadioGraphics. 1998;18:1425–1440. doi: 10.1148/radiographics.18.6.9821192. [DOI] [PubMed] [Google Scholar]
- 4.Bovio S, Cataldi A, Reimondo G, et al. Prevalence of adrenal incidentaloma in a contemporary computerized tomography series. J Endocrinol Invest. 2006;29:298–302. doi: 10.1007/BF03344099. [DOI] [PubMed] [Google Scholar]
- 5.Kuruba R, Gallagher SF. Current management of adrenal tumors. Curr Opin Oncol. 2008;20:34–46. doi: 10.1097/CCO.0b013e3282f301fd. [DOI] [PubMed] [Google Scholar]
- 6.DeWitt J, Alsatie M, LeBlanc J, et al. Endoscopic ultrasound-guided fine-needle aspiration of left adrenal gland masses. Endoscopy. 2007;39:65–71. doi: 10.1055/s-2006-945042. [DOI] [PubMed] [Google Scholar]
- 7.Harisinghani MG, Maher MM, Hahn PF, et al. Predictive value of benign percutaneous adrenal biopsies in oncology patients. Clin Radiol. 2002;57:898–901. doi: 10.1053/crad.2002.1054. [DOI] [PubMed] [Google Scholar]
- 8.Bodtger U, Vilmann P, Clementsen P, et al. Clinical impact of endoscopic ultrasound-fine needle aspiration of left adrenal masses in established or suspected lung cancer. J Thorac Oncol. 2009;4:1485–1489. doi: 10.1097/JTO.0b013e3181b9e848. [DOI] [PubMed] [Google Scholar]
- 9.Pantalone KM, Gopan T, Remer EM, et al. Change in adrenal mass size as a predictor of a malignant tumor. Endocr Pract. 2010;16:577–587. doi: 10.4158/EP09351.OR. [DOI] [PubMed] [Google Scholar]
- 10.Sharma KV, Venkatesan AM, Swerdlow D, et al. Image-guided adrenal and renal biopsy. Tech Vasc Interv Radiol. 2010;13:100–109. doi: 10.1053/j.tvir.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumachi F, Borsato S, Brandes AA, et al. Fine-needle aspiration cytology of adrenal masses in non-cancer patients:clinic-radiologic and histologic correlations in functioning and nonfunctioning tumors. Cancer. 2001;93:323–329. doi: 10.1002/cncr.9047. [DOI] [PubMed] [Google Scholar]
- 12.Eloubeidi MA, Black KR, Tamhane A, et al. A large single-center experience of EUS-guided FNA of the left and right adrenal glands:diagnostic utility and impact on patient management. Gastrointest Endosc. 2010;71:745–753. doi: 10.1016/j.gie.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Arellano RS, Garcia RG, Gervais DA, et al. Percutaneous CT-guided radiofrequency ablation of renal cell carcinoma:efficacy of organ displacement by injection of 5% dextrose in water into the retroperitoneum. AJR Am J Roentgenol. 2009;193:1686–1690. doi: 10.2214/AJR.09.2904. [DOI] [PubMed] [Google Scholar]
- 14.Eloubeidi MA, Beydoun M, Jurdi N, et al. Transduodenal EUS-guided FNA of the right adrenal gland to diagnose lung cancer where percutaneous approach was not possible. J Med Liban. 2011;59:173–175. [PubMed] [Google Scholar]
- 15.Bang JY, Hebert-Magee S, Varadarajulu S. Diagnosis of bilateral adrenal metastases secondary to malignant melanoma by EUS-guided FNA. Am J Gastroenterol. 2011;106:1862–1863. doi: 10.1038/ajg.2011.176. [DOI] [PubMed] [Google Scholar]
- 16.DeWitt JM. Endoscopic ultrasound-guided fine-needle aspiration of right adrenal masses:report of 2 cases. J Ultrasound Med. 2008;27:261–267. doi: 10.7863/jum.2008.27.2.261. [DOI] [PubMed] [Google Scholar]
- 17.Tsitouridis I, Michaelides M, Stratilati S, et al. CT guided percutaneous ad- renal biopsy for lesions with equivocal findings in chemical shift MR imaging. Hippokratia. 2008;12:37–42. [PMC free article] [PubMed] [Google Scholar]
- 18.Uemura S, Yasuda I, Kato T, et al. Preoperative routine evaluation of bilateral adrenal glands by endoscopic ultrasound and fine-needle aspiration in patients with potentially resectable lung cancer. Endoscopy. 2013;45:195–201. doi: 10.1055/s-0032-1325988. [DOI] [PubMed] [Google Scholar]
- 19.Wu HH, Cramer HM, Kho J, et al. Fine needle aspiration cytology of benign adrenal cortical nodules. A comparison of cytologic findings with those of primary and metastatic adrenal malignancies. Acta Cytol. 1998;42:1352–1358. doi: 10.1159/000332167. [DOI] [PubMed] [Google Scholar]
- 20.Schuurbiers OC, Tournoy KG, Schoppers HJ, et al. EUS-FNA for the detection of left adrenal metastasis in patients with lung cancer. Lung Cancer. 2011;73:310–315. doi: 10.1016/j.lungcan.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Sood SK, Balasubramanian SP, Harrison BJ. Percutaneous biopsy of adrenal and extra-adrenal retroperitoneal lesions:beware of catecholamine secreting tumours! Surgeon. 2007;5:279–281. doi: 10.1016/s1479-666x(07)80026-6. [DOI] [PubMed] [Google Scholar]
- 22.Young Jr WF. Clinical practice:The incidentally discovered adrenal mass. N Engl J Med. 2007;356:601–610. doi: 10.1056/NEJMcp065470. [DOI] [PubMed] [Google Scholar]
- 23.Angeli A, Osella G, Ali A, Terzolo M. Adrenal incidentaloma:an overview of clinical and epidemiological data from the National Italian Study Group. Horm Res. 1997;47:279–283. doi: 10.1159/000185477. [DOI] [PubMed] [Google Scholar]
- 24.Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentaloma”) Ann Intern Med. 2003;138:424–429. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 25.Arellano RS, Harisinghani MG, Gervais DA, Hahn PF, Mueller PR. Image-guided percutaneous biopsy of the adrenal gland:review of indications, technique, and complications. Curr Probl Diagn Radiol. 2003;32:3–10. doi: 10.1067/cdr.2003.120002. [DOI] [PubMed] [Google Scholar]
- 26.Casola G, Nicolet V, vanSonnenberg E, et al. Unsuspected pheochromocytoma:risk of blood-pressure alterations during percutaneous adrenal biopsy. Radiology. 1986;159:733–735. doi: 10.1148/radiology.159.3.3517958. [DOI] [PubMed] [Google Scholar]
- 27.Dietrich CF, Wehrmann T, Hoffmann C, et al. Detection of the adrenal glands by endoscopic or transabdominal ultrasound. Endoscopy. 1997;29:859–864. doi: 10.1055/s-2007-1004322. [DOI] [PubMed] [Google Scholar]
- 28.Sharma R, Ou S, Ullah A, et al. Endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) of the right adrenal gland. Endoscopy. 2012;44(Suppl 2 UCTN):E385–E386. doi: 10.1055/s-0032-1310145. [DOI] [PubMed] [Google Scholar]
- 29.Ang TL, Chua TS, Fock KM, et al. EUS-FNA of the left adrenal gland is safe and useful. Ann Acad Med Singapore. 2007;36:954–957. [PubMed] [Google Scholar]
- 30.Eloubeidi MA, Luz LP, Crowe DR, Snowden C, Morgan DE, Arnoletti PJ. Bilateral adrenal gland enlargement secondary to histoplasmosis mimicking adrenal metastases:diagnosis with EUS-guided FNA. Diagn Cytopathol. 2010;38:357–359. doi: 10.1002/dc.21210. [DOI] [PubMed] [Google Scholar]
- 31.Azhar J, Jacqueline H, Tony L, et al. Bilateral adrenal histoplasmosis:endoscopic ultrasound-guided fine needle aspiration as a method of diagnosis and assessment. Med J Malaysia. 2011;66:504–506. [PubMed] [Google Scholar]
- 32.Eloubeidi M, Seewald S, Tamhane A, et al. EUS-guided FNA of the left adrenal gland in patients with thoracic or GI malignancies. Gastrointest Endosc. 2004;59:627–633. doi: 10.1016/s0016-5107(04)00296-2. [DOI] [PubMed] [Google Scholar]
- 33.Martinez M, LeBlanc J, Al-Haddad M, et al. Role of endoscopic ultrasound fine-needle aspiration evaluating adrenal gland enlargement or mass. World J Nephrol. 2014;3:92–100. doi: 10.5527/wjn.v3.i3.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerke H, Robinson R, Luo P. Diagnosis of focal metastasis to the adrenal gland by EUS-guided core biopsy. Gastrointest Endosc. 2005;62:469–471. doi: 10.1016/s0016-5107(05)01578-6. [DOI] [PubMed] [Google Scholar]
- 35.Haseganu L, Diehl D. Left adrenal gland hemorrhage as a complication of EUS-FNA. Gastrointest Endosc. 2009;69:E51–E52. doi: 10.1016/j.gie.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 36.Jhala NC, Jhala D, Eloubeidi MA, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy of the adrenal glands:analysis of 24 patients. Cancer. 2004;102:308–314. doi: 10.1002/cncr.20498. [DOI] [PubMed] [Google Scholar]
- 37.Puri R, Thandaserry R, Choudhary N, et al. Endoscopic ultrasound-guided fine-needle aspiration of the adrenal glands:analysis of 21 patients. Clin Endosc. 2015;48:165–170. doi: 10.5946/ce.2015.48.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]


