Abstract
Background
The negative effect of diabetes mellitus (DM) on the colonoscopy preparation has been previously established. Metabolic syndrome has been shown to increase risk for malignancy and possibly for premalignant lesions. This study aimed to investigate the impact of DM control on colonoscopy outcomes including bowel preparation and adenoma detection rate (ADR).
Methods
We included patients with DM who underwent colonoscopy in our hospital and had a documented glycated hemoglobin (HbA1C) within 3 months. Patients were categorized into three groups based on their HbA1C level. The clinical and endoscopic data were collected and analyzed.
Results
Our cohort included 352 DM patients. The mean age was 63.5 years. When patients were analyzed based on HBA1C, bowel preparation was poor in 46.7% of patients with good glycemic control, 52.1% of patients with fair control and 50% of patients with poor control. ADR was 24.3% in patients with good glycemic control, 20.2% in patients with fair glycemic control and 27.1% in patients with poor glycemic control. There was no statistically significant difference in the quality of preparation or adenoma detection amongst the groups.
Conclusion
The degree of glycemic control did not impact the quality of bowel preparation or ADR.
Keywords: Adenoma detection rate, diabetes mellitus, bowel preparation, colonoscopy, glycosylated hemoglobin
Introduction
Colorectal cancer (CRC) is a leading cause of cancer-related mortality in the United States. Early detection and removal of adenomatous polyps reduces the risk of future CRC [1]. Colonoscopy is the gold standard in diagnosis of adenomatous polyps and CRC because of its efficacy in detecting lesions and its ability to concomitantly resect these lesions [1]. The value of colonoscopy in screening for CRC is dependent on the adenoma detection rate (ADR) which is directly related to the endoscopist’s skills and the bowel preparation quality [2]. Published literature have shown that inadequate bowel preparation is linked to missed adenomas, increased interval cancer rates, increased procedure time, and increased healthcare costs resulting from repeating colonoscopic exams [2].
A variety of factors can influence the quality of bowel preparation among adult patients undergoing colonoscopy [3]. These factors can be categorized into either patient-related or procedure-related. Multiple studies have identified patient-related factors such as advanced age, male sex, comorbid medical illness, and low socio-economic status to be associated with poor bowel preparation [2,3]. Furthermore, procedure-related factors such as timing of preparation administration and the waiting period on the day of colonoscopy were found to influence the quality of colon cleansing. Understanding these factors can provide guidance in developing strategies targeted towards improvement in the quality of colonoscopy performance [3].
Diabetes mellitus (DM) has been established as an independent factor affecting the quality of bowel preparation in few studies [3-5]. DM is known to impair colonic and general gastrointestinal transit [4]. The mechanism of delayed colonic transit in DM is still unclear, however, amongst the suggested mechanisms, autonomic neuropathy, which is a complication of long-standing DM, has been widely accepted as the main underlying etiology. Autonomic neuropathy may lead to absence of normal postprandial gastro-colonic response, resulting in delayed colonic transit time [4,5]. Multiple nerve conduction studies showed that stage of neuropathy is highly correlated to the glycated hemoglobin (HbA1C) representing the level of glycemic control [6]. The effect of diabetes control on the quality of bowel preparation has not been studied previously. In this study, we aimed to investigate the impact of diabetes control on colonoscopy outcomes including bowel preparation and ADR.
Patients and methods
Setting and patient selection
The cohort was selected out of colonoscopy outcomes in minority population registry (COMP), a teaching community hospital-based database in New York City. Of 1186 patients who underwent average risk outpatient screening colonoscopy evaluation during January 1, 2008 to August 30, 2012, 352 were found to be diabetic. The diagnosis of DM was based on ICD-9 code documented in these patients charts. Patients included in the study were adults 50-85 years old who had used the standard bowel preparation of 1 gallon polyethylene glycol solution. Patients were excluded from the study if they used another type of bowel preparation regimen or had a repeat colonoscopy during the study period secondary to inadequate bowel preparation. The study protocol was approved by the Institutional Review Board.
The patients’ records were retrospectively reviewed for demographic, colonoscopic and pathological variables. Based on the available data, the level of HbA1C was used as a reliable surrogate for diabetes control [7]. The patients with documented HBA1C within 3 months of the colonoscopy were further categorized into three groups based on their HbA1C (good glycemic control A <7, fair glycemic control B 7-9, poor glycemic control C >9). The bowel preparation was also classified to two subclasses of optimal (excellent or good) and suboptimal (fair, poor, or inadequate) based on the Ottawa scale. The patients in this cohort used the prior evening preparation, which was the standard in our institution at that time, with an estimated time lag of 12 hours between complete administration of the preparation and performing colonoscopy. Patient data were collected and analyzed retrospectively.
Statistical analysis
The primary endpoint analyzed was quality of bowel preparation. Secondary endpoints included ADR and advanced ADR (aADR). Categorical variables were expressed as valid percentages and compared using chi square test. Subgroups analyzed were patients with good, fair, or poor glycemic control, patients with optimal or suboptimal bowel preparation, and patients with or without adenoma detected on colonoscopy. P values <0.05 were considered significant. Analysis was carried out using SPSS 18.0 (SPSS, Chicago, IL).
Results
Our cohort included 352 diabetic patients. 58.4% of the study population was African Americans, 31.9% were Hispanics, 7.9% were whites and 1.7% Asians (Table 1). Patients with good glycemic control represented 59.7% of study population, whereas patients with fair glycemic control and poor glycemic control represented 26.7% and 13.6%, respectively. The quality of bowel preparation was relatively similar across all study groups (Fig. 1). Optimal bowel preparation was observed in 53.3% of patients with good glycemic control, while 47.9% of patients with fair glycemic control were found to have optimal bowel preparation, and 50% of patients with poor glycemic control had optimal bowel preparation (P=0.6).
Table 1.
Study population characteristics
Figure 1.
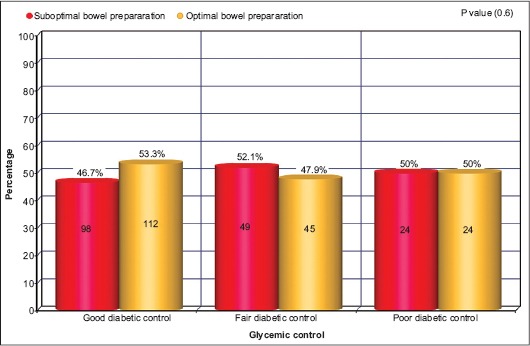
Percentage of suboptimal/optimal bowel preparation in diabetic patients with different levels of glycemic control
ADR was 24.3% in patients with good glycemic control, whereas in patients with fair glycemic control and in patients with poor glycemic control, ADRs were 20.2% and 27.1%, respectively (Fig. 2) (P=0.6). Among patients with good glycemic control aADR was 12.9%, while in patients with fair glycemic control and poor glycemic control aADRs were 11.7% and 18.8%, respectively (Fig. 2) (P=0.4).
Figure 2.
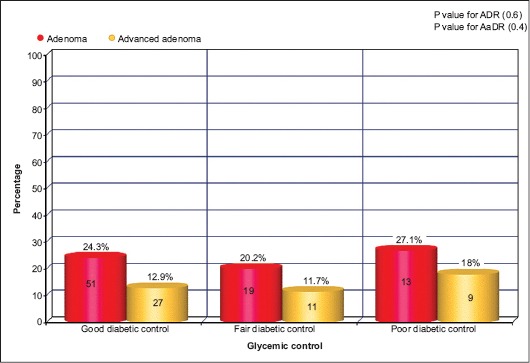
Percentage of adenoma detection rate/advanced adenoma detection rate in diabetic patients with different levels of glycemic control
Discussion
This is the first study to evaluate the impact of DM control on colonoscopy bowel preparation in a minority population. This study also evaluated the impact of long-term glycemic control on ADR and aADR in a population that predominantly African Americans and Hispanics. Previous studies of this kind had included mostly non-African American non-Hispanic populations [8]. The quality of bowel prep is a key element to determine the ADR. Poorly prepared patients during colonoscopy usually undergo longer, more difficult procedures and a lower diagnostic yield for polyps. Previous studies have shown an inverse relationship between diabetes and quality of bowel preparation for colonoscopy [9].
Taylor C has conducted a study to examine the factors that affect poor bowel preparation [10]. On a subgroup analysis, the diabetic patients, irrespective of diabetic control, have been shown to have significantly poor response to standard polyethylene glycol preparation compared to non-diabetics. However, this study was limited by the small number of patients (54 patients) and by the retrospective design. In our study, we compared the bowel preparation quality amongst three groups of diabetics (good, fair and poor glycemic control) and no significant difference in the preparation quality was found. Since previous studies have postulated that the poor bowel prep observed in diabetics could be related to colonic atony [10,11], our findings amongst different groups of diabetics could suggest that colonic atony is a de novo process that can happen regardless of the stage or control of DM.
DM is associated with an increased ADR [12,13]. There are many theories which could explain the increased risk of colorectal adenoma or cancer in people with DM [14,15]. In patients with type 2 DM, hyperinsulinemia is present early in the disease in response to peripheral resistance to insulin [16]. One theory proposes that hyperinsulinemia and insulin like growth factor (IGF-1) contribute to the proliferation of colon epithelial cells and colon carcinoma cells, leading to CRC [14].
A prior study done by Rodney Eddi et al in a predominantly white population showed that there was significant difference in ADR or aADR between well controlled and poorly controlled diabetic patients [13]. Our study classification was more comprehensive as we categorized patients into three glycemic control categories, however, our findings showed no significant difference in ADR and aADR amongst the groups. Siddiqui et al found that the worse the control of DM, the higher incidence of CRC [17]. Another study done by Kanadiya et al supported an association between DM and colonic adenomas and showed a reduction in this risk in those who took metformin to control their DM, however, the study did not analyze the level of glycemic control in the cohort and it was unclear if the reduction in ADR is related to changes in glycemic control or to the presumed anticancer effects of metformin [18]. Furthermore, this study did not specify whether patients had advanced adenomas.
The strengths of our study are that it is the largest study that investigated the influence of DM control on the quality of bowel prep and it is the first of its kind that was done in minority populations, a subgroup that is often underrepresented. Our study limitations are represented by its retrospective design, and that it did not evaluate some other potential confounders, i.e. level of education, smoking history, alcohol abuse, location of the colonic adenoma, and other comorbidities (i.e. obesity, sedentary lifestyle and metabolic syndrome). Despite the fact that HbA1C is an acceptable marker for glycemic control over a 12-week period, it cannot surely show the length of the time that the patient has been exposed to high levels of insulin, an agent which later can increase the risk of colonic adenoma or CRC.
In conclusion, DM has been associated with suboptimal bowel preparation. Outcomes of colonoscopy in diabetics represented by quality of bowel preparation, as well as ADR and aADR could be independent of the level of glycemic control. Therefore, diabetic patients might need a more intensive bowel preparation regimen regardless of their glycemic control. These findings need to be validated by larger studies to explore the underlying pathophysiology for these findings. Interventions to improve bowel preparation in diabetic subjects need to be evaluated.
Summary Box.
What is already known:
Suboptimal colonoscopy preparation can result in missed adenomatous lesions
Diabetes mellitus (DM) is associated with suboptimal colonoscopy preparation
DM may be associated with increased adenoma detection rate (ADR)
What the new fi ndings are:
The quality of colonoscopy preparation in diabetics could be unrelated to the degree of glycemic control in these patients
The degree of glycemic control might have no impact on ADR and advanced ADR
Intense bowel preparation regimens should be considered in diabetics regardless of how well controlled the diabetes is
Biography
Montefiore Medical Center, Bronx, NY; The Brooklyn Hospital Center, Brooklyn, NY; Atlanticare Regional Medical Center, Atlantic City, NJ; The Brookdale University Hospital and Medical Center, Brooklyn, NY; University of Texas Health Science Center at Houston, USA
Footnotes
Conflict of Interest: None
References
- 1.Geiger TM, Ricciardi R. Screening options and recommendations for colorectal cancer. Clin Colon Rectal Surg. 2009;22:209–217. doi: 10.1055/s-0029-1242460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serper M, Gawron AJ, Smith SG, et al. Patient factors that affect quality of colonoscopy preparation. Clin Gastroenterol Hepatol. 2014;12:451–457. doi: 10.1016/j.cgh.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero RV, Mahadeva S. Factor’s influencing quality of bowel preparation for colonoscopy. World J Gastrointest Endosc. 2013;5:39–46. doi: 10.4253/wjge.v5.i2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozturk NA, Gokturk HS, Demir M, et al. The effect of autonomous neuropathy on bowel preparation in type 2 diabetes mellitus. Int J Colorectal Dis. 2009;24:1407–1412. doi: 10.1007/s00384-009-0757-4. [DOI] [PubMed] [Google Scholar]
- 5.Jung HK, Kim DY, Moon IH, Hong YS. Colonic transit time in diabetic patients--comparison with healthy subjects and the effect of autonomic neuropathy. Yonsei Med J. 2003;44:265–272. doi: 10.3349/ymj.2003.44.2.265. [DOI] [PubMed] [Google Scholar]
- 6.El-Salem K, Ammari F, Khader Y, Dhaimat O. Elevated glycosylated hemoglobin is associated with subclinical neuropathy in neurologically asymptomatic diabetic patients:a prospective study. J Clin Neurophysiol. 2009;26:50–53. doi: 10.1097/WNP.0b013e31819862ee. [DOI] [PubMed] [Google Scholar]
- 7.Saudek CD, Brick JC. The clinical use of hemoglobin A1C. J Diabetes Sci Technol. 2009;3:629–634. doi: 10.1177/193229680900300402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong SN, Sung IK, Kim JH, et al. The effect of the bowel preparation status on the risk of missing polyp and adenoma during screening colonoscopy:a Tandem Colonoscopic Study. Clin Endosc. 2012;45:404–411. doi: 10.5946/ce.2012.45.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung YW, Han DS, Park KH, et al. Patient factors predictive of inadequate bowel preparation using polyethylene glycol:a prospective study in Korea. J Clin Gastroenterol. 2009;43:448–452. doi: 10.1097/MCG.0b013e3181662442. [DOI] [PubMed] [Google Scholar]
- 10.Taylor C, Schubert ML. Decreased efficacy of polyethylene glycol lavage solution (golytely) in the preparation of diabetic patients for outpatient Colonoscopy:a prospective and blinded study. Am J Gastroenterol. 2001;96:710–714. doi: 10.1111/j.1572-0241.2001.03610.x. [DOI] [PubMed] [Google Scholar]
- 11.Dziemidok P, Szcześniak G, Kostrzewa-Zabłocka E, Paprzycki P, Korzon-Burakowska A. Current glycaemic control has no impact on the advancement of diabetic neuropathy. Ann Agric Environ Med. 2012;19:742–745. [PubMed] [Google Scholar]
- 12.Vu HT, Ufere N, Yan Y, Wang JS, Early DS, Elwing JE. Diabetes mellitus Increases risk for colorectal adenomas in younger patients. World J Gastroenterol. 2014;20:6946–6952. doi: 10.3748/wjg.v20.i22.6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eddi R, Karki A, Shah A, DeBari VA, DePasquale JR. Association of type 2 diabetes and colon adenomas. J Gastrointest Cancer. 2012;43:87–92. doi: 10.1007/s12029-011-9316-7. [DOI] [PubMed] [Google Scholar]
- 14.Giouleme O, Diamantidis MD, Katsaros MG. Is diabetes a causal agent for colorectal cancer? Pathophysiological and molecular mechanisms. World J Gastroenterol. 2011;17:444–448. doi: 10.3748/wjg.v17.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;1060:1911–1921. doi: 10.1038/ajg.2011.301. quiz 1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 17.Siddiqui AA, Spechler SJ, Huerta S, Dredar S, Little BB, Cryer B. Elevated HbA1c is an independent predictor of aggressive clinical behavior in patients with colorectal cancer:a case-control study. Dig Dis Sci. 2008;53:2486–2494. doi: 10.1007/s10620-008-0264-4. [DOI] [PubMed] [Google Scholar]
- 18.Kanadiya MK, Gohel TD, Sanaka MR, Thota PN, Shubrook JH Jr. Relationship between type-2 diabetes and use of metformin with risk of colorectal adenoma in an American population receiving colonoscopy. J Diabetes Complications. 2013;27:463–466. doi: 10.1016/j.jdiacomp.2013.04.010. [DOI] [PubMed] [Google Scholar]


