Abstract
Psychological stress during pregnancy increases the risk of childhood wheeze and asthma. However, the transmitting mechanisms remain largely unknown. Since epigenetic alterations have emerged as a link between perturbations in the prenatal environment and an increased disease risk we used whole genome bisulfite sequencing (WGBS) to analyze changes in DNA methylation in mothers and their children related to prenatal psychosocial stress and assessed its role in the development of wheeze in the child. We evaluated genomic regions altered in their methylation level due to maternal stress based of WGBS data of 10 mother-child-pairs. These data were complemented by longitudinal targeted methylation and transcriptional analyses in children from our prospective mother-child cohort LINA for whom maternal stress and wheezing information was available (n = 443). High maternal stress was associated with an increased risk for persistent wheezing in the child until the age of 5. Both mothers and children showed genome-wide alterations in DNA-methylation specifically in enhancer elements. Deregulated neuroendocrine and neurotransmitter receptor interactions were observed in stressed mothers and their children. In children but not in mothers, calcium- and Wnt-signaling required for lung maturation in the prenatal period were epigenetically deregulated and could be linked with wheezing later in children’s life.
The psychosocial health of a pregnant woman can significantly impact the growing fetus and predispose offspring toward increased risk of developing obstructive airway diseases later in life. Although maternal stress during pregnancy has been linked to the development of repeated wheeze, asthma, or airway hyper-responsiveness1,2,3,4,5, the mechanisms involved remain unknown. Evidence largely from animal studies suggests that stress modulates neuroendocrine and autonomic nervous system (ANS) responses to impact immune system functioning and lung development6,7,8,9. There is growing evidence in humans that prenatal stress alters immune function in the developing fetus as indicated by perturbed stimulated cytokine responses and elevated IgE levels at birth2,10. It has been suggested that an aberrant or excessive pro-inflammatory immune response contributes to lung structure-function changes promoting the development of wheezing syndromes later in life11,12.
In the current study we assessed the perceived stress level of women during the course of their pregnancy and were able to confirm previous findings showing a positive association of the prenatal maternal stress levels with an increase in risk for the child to develop persistent wheezing. Our central aim was to assess possible biological mechanisms by which prenatal stress translates into lung dysfunction in the child.
It is hypothesized that transient effects of environmental exposure early in life, in this case prenatal maternal stress, can be preserved by epigenetic mechanisms (e.g. DNA methylation) that silence or activate disease relevant signaling pathways in a persistent way and thereby may modify disease susceptibility13. Such stress induced epigenetic programming leading to adverse respiratory outcomes may be influenced by a variety of pathways involved in lung development beyond the suggested aberrant immune response. There is a lack of comprehensive studies on genome-wide stress-dependent epigenetic perturbations. To fill this gap we chose a well-defined set of mothers and their newborn children to study stress-dependent DNA methylation changes in an unbiased manner by whole genome bisulfite sequencing covering every single base pair of the genome. Based on this genome-wide analysis a hypothesis was developed for the link between prenatal stress and respiratory outcomes and validated by targeted methylation and RNA expression analysis in the entire LINA cohort.
Methods
Further information and details are available in the Supplementary Information.
Study Design
This study is based on a subset of the prospective mother-child cohort LINA (Lifestyle and environmental factors and their Influence on Newborns Allergy risk), which originally included 629 mother–child pairs (622 mothers, 629 children; 7 twin-pairs) recruited between May 2006 and December 2008 in Leipzig, Germany14. These analyses included 443 mother-child pairs for which information on maternal stress level, and prevalence of wheeze of the child up to the age of 5 years was available (Table 1). Wheezing phenotypes were classified according to Martinez et al.15. Information on possible confounders was obtained from questionnaires completed by mothers during pregnancy and around their child’s first birthday16. Study participation was voluntary, written informed consent from all participants and institutional review board approval was obtained (University of Leipzig, 046-2006, 160-2008, 160-2008, 160b/2008, 144-10-31052010, 113-11-18042011). The methods applied in this study were in accordance with the approved guidelines.
Table 1. Characteristics of the analysed subcohort and the entire LINA cohort.
| Parameters | Discovery cohort | Analysed subcohort | Entire LINA cohort | P value* |
|---|---|---|---|---|
| Pregnancy and birth | ||||
| n (%), N = 10 | n (%), N = 443 | n (%), N = 629 | ||
| Gender of the child | ||||
| male | 7 (70) | 227 (51.2) | 327 (52.0) | 0.858 |
| female | 3 (30) | 216 (48.8) | 302 (48.0) | |
| Parental history of atopya | ||||
| negative | 5 (50) | 149 (33.6) | 212 (33.3) | 0.913 |
| single positive | 4 (40) | 213 (48.1) | 296 (46.5) | |
| double positive | 1 (10) | 81 (18.3) | 121 (19.0) | |
| Parental educationb | ||||
| low | 0 (0) | 5 (1.1) | 16 (2.5) | 0.297 |
| intermediate | 1 (10) | 98 (22.1) | 144 (22.6) | |
| high | 9 (90) | 340 (76.8) | 469 (73.7) | |
| Pet keeping (cat) | ||||
| no | 7 (70) | 380 (85.8) | 511 (80.3) | 0.573 |
| yes | 3 (30) | 63 (14.2) | 95 (14.9) | |
| Smoking during pregnancy | ||||
| never | 9 (90) | 388 (87.6) | 534 (89.9) | 0.575 |
| occasionally | 1 (10) | 28 (6.3) | 43 (6.8) | |
| once per week | 0 (0) | 2 (0.5) | 4 (0.76) | |
| daily | 0 (0) | 25 (5.6) | 48 (7.5) | |
| ETS exposure after birth | ||||
| no | 10 (10) | 422 (95.3) | 568 (93.9) | 0.326 |
| yes | 0 (0) | 20 (4.5) | 37 (6.1) | |
| Maternal stress during pregnancy | ||||
| low | 5 (50) | 110 (24.8) | 151 (24.4) | 0.947 |
| medium | 0 (0) | 221 (49.9) | 306 (49.5) | |
| high | 5 (50) | 112 (25.3) | 161 (26.1) | |
*P value from chi squared test for cross relationship, analysed subcohort against entire cohort.
ahistory of atopy is defined as: occurrence of asthma or atopic dermatitis or hay fever.
blow, 9 yrs of schooling or less “Hauptschulabschluss”; intermediate, 10 yrs of schooling “Mittlere Reife”; high, 12 yrs of schooling or more “(Fach-) hochschulreife”.
Perceived Stress Assessment
Maternal stress was assessed by a 20-item perceived stress questionnaire (PSQ)17,18 evaluating how often certain experiences of stress (worries, tension, loss of joy, demands) occurred on a 4-point scale (1 through 4, Table E1)17. A total score was calculated by summing the scored answers of each item. The resulting total scores were subsequently categorized into quartiles. Mothers with a total stress score below the 25th quartile were defined as lowly, above the 75th quartile as highly stressed. The second and third quartiles were summarized as medium stress. To validate this categorization the concentration of the stress marker homovanillic acid normalized to creatinine19,20 was determined in the maternal urine at 36th week of gestation as previously described21.
Whole Genome Bisulfite Sequencing
Whole blood samples of 5 mother-child pairs with a low stress score compared to 5 pairs with a high stress score were evaluated. Bisulfite converted DNA from cord blood and venous blood of mothers obtained at the 36th week of gestation were subjected to whole genome bisulfite sequencing (WGBS, Table E2). Using the bsseq v0.10 package for R statistical software v3.0.0 we followed a slightly adapted approach22 as described by Hansen et al.23 to identify regions of differential DNA methylation (DMRs) since they have been reported to be more informative for a phenotype24,25 compared to single differentially methylated CpGs (Supplementary Information).
Validation analysis: MassARRAY and qPCR
Methylation differences of selected differentially methylated regions (DMRs) were assessed using the MassARRAY system (Sequenom Inc./Agena Bioscience GmbH, Hamburg Germany), a mass spectrometry based approach for targeted methylation analysis26. Since DNA samples were not available for all children, the methylation analyses were restricted to 324 and 217 children at time of birth and at year four, respectively (Figure E1, Table E3). Differential transcription was assessed using the 96.96 Dynamic Array integrated fluidic circuits (Fluidigm, San Francisco, CA, USA).
Statistics
Equal parameter distribution in our sub- and the entire LINA cohort was tested using Chi-square. To assess whether maternal stress, or DNA methylation and gene expression changes observed in our subcohort contribute to an increased risk for the child to develop persistent wheeze logistic regression models were implemented adjusting for known confounding factors of lung disease in early childhood27 (gender of the child, siblings, smoking during pregnancy, ETS exposure after birth, cat keeping, parental history of atopy and parental educational level).
After logarithmic transformation of DNA methylation and gene expression values adjusted mean ratios (MR), i.e. the ratio of the geometric mean, were calculated to evaluate their relationship to the maternal stress level. Confounding factors used were: gender of the child, smoking during pregnancy, ETS exposure after birth (where applicable), age of the mother at time of birth, birth week, mode of delivery, maternal medication during pregnancy28 and cell composition (Supplementary Information). All calculations were performed in STATISTICA for Windows, Version 10 (Statsoft Inc., Tulsa, OK, USA). P-values < 0.05 were considered significant.
Results
Prenatal maternal stress and persistent childhood wheeze
The median total stress score of mothers in our subcohort was 1.85 (min = 1.00, max = 3.55). Total stress score grouping into quartiles resulted in n = 110, 221 and 112 mothers with a low, medium and high stress level respectively (Table 1).
Urinary homovanillic acid concentration was significantly elevated in mothers with high compared to mothers reporting low stress. This effect sustained even after adjusting for maternal medication a possible influencing factor of homovanillic acid production (adj. p-value = 0.023, Fig. 1). High levels of maternal stress during pregnancy were associated with an increased risk of persistent childhood wheeze (OR = 2.44; 95% CI: 1.09–5.46). Adjustment for possible confounders27 slightly increased the calculated OR (OR = 2.73; 95% CI: 1.13–6.55, Table 2).
Figure 1. Concentration of homovanillic acid for different stress scores.
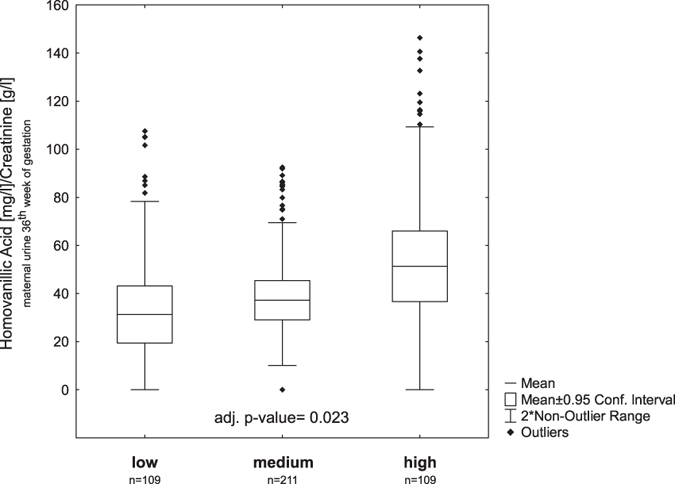
Homovanillic acid was measured in urine of mothers at 36th weeks of gestation and normalized to creatinine19,20,21. The concentration of the stress metabolite homovanillic acid is significantly higher in mothers with a high stress score compared to those with the lowest stress score supporting stress categorization based on our questionnaire. A significant relationship of homovanillic acid concentration and maternal stress score was determined by multiple regression analysis. Mean ratios were adjusted for maternal medication information derived from the same samples28 (box plots represent: mean +/− CI, whiskers +/− non-outlier range).
Table 2. Relationship between maternal stress during pregnancy assessed using the perceived stress questionnaire (PSQ) and children’s persistent wheeze up to the age of 5 years.
| Maternal stress# | % (n/N) | Persistent Wheeze | |
|---|---|---|---|
| Raw OR (95% CI) | Adjusted OR (95% CI)* | ||
| Low | 9.1% (10/110) | 1.00 | 1.00 |
| Medium | 12.2% (27/221) | 1.39 (0.65–3.00) | 1.39 (0.61–3.15) |
| High | 19.6% (22/112) | 2.44 (1.09–5.46) | 2.73 (1.13–6.55) |
*Adjusted odds ratios for parental history of atopy, parental educational level, gender of the child, siblings, smoking during pregnancy, ETS exposure after birth, cat keeping.
#Maternal stress was assessed by the 20-item PSQ17,18. A total score was calculated by summing the scored answers of each item. The resulting total scores were subsequently categorized into quartiles. While mothers with a total stress score below the 25th quartile were defined as lowly stressed, those above the 75th quartile were defined as highly stressed.
Stress and DNA methylation changes
Comparison of five mother-child pairs with low and five pairs with high stress levels by WGBS revealed a substantial genome-wide perturbation in DNA methylation (Table E4). A total of 2306 stress-dependent differentially methylated regions (DMRs, ≥3 consecutive CpGs, Δmeth >10%, t > +/− 4.523) were identified in children and 2495 in mothers with a false discovery rate of 1.9% and 2.5% respectively (Supplementary Information). It is noteworthy that the majority of the DNA methylation changes we detected would not have been observed using a 450 K bead array approach, since only about 5% of DMRs overlapped with at least one CpG sites covered by the array (mothers: n = 130 (5.2%); children: n = 114 (4.9%), Table E4).
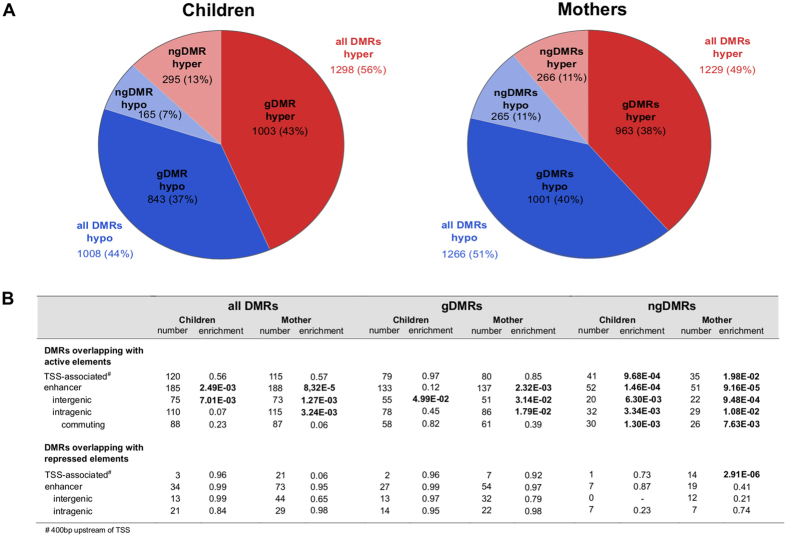
DNA methylation cannot only be affected by environmental factors but is also related to the individual genotype. Besides single nucleotide polymorphisms (SNP) that may destroy the CpG context and thereby directly induce differential DNA methylation, SNPs may also create or disrupt transcription factor binding sites (TFBS) affecting the level of DNA methylation29. To take this into account we performed SNP-calling for each individual bisulfite-sequencing sample using Bis-SNPs30 followed by DMR classification. Whenever a SNP in the neighborhood (+/− 5 kb) of a DMR was statistically significantly correlated with methylation of this DMR, this SNP was classified as a meQTL-SNP (methylation quantitative trait locus) for this DMR. The corresponding potentially genetically influenced DMR was abbreviated as gDMR. All DMRs that were not associated with any meQTL-SNP in their neighborhood and thus presumably not influenced by the genotype were categorized as non-genetically influenced DMRs (ngDMR). This conservative classification still retained about 20% (460/2306 in children and 531/2495 in mothers) of all DMRs as ngDMRs that do not have an apparent correlation with the underlying genetic sequence variation (Fig. 2A).
Figure 2. Global overview of differential methylation in children and mothers.
(A) Pie charts represent the number and percentage of DMRs observed in mothers and children. Two classes of DMRs were differentiated: gDMRs for which methylation significantly correlated with a meQTL-SNP and ngDMRs where the underlying sequence variation did not influence methylation (red: hypermethylation, blue: hypomethylation). (B) The table summarizes the functional genomic regions enriched for an overlap with these DMRs. Active regulatory genomic regions in particular enhancer elements were significantly enriched in overlapping DMRs irrespective of their type (gDMR or ngDMR).
The total number of DMRs was similar in mothers and children. In children the number of hypermethylated DMRs was significantly higher than in mothers (Chi-square, p < 0.001, power = 1.0, Fig. 2A/B). Only about 8% of DMRs were shared between mothers and children (n = 187, Table E3). The genomic distribution was similar in mothers and children with approximately half of the detected DMRs located intergenically (1286 (52%) and 1256 (55%) in mothers and children respectively, Table E4). Of the remaining DMRs almost all were found in introns (mothers: 44%, children: 41%, Table E4).
DMRs-irrespective of their type (gDMRs or ngDMRs)-preferentially overlapped with functional regulatory regions (Fig. 2B, Table E5). ChIP-Seq data of several histone modifications in a selected set of mothers and children of the LINA cohort31 revealed that in particular ngDMRs were enriched in TSS-associated (400 bp upstream of TSS) active regulatory elements and enhancers for both mothers and children (Fig. 2B). The majority of enhancers overlapping with a ngDMR were intragenic (children: 110/185, mothers: 115/188), with more than 75% of these enhancers interacting not only with their host gene but also with distal genes (commuting enhancer, Fig. 2B).
Analysis of DMR overlap with tissue/-cell type specific enhancer regions of the ENCODE roadmap32 revealed an anticipated strong enrichment in blood cell specific enhancers (Figure E2, Supplementary Information). This enrichment was particularly pronounced in ngDMRS while gDMRs were also found in enhancer regions characteristic also for other cell types.
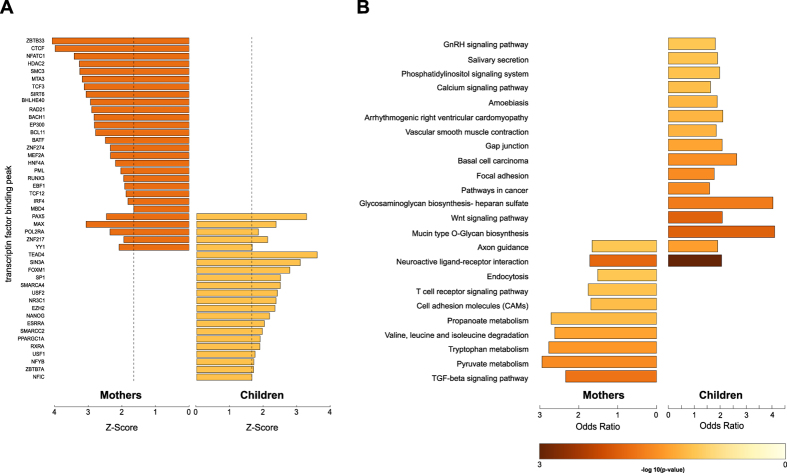
In a subsequent analysis we evaluated the overlap of DMRs with TFBS (Fig. 3A, Supplementary Information). Using ENCODE ChIP-Seq data of transcription factor binding32,33,34 we determined a significant overlap of DMRs with POL2RA binding sites in mothers and children supporting our observation that DNA methylation changes not occur arbitrary but particularly affect functional regulatory regions.
Figure 3. DMR overlap with transcription factor binding sites and KEGG pathways.
(A) Z-scores of DMR overlaps with transcription factor binding peak sets from ENCODE. For each transcription factor, z-scores were calculated using the mean value and the standard deviation from the overlap distribution of randomly shuffled DMRs with the corresponding transcription factor binding factor binding peak set. The dashed line represents the 5% upper tail of the standard-normal distribution. Only z-scores are shown that are above the 10% upper tail threshold. (B) Enrichment of DMRs in KEGG pathways.
In addition, DNA binding sites of the glucocorticoid receptor (NR3C1), the main regulator of the glucocorticoid (cortisol in humans) mediated stress response, were significantly enriched for an overlap with DMRs in children (Fig. 3A).
Minimal functional overlap between mothers and children
DMRs of mothers and children were subjected to an enrichment analysis for KEGG pathways. There was only marginal overlap between enriched pathways while the majority was unique to mothers or their children (Fig. 3B). Most of the receptors in the “Neuroactive ligand receptor interaction” list affected by differential methylation are G protein coupled receptors (GPCR, Fig. 3B) of the neuroendocrine system. Although the number of DMRs in this list was almost identical in mothers and children only eleven genes were consistently affected by DMRs (Figure E3, Table E5A) and of these only five DMRs were shared (Table E5B).
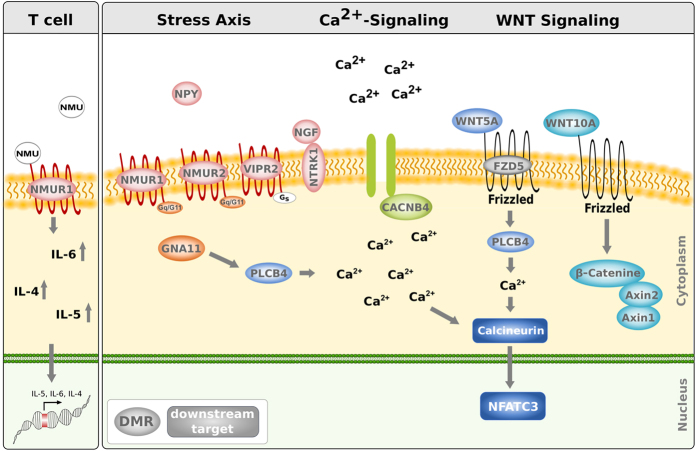
A subclass of neuroactive GPCRs promotes calcium release upon activation and is directly linked to the “Calcium signaling pathway” (Figure E4A) enriched only in children for stress-induced DMRs (Fig. 3B). Furthermore, the “Wnt signaling pathway” another pathway mediating intracellular calcium release (Figure E4B) was enriched in DMRs observed in children of highly stressed mothers. We were particularly interested in changes of the epigenetic landscape, which might contribute to the adverse respiratory outcome observed in children. Since calcium homeostasis and Wnt signaling have previously been described as being important for lung development and showed a close interconnection also to the upstream GPCRs mediating stress response we chose candidate genes in the overlap of these pathways (Fig. 4)35,36. We specifically selected DMRs located in regulatory genomic elements since such perturbations in DNA methylation are more likely to translate into transcriptional changes.
Figure 4. Pathways intertwined by stress induced differential methylation.
NMUR1, neuroendocrine system and immune response
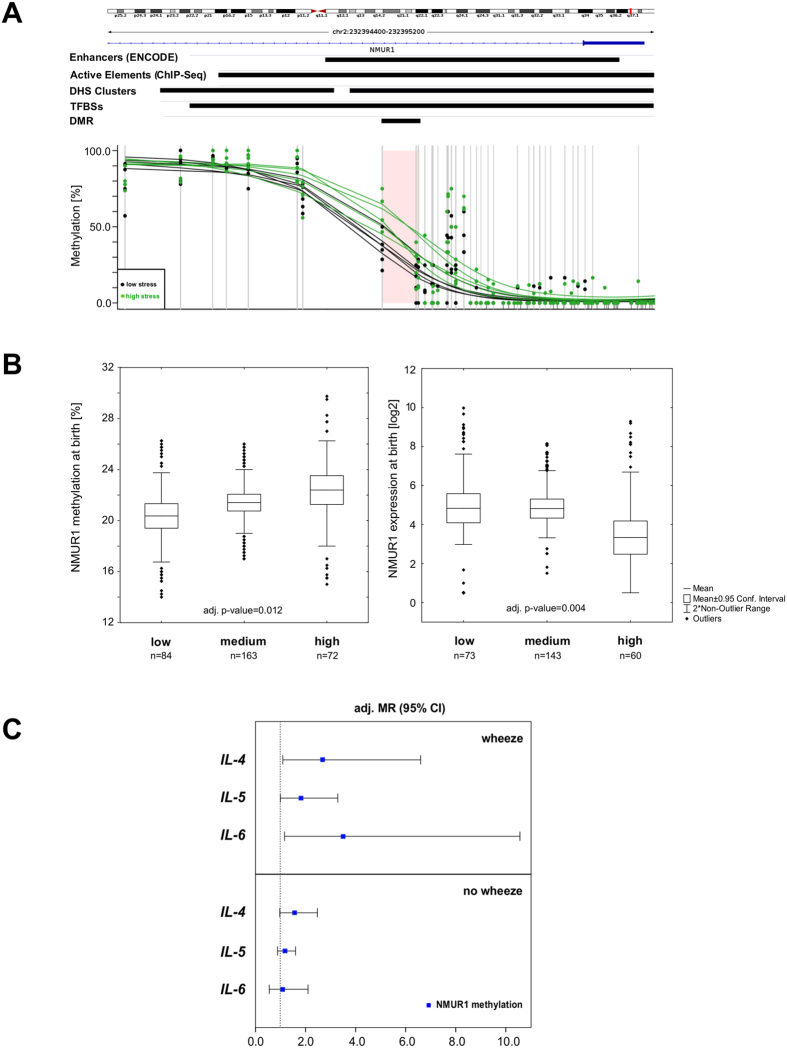
In children, we determined a hypermethylated ngDMR in the gene of neuromedin U receptor 1 (NMUR1), a GPCR known to be involved in hypothalamic-pituitary-adrenal axis (HPA) response37,38. The DMR (WGBS: Δmeth = 28%, p-value = 0.030) consisted of three CpGs located in the first intron and overlapped with an ENCODE enhancer region (Fig. 5A). For validation analysis in the entire cohort by MassARRAY, we extended the region to include seven subsequent CpGs. At time of birth methylation in the NMUR1 DMR significantly increased dependent on the maternal stress score (Δmeth = 2%, adj. p-value = 0.012), although with a much smaller effect size than observed by WGBS. Comparing methylation levels in the same children obtained by WGBS and MassARRAY respectively reveals that this is most likely related to a much smaller dynamic range of the validation method in this region (Figure E5). Correspondingly, a significant decrease in the expression of NMUR1 in children born to highly stressed compared to those born to lowly stressed mothers was observed (adj. p-value = 0.0034), Fig. 5B).
Figure 5. Neuromedin U Receptor 1 is differentially methylated and expressed in children experiencing prenatal maternal stress.
(A) Based on the histone modification data the DMR (WGBS: Δmeth = 28%) identified in the NMUR1 gene is located in an enhancer region (ENCODE/Active Elements (own ChIP-Seq data)). (B) Validation of the NMUR1 DMR (7 CpGs, chr2: 232,394,701–232,394,805) in the total cohort by MassARRAY shows that an increase of the maternal stress level leads to a significant elevation in the methylation level in children at time of birth. In highly stressed children this methylation increase corresponds to a significantly decreased mRNA expression of NMUR1 (mean +/− 95% CI, whiskers +/− non-outlier range). Relationship between NMUR1 methylation and maternal stress score were determined by multiple regression analysis adjusted for gender of the child, birth week, age of the mother, mode of delivery, maternal smoking/-medication during pregnancy, parental history of atopy and cell composition. (C) Relationship of interleukin-4, -5 and -6 secretion at time of birth and NMUR1 methylation. Data are presented as ratios of the mean (MR) and 95% confidence intervals. Models were adjusted for known confounders of interleukin concentrations in cord blood (month of birth, mode of delivery, gender of the child, parental history of atopy, smoking during pregnancy and cell composition).
Since activation of NMUR1 by its ligand neuromedin U is known to elicit Th2-cytokine release39, we further investigated the relationship of NMUR1 methylation and the concentration of IL-4, IL-5 and IL-6 measured in maternal blood four weeks before birth and cord blood. While no stress-dependent changes in IL-4, IL-5, and IL-6 were observed in mothers, children exposed to high maternal stress during pregnancy showed a significantly higher concentration in cord blood of all three cytokines than children exposed to low maternal stress (Figure E6). Applying linear regression models adjusted for possible confounders of interleukin concentrations observed in cord blood revealed a significant relationship between NMUR1 methylation and IL-4, and IL-6 concentrations at time of birth only in those children developing late or persistent wheeze later in their life’s (Fig. 5C).
Together with the perturbed protein secretion of IL-4, IL-5, and IL-6 the differential methylation in the NMUR1 enhancer region had subsided in four-year-old children (Figure E7).
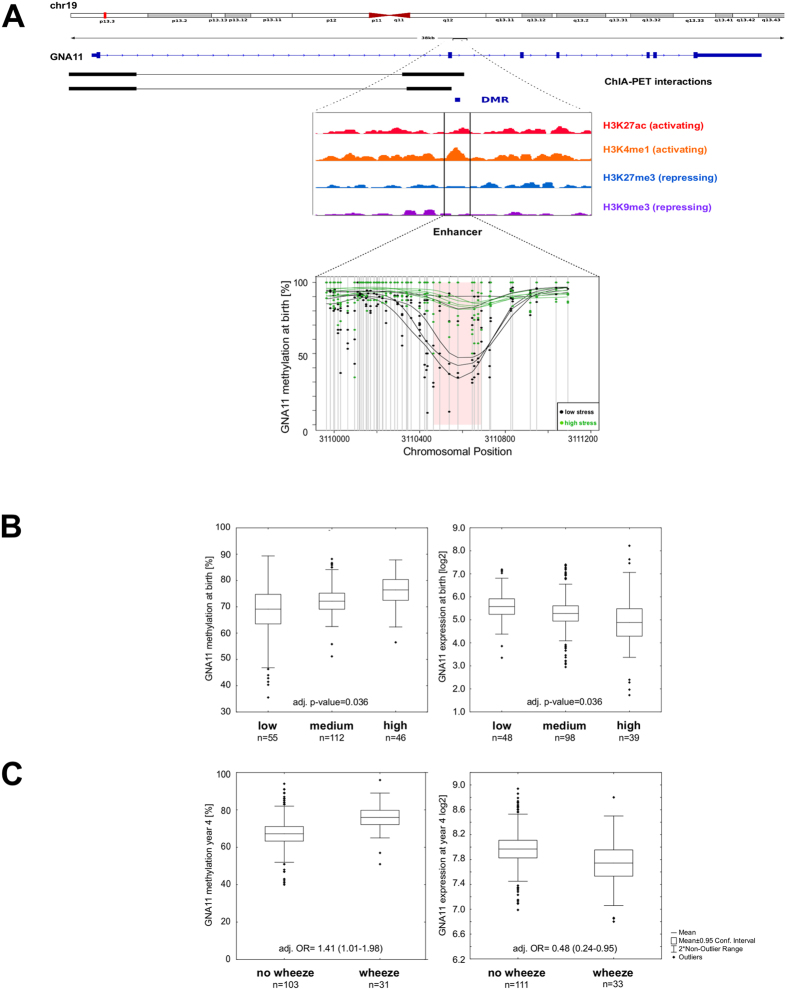
Perturbed calcium signaling and wheezing
In total 19 DMRs in children were assigned to the “Calcium signaling pathway”. One of these DMRs was located in an intron of the GNA11 gene coding for the alpha subunit of the G protein of the G11 class. GNA11 is assigned to the KEGG “Calcium signaling pathway” linking neuroendocrine/-transmitter signaling to calcium release (Figure E4A). The GNA11 gDMR contained eight subsequent CpGs in a poised enhancer region (containing the active mark H3K4me1, Fig. 5A)31. Based on ENCODE ChIA-PET data this differentially methylated enhancer interacts with the promoter of GNA11 (Fig. 5A).
Mean methylation of this GNA11 enhancer was significantly greater in children of mothers reporting high compared to those reporting low stress (WGBS: Δmeth = 22%, p-value = 0.042) (Fig. 6A). High levels of prenatal stress were associated with a significant higher methylation level in this region with a concomitant decrease in gene expression (Fig. 6B). Among persistently wheezing children assessed at age 4, we observed a significantly higher methylation level in the GNA11 gDMR (adj. OR = 1.41 (1.01–1.98)) with a concomitant lower level of GNA11 gene expression (adj. OR = 0.48 (0.24–0.95)) when compared to non-symptomatic children (Fig. 6C).
Figure 6. G protein subunit alpha 11 is epigenetically perturbed in children by prenatal maternal stress.
(A) Although found in an intron the DMR (WGBS: Δmeth = 22%) in the GNA11 gene is located in an enhancer region according to our own ChIP-Seq data. ChIA-PET data suggest this enhancer regulates its host gene. (B) Maternal stress and the methylation level in the GNA11 DMR show a significant relationship at time of birth while at the same time a significant reverse relationship is observed for the expression of GNA11 when including all children in our cohort for whom persistent or never persistent wheeze was reported (mean +/− 95% CI, whiskers +/− non-outlier range, p-values adjusted for: gender of the child, birth week, age of the mother, mode of delivery, maternal smoking/-medication during pregnancy, parental history of atopy and cell composition). (C) In four-year-old children increased methylation in the GNA11 enhancer (chr19: 3110675) is associated with an increased risk for persistent wheeze. Corresponding results are observed for GNA11 expression. Odds ratios were calculated using logistic regression models adjusted for known confounders of wheezing (parental history of atopy, parental educational level, gender, siblings, smoking during pregnancy, ETS exposure after birth, cat keeping and maternal stress score).
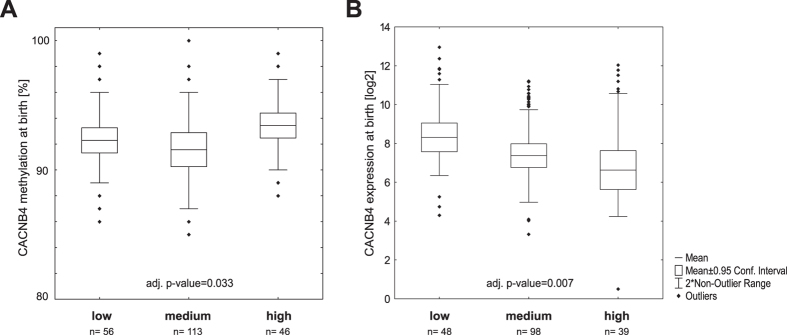
Among the 18 additional DMRs associated to “Calcium signaling pathway”, several are related to calcium channels including CACNB4. The hypermethylated ngDMR located in a weak enhancer region in the CACNB4 gene likely interacts with its host gene promoter based on ENCODE ChIA-PET data (Table E3). Multiple regression analysis revealed a significant relationship of CACNB4 methylation and maternal stress score at time of birth (adj. p-value = 0.033, Fig. 6A) with an increase in methylation concomitant with the stress score. At the same time transcription of CACNB4 was significantly decreased (adj. p-value = 0.007, Fig. 7B).
Figure 7. Peripheral beta 4 subunit of voltage gated calcium channel in children is altered by maternal stress.
(A) Methylation could only be assessed for one CpG in the CACNB4 ngDMR. Amplification of the entire region yielded no PCR product therefore we could only consider two CpGs (chr2: 152898920–152899112). Since one of the two CpGs was a CpG destroying SNP further analysis was restricted to a single position. (A) At time of birth methylation of this CpG shows a significant relationship to the maternal stress score (considering all children either persistently wheezing or showing no respiratory symptoms), (B) while in the same children expression of CACNB4 is decreased to maternal stress. Adjusted p-values were calculated including gender of the child, birth week, age of the mother, mode of delivery, maternal smoking/-medication during pregnancy, parental history of atopy and cell composition as confounding variables.
Wnt/Ca2+ -signaling pathway and stress-related wheeze
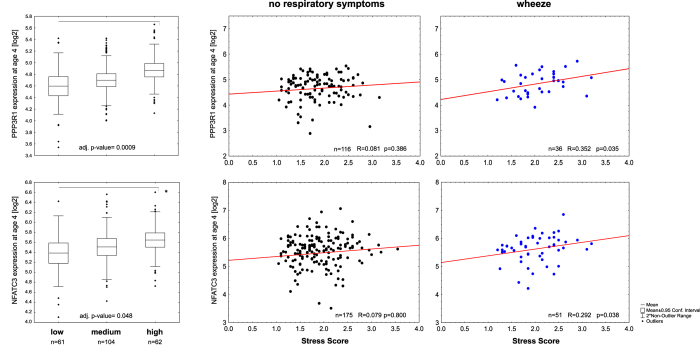
We identified several DMRs related to members of the canonical and calcium-dependent Wnt-signaling pathway (Table E6A) To elucidate the functional impact of these DMRs, we determined transcriptional changes of their downstream targets. For the Wnt/Ca2+ -signaling pathway we evaluated transcription of PPP3R1/PPP3CA (calcineurin) and NFATC3. While no differential transcription was observed for PPP3CA (data not shown), PPP3R1 was significantly elevated in four-year-old children dependent on maternal stress (adj. p-value = 0.0009, Fig. 8). Expression was positively correlated with the stress score only in those children who persistently wheezed. In children of highly stressed mothers, NFATC3 transcription significantly increased dependent on the maternal stress score in four-year-old children (adj. p-value = 0.048). A positive correlation of the stress score with NFATC3 expression was only observed in late and persistently wheezing children. For both PPP3R1 and NFATC3 no correlation of maternal stress score and transcription was observed in children without any respiratory symptoms (Fig. 8).
Figure 8. Downstream targets of the Wnt5A/Ca2+ -signaling pathway are activated in prenatally stressed children.
mRNA expression in prenatally highly stressed children is significantly increased for the calcium binding beta subunit of calcineurin PPP3R1 and its downstream target NFATC3 in four year old children (given are p-values adjusted for: gender of the child, birth week, age of the mother, mode of delivery, maternal smoking/-medication during pregnancy and parental history of atopy; +/− 95% CI, whiskers +/− non-outlier range). A positive correlation of the stress score with NFATC3 expression was only observed in late and persistently wheezing children, no correlation of maternal stress score and transcription was observed in children without any respiratory symptoms for both PPP3R1 and NFATC3.
While CTNNB1 (beta-catenin) showed a stress-dependent increase of transcription already at time of birth (Figure E8A, adj. p-value = 0.024), in four-year-old children expression of both CTNNB1 and AXIN2 (canonical Wnt-signaling) were significantly elevated in children of highly stressed mothers (Figure E8A/B, adj. p-value = 0.002, 0.004) compared to those of low stressed mothers. Furthermore, elevated CTNNB1 expression levels at time of birth were associated with an increased risk for the child to develop late or persistent wheeze later in life (adj. OR = 1.78 (1.28–2.89)). At year four differential transcription related to late or persistent wheeze was no longer observed neither for CTNNB1 nor for AXIN2 (Figure E8A/B).
Discussion
Maternal distress during pregnancy has been shown to persistently affect the health of the child in different ways. Premature birth, low birth weight40 and an increased risk for childhood adiposity41 have been attributed to prenatal maternal stress. Well studied are the long-term neurobiological or behavioral consequences for the child including the development of autism42,43, depression, or schizophrenia44. While these pathophysiological changes in the offspring have been mainly ascribed to reprogramming of neurodevelopment and function of the HPA axis45,46,47 or metabolism on the cellular level48 little is known about the pathways contributing to the development of adverse respiratory outcomes like wheezing1,49, or asthma50,51,52.
Given that the prenatal period is susceptible to external stimuli that can shape the epigenetic landscape and thereby determine disease susceptibility later in life the aim of the current study was to evaluate global DNA methylation changes relating prenatal maternal stress to the increased risk for the child to develop wheezing.
Global methylation changes
We studied differential DNA methylation at time of birth by WGBS of 10 mother-child-pairs complemented by longitudinal targeted methylation and transcriptional analysis in over 300 children. Our WGBS approach offered us the advantage of a global evaluation of DNA methylation changes not restricted to a selected set of CpGs as in commonly used DNA methylation arrays. This is noteworthy since only 5% of our identified DMRs showed an overlap with CpG sites covered by the Infinium HumanMethylation450 BeadChip array, which would have left the vast majority of the maternal-stress-dependent DMRs undiscovered.
Both mothers and children showed genome-wide perturbations in DNA-methylation affecting genomic regulatory elements in particular enhancer elements. Although this preferential deregulation in DNA methylation has already been described in disease states especially in cancer53,54, our results show that also changes in the prenatal environment can lead to perturbed enhancer methylation already at a time at which no disease phenotype has yet developed. Epigenetic perturbations in DNA methylation by stress are not random but rather preferentially occur in enhancer elements regulating more than one gene in the genome, which might contribute to the broad ramifications for children’s health attributed to maternal prenatal stress.
A variety of different biological pathways showed an enrichment of DMRs with a minimal overlap between mothers and children. DNA methylation is thought to be subjected to age-dependent changes55,56 therefore it is possible that the same stressor might lead to distinct perturbations in DNA methylation in different age groups. This is in line with our observation that only genes related to axon guidance and neuroendocrine/neurotransmitter receptor interactions were affected both in mothers and their children with a marginal overlap of affected regions. Since axon guidance is fundamental to neuron wiring modification of the epigenetic signature of this pathway-in particular during brain development57-might influence plasticity of the brain and thereby contribute to the adverse psychological conditions described above for maternal stress exposed offspring.
Stressful life events have been widely related to changes in the cortisol mediated stress response58. DNA methylation of NR3C1 (“glucocorticoid receptor”) as the key mediator of this response has been associated with different types of stressful life events. However, the extent and direction of the alteration in DNA methylation vary dependent on the kind of stress experienced59,60,61 and significant differential DNA methylation seems to be observed only in cases of severe psychological trauma62,63. Previous studies in cord blood showed association of DNA methylation in the 1F region of NR3C1 with maternal depression or pregnancy related anxiety however no differential methylation was observed related to maternal stress assessment64,65. Correspondingly our approach based on the identification of differential DNA methylation did not reveal a NR3C1 related DMR. However, we found a significant enrichment of DMRs overlapping with binding sites of NR3C1. This suggests that perturbations in cortisol mediated signaling might also occur independent of a direct effect of maternal stress on NR3C1 methylation in the child.
Perturbed pathways are intertwined
It has been accepted that perturbations in the mammalian stress response contribute to an aberrant immune response and may adversely affect lung development in prenatally exposed children13,66. We therefore focused our validation analysis on DMRs relating to genes connecting the stress response to downstream pathways possibly involved in immune regulation and lung development or function (Fig. 4).
Calcineurin/NFAT signaling is activated by GPCR-mediated intracellular calcium increase. In knockout mice it has been shown that calcineurin/NFAT signaling controls lung maturation prior to birth and deletion of the calcium binding subunit calcineurin b (PPP3R1) results in immaturity of the lung67. We observed hypermethylation and transcriptional repression for a variety of calcineurin/NFAT upstream regulators related to prenatal maternal stress suggesting an impaired activation of calcineurin/NFAT already during the prenatal period.
The GPCRs NMUR1 and -2 play a role in central and peripheral stress response. Binding of NMU to its receptors mediates intracellular calcium signaling68, which in T cells promotes synthesis and release of the in allergic inflammation involved Th2 cytokines IL-4, 5, and 639. Our observations linking maternal stress, NMUR1 methylation, and elevated IL-4, -5, and IL-6 cord blood levels support these findings.
The hypermethylated DMR in an enhancer of NMUR1 we identified in children of highly stressed mothers was confirmed in the entire LINA cohort. Although the observed methylation difference in the larger sample subset was rather small, this seems to be related to a much narrower distribution of methylation values observed by MassARRAY compared to WGBS. The around 5% higher methylation found in prenatally stressed children might be related to a deregulated subset of T cells producing Th2 cytokines. NMUR1 enhancer hypermethylation was accompanied by repressed transcription of NMUR1 in prenatally stressed children. This result is in accordance with data suggesting a decrease in NMUR1 expression in response to stress69,70. A negative feedback in stress activated receptor signaling is common71. Therefore it seems likely that hyperactivation of NMU mediated stress response perturbed NMUR1 methylation and transcription. When exposure to maternal stress is ceased, the methylation and transcription changes of NMUR1 subside together with the altered immune response (elevated IL-4, -5, -6) observed at birth.
Activation of NMUR1/2, coupled to G alpha q/11 (GNA11) and G alpha i72, can lead to activation of phospholipase C mediated increase in cytoplasmic calcium. Both GNA11 and PLCB4 “phospholipase C beta 4” showed a hypermethylated enhancer regions related to prenatal maternal stress. For GNA11 this was associated with decreased expression in both prenatally stressed and children with wheezing symptoms. Similar observations for the calcium channel coding CACNB4 gene further suggest an involvement of calcium signaling in impaired lung function.
Wnt-signaling plays a widely established role in lung organogenesis. In particular canonical Wnt-signaling is involved in cell fate decisions and differentiation of lung cells73. Although the role of non-canonical/WNT5A signaling upstream of calcineurin/NFAT in lung development is less clear, Wnt5a knockout mice are characterized by morphologically smaller lungs, thickened mesenchyme and a delayed alveolar development74,75. Albeit a variety of DMRs related to members of both Wnt-signaling pathways observed, our results point to calcium-dependent Wnt-signaling as related to the development of wheezing symptoms. We did not observe long-term epigenetic memory in the investigated regions, which were mostly ngDMRs. However epigenetic modifications upstream of calcineurin/NFAT signaling in the prenatal period might facilitate altered lung development contributing to the wheezing phenotype observed in children exposed to high levels of prenatal maternal stress.
Our approach revealed extensive alterations in the epigenome of mothers and children preferentially in enhancer region, which might contribute to the broad ramifications attributed to stress exposure. Although we report blood-derived DNA methylation changes, which may not always reflect methylation within other tissues.
Based on WGBS data from 10 mother-child pairs we derived a hypothesis how maternal stress in the prenatal period might be translated into impaired lung function in the child. We identified potentially involved signaling pathways and targets, which were subsequently validated in a larger sample set from the entire LINA cohort using targeted approaches. Since the focus of the present study was on prenatal stress and respiratory outcomes, pathways and targets involved in stress signaling and lung development were selected for further validation analyses thereby ignoring stress-related epigenetic modifications in pathways potentially linked to further outcomes, such as overweight development or adverse psychological conditions. Addressing all adverse outcomes described to occur in children exposed to maternal prenatal stress was beyond the scope of this study but certainly deserves further in depth analysis in follow-up studies.
Additional Information
How to cite this article: Trump, S. et al. Prenatal maternal stress and wheeze in children: novel insights into epigenetic regulation. Sci. Rep. 6, 28616; doi: 10.1038/srep28616 (2016).
Supplementary Material
Acknowledgments
We would like to thank Stephan Wolf (DKFZ Genomics and Proteomics Core Facility) for excellent support in whole genome bisulfite sequencing and expertise. Further, we are grateful to Marion Bähr and Monika Helf who provided support in MassARRAY validation. We cordially thank the participants of the LINA study as well as Beate Fink, Anne Hain, Livia Sztraka and Melanie Nowak for their excellent technical assistance and fieldwork. Whole-genome bisulfite sequencing data have been deposited at the European Genome-Phenome Archive under accession number EGAS00001000455. This work was supported by the German Cancer Research Center–Heidelberg Center for Personalized Oncology (DKFZ-HIPO) and the Helmholtz Initiative on Personalized Medicine (iMed).
Footnotes
Author Contributions S.T., M.Ba., D.W., L.T., G.H., M.S., M.E., D.K.W., C.P., C.L., M.v.B., M.Bo., R.J.W., R.E. and I.L. performed and/or coordinated experimental work. M.B., S.T., N.I., L.G., S.R., T.B., M.Ba., N.D., O.M., H.-D.E., C.H., G.H., M.S., Z.G. performed data analysis. M.Bo., S.R., G.H. and I.L. collected data and provided proband materials. S.T., M.B., Z.G., L.T., D.W., C.P., D.K.W., T.B., S.R., R.E. and I.L. prepared the initial manuscript and figures. R.W., M.Bo., C.H., C.P., R.E. and I.L. provided project leadership.
References
- Mathilda Chiu Y. H., Coull B. A., Cohen S., Wooley A. & Wright R. J. Prenatal and postnatal maternal stress and wheeze in urban children: effect of maternal sensitization. American Journal of Respiratory and Critical Care Medicine 186, 147–154, doi: 10.1164/rccm.201201-0162OC (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. J. Prenatal maternal stress and early caregiving experiences: implications for childhood asthma risk. Paediatric and Perinatal Epidemiology 21 Suppl 3, 8–14, doi: 10.1111/j.1365-3016.2007.00879.x (2007). [DOI] [PubMed] [Google Scholar]
- de Marco R. et al. Foetal exposure to maternal stressful events increases the risk of having asthma and atopic diseases in childhood. Pediatric Allergy and Immunology: Official Publication of the European Society of Pediatric Allergy and Immunology 23, 724–729, doi: 10.1111/j.1399-3038.2012.01346.x (2012). [DOI] [PubMed] [Google Scholar]
- Khashan A. S. et al. Prenatal stress and risk of asthma hospitalization in the offspring: a Swedish population-based study. Psychosomatic Medicine 74, 635–641, doi: 10.1097/PSY.0b013e31825ac5e7 (2012). [DOI] [PubMed] [Google Scholar]
- Peters J. L. et al. Prenatal negative life events increases cord blood IgE: interactions with dust mite allergen and maternal atopy. Allergy 67, 545–551, doi: 10.1111/j.1398-9995.2012.02791.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot E., Couret D. & Otten W. Prenatal stress, fetal imprinting and immunity. Brain, Behavior, and Immunity 22, 42–51, doi: 10.1016/j.bbi.2007.05.007 (2008). [DOI] [PubMed] [Google Scholar]
- Couret D., Jamin A., Kuntz-Simon G., Prunier A. & Merlot E. Maternal stress during late gestation has moderate but long-lasting effects on the immune system of the piglets. Veterinary Immunology and Immunopathology 131, 17–24, doi: 10.1016/j.vetimm.2009.03.003 (2009). [DOI] [PubMed] [Google Scholar]
- Kinkead R. et al. Neonatal maternal separation and early life programming of the hypoxic ventilatory response in rats. Respiratory Physiology & Neurobiology 149, 313–324, doi: 10.1016/j.resp.2005.04.014 (2005). [DOI] [PubMed] [Google Scholar]
- Kinkead R., Dupenloup L., Valois N. & Gulemetova R. Stress-induced attenuation of the hypercapnic ventilatory response in awake rats. Journal of Applied Physiology 90, 1729–1735 (2001). [DOI] [PubMed] [Google Scholar]
- Lin Y. C., Wen H. J., Lee Y. L. & Guo Y. L. Are maternal psychosocial factors associated with cord immunoglobulin E in addition to family atopic history and mother immunoglobulin E? Clinical and Experimental Allergy: Journal of the BRITISH Society for Allergy and Clinical Immunology 34, 548–554, doi: 10.1111/j.1365-2222.2004.1928.x (2004). [DOI] [PubMed] [Google Scholar]
- Holt P. G., Upham J. W. & Sly P. D. Contemporaneous maturation of immunologic and respiratory functions during early childhood: implications for development of asthma prevention strategies. The Journal of Allergy and Clinical Immunology 116, 16–24; quiz 25, doi: 10.1016/j.jaci.2005.04.017 (2005). [DOI] [PubMed] [Google Scholar]
- Prescott S. L. The development of respiratory inflammation in children. Paediatric Respiratory Reviews 7, 89–96, doi: 10.1016/j.prrv.2006.03.001 (2006). [DOI] [PubMed] [Google Scholar]
- Rosenberg S. L., Miller G. E., Brehm J. M. & Celedon J. C. Stress and asthma: Novel insights on genetic, epigenetic, and immunologic mechanisms. The Journal of Allergy and Clinical Immunology 134, 1009–1015, doi: 10.1016/j.jaci.2014.07.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz D. et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy 67, 380–389, doi: 10.1111/j.1398-9995.2011.02767.x (2012). [DOI] [PubMed] [Google Scholar]
- Martinez F. D., W. A. L., T. L. M., Holberg C. J., Halonen M. & Morgan W. J. Asthma and wheezing in the first six years of life. The Group Health Mediacl Associates. The New England Journal of Medicine 332, 133–138 (1995). [DOI] [PubMed] [Google Scholar]
- Herberth G. et al. Maternal immune status in pregnancy is related to offspring’s immune responses and atopy risk. Allergy 66, 1065–1074, doi: 10.1111/j.1398-9995.2011.02587.x (2011). [DOI] [PubMed] [Google Scholar]
- Fliege H. et al. The Perceived Stress Questionnaire (PSQ) reconsidered: validation and reference values from different clinical and healthy adult samples. Psychosomatic Medicine 67, 78–88, doi: 10.1097/01.psy.0000151491.80178.78 (2005). [DOI] [PubMed] [Google Scholar]
- Levenstein S. et al. Development of the Perceived Stress Questionnaire: a new tool for psychosomatic research. Journal of Psychosomatic Research 37, 19–32 (1993). [DOI] [PubMed] [Google Scholar]
- Rauste-von Wright M. & Frankenhaeuser M. Females’ emotionality as reflected in the excretion of the dopamine metabolite HVA during mental stress. Psychological Reports 64, 856–858, doi: 10.2466/pr0.1989.64.3.856 (1989). [DOI] [PubMed] [Google Scholar]
- Tuchman M., Ramnaraine M. L., Woods W. G. & Krivit W. Three years of experience with random urinary homovanillic and vanillylmandelic acid levels in the diagnosis of neuroblastoma. Pediatrics 79, 203–205 (1987). [PubMed] [Google Scholar]
- Remane D. et al. Validation of a multi-analyte HPLC-DAD method for determination of uric acid, creatinine, homovanillic acid, niacinamide, hippuric acid, indole-3-acetic acid and 2-methylhippuric acid in human urine. J Chromatogr B Analyt Technol Biomed Life Sci 998–999, 40–44, doi: 10.1016/j.jchromb.2015.06.021 (2015). [DOI] [PubMed] [Google Scholar]
- Junge K. M. et al. Increased vitamin D levels at birth and in early infancy increase offspring allergy risk-evidence for involvement of epigenetic mechanisms. The Journal of Allergy and Clinical Immunology, doi: 10.1016/j.jaci.2015.06.040 (2015). [DOI] [PubMed] [Google Scholar]
- Hansen K. D., Langmead B. & Irizarry R. A. BSmooth: from whole genome bisulfite sequencing reads to differentially methylated regions. Genome Biol 13, R83, doi: 10.1186/gb-2012-13-10-r83 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A. E. et al. Bump hunting to identify differentially methylated regions in epigenetic epidemiology studies. Int J Epidemiol 41, 200–209, doi: 10.1093/ije/dyr238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D. et al. Statistical methods for detecting differentially methylated loci and regions. Front Genet 5, 324, doi: 10.3389/fgene.2014.00324 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrich M. et al. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proceedings of the National Academy of Sciences of the United States of America 102, 15785–15790, doi: 10.1073/pnas.0507816102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck U. et al. Prenatal VOC exposure and redecoration are related to wheezing in early infancy. Environment International 73, 393–401, doi: 10.1016/j.envint.2014.08.013 (2014). [DOI] [PubMed] [Google Scholar]
- Hoeke H. et al. Monitoring of drug intake during pregnancy by questionnaires and LC-MS/MS drug urine screening: evaluation of both monitoring methods. Drug Testing and Analysis 7, 695–702, doi: 10.1002/dta.1767 (2015). [DOI] [PubMed] [Google Scholar]
- Gutierrez-Arcelus M. et al. Passive and active DNA methylation and the interplay with genetic variation in gene regulation. eLife 2, e00523, doi: 10.7554/eLife.00523 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Siegmund K. D., Laird P. W. & Berman B. P. Bis-SNP: combined DNA methylation and SNP calling for Bisulfite-seq data. Genome Biol 13, R61, doi: 10.1186/gb-2012-13-7-r61 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer T. et al. Environment-induced epigenetic reprogramming in genomic regulatory elements in smoking mothers and their children. Molecular Systems Biology 12, 861, doi: 10.15252/msb.20156520 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roadmap Epigenomics C. et al. Integrative analysis of 111 reference human epigenomes. Nature 518, 317–330, doi: 10.1038/nature14248 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium E. P. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74, doi: 10.1038/nature11247 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom K. R. et al. ENCODE data in the UCSC Genome Browser: year 5 update. Nucleic Acids Research 41, D56–63, doi: 10.1093/nar/gks1172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongracz J. E. & Stockley R. A. Wnt signalling in lung development and diseases. Respiratory Research 7, 15, doi: 10.1186/1465-9921-7-15 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan S. C. et al. Fetal calcium regulates branching morphogenesis in the developing human and mouse lung: involvement of voltage-gated calcium channels. PloS one 8, e80294, doi: 10.1371/journal.pone.0080294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. L. et al. Chronic administration of NMU into the paraventricular nucleus stimulates the HPA axis but does not influence food intake or body weight. Biochemical and Biophysical Research Communications 323, 65–71, doi: 10.1016/j.bbrc.2004.08.058 (2004). [DOI] [PubMed] [Google Scholar]
- Malendowicz L. K., Ziolkowska A. & Rucinski M. Neuromedins U and S involvement in the regulation of the hypothalamo-pituitary-adrenal axis. Frontiers in Endocrinology 3, 156, doi: 10.3389/fendo.2012.00156 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. N. et al. Neuromedin U elicits cytokine release in murine Th2-type T cell clone D10.G4.1. Journal of imMunology 173, 7230–7238 (2004). [DOI] [PubMed] [Google Scholar]
- Su Q. et al. Maternal Stress in Gestation: Birth Outcomes and Stress-Related Hormone Response of the Neonates. Pediatrics and Neonatology, doi: 10.1016/j.pedneo.2015.02.002 (2015). [DOI] [PubMed] [Google Scholar]
- Dancause K. N. et al. Prenatal stress due to a natural disaster predicts adiposity in childhood: the Iowa Flood Study. J Obes 2015, 570541, doi: 10.1155/2015/570541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney D., Miller A., Crowley D., Huang E. & Gerber E. Autism Prevalence Following Prenatal Exposure to Hurricanes and Tropical Storms in Louisiana. J Autism Dev Disord 38, 481–488, doi: 10.1007/s10803-007-0414-0 (2008). [DOI] [PubMed] [Google Scholar]
- Walder D. J. et al. Prenatal maternal stress predicts autism traits in 6(1/2) year-old children: Project Ice Storm. Psychiatry Research 219, 353–360, doi: 10.1016/j.psychres.2014.04.034 (2014). [DOI] [PubMed] [Google Scholar]
- Debnath M., Venkatasubramanian G. & Berk M. Fetal programming of schizophrenia: select mechanisms. Neuroscience and Biobehavioral reviews 49, 90–104, doi: 10.1016/j.neubiorev.2014.12.003 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V., O’Connor T. G. & O’Donnell K. Prenatal stress and the programming of the HPA axis. Neuroscience and Biobehavioral reviews 35, 17–22, doi: 10.1016/j.neubiorev.2009.11.008 (2010). [DOI] [PubMed] [Google Scholar]
- Cottrell E. C. & Seckl J. R. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3, 19, doi: 10.3389/neuro.08.019.2009 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain L. et al. Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high-fat-sucrose diet in prenatally stressed female rats. Neuroendocrinology 96, 249–260, doi: 10.1159/000341684 (2012). [DOI] [PubMed] [Google Scholar]
- Campbell J. E., Peckett A. J., D’Souza A. M., Hawke T. J. & Riddell M. C. Adipogenic and lipolytic effects of chronic glucocorticoid exposure. American Journal of Physiology. Cell Physiology 300, C198–209, doi: 10.1152/ajpcell.00045.2010 (2011). [DOI] [PubMed] [Google Scholar]
- Sternthal M. J., Coull B. A., Chiu Y. H., Cohen S. & Wright R. J. Associations among maternal childhood socioeconomic status, cord blood IgE levels, and repeated wheeze in urban children. The Journal of Allergy and Clinical Immunology 128, 337–345 e331, doi: 10.1016/j.jaci.2011.05.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu Y. H. et al. Effects of prenatal community violence and ambient air pollution on childhood wheeze in an urban population. The Journal of Allergy and Clinical Immunology 133, 713–722 e714, doi: 10.1016/j.jaci.2013.09.023 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. J. et al. Prenatal maternal stress and cord blood innate and adaptive cytokine responses in an inner-city cohort. American Journal of Respiratory and Critical Care Medicine 182, 25–33, doi: 10.1164/rccm.200904-0637OC (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange N. E. et al. Parental psychosocial stress and asthma morbidity in Puerto Rican twins. The Journal of Allergy and Clinical Immunology 127, 734–740 e731–737, doi: 10.1016/j.jaci.2010.11.010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agirre X. et al. Whole-epigenome analysis in multiple myeloma reveals DNA hypermethylation of B cell-specific enhancers. Genome Research 25, 478–487, doi: 10.1101/gr.180240.114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone A. et al. DNA methylation of oestrogen-regulated enhancers defines endocrine sensitivity in breast cancer. Nature Communications 6, 7758, doi: 10.1038/ncomms8758 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn H. et al. Distinct DNA methylomes of newborns and centenarians. Proceedings of the National Academy of Sciences of the United States of America 109, 10522–10527, doi: 10.1073/pnas.1120658109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnefeld M. & Lyko F. The aging epigenome: DNA methylation from the cradle to the grave. Genome Biology 13, 165, doi: 10.1186/gb4033 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedotal A. & Richards L. J. Wiring the brain: the biology of neuronal guidance. Cold Spring Harbor Perspectives in Biology 2, a001917, doi: 10.1101/cshperspect.a001917 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl J. R. & Meaney M. J. Glucocorticoid programming. Annals of the New York Academy of Sciences 1032, 63–84, doi: 10.1196/annals.1314.006 (2004). [DOI] [PubMed] [Google Scholar]
- Turecki G. & Meaney M. J. Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biol Psychiatry, doi: 10.1016/j.biopsych.2014.11.022 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma-Gudiel H., Cordova-Palomera A., Leza J. C. & Fananas L. Glucocorticoid receptor gene (NR3C1) methylation processes as mediators of early adversity in stress-related disorders causality: A critical review. Neuroscience and Biobehavioral Reviews 55, 520–535, doi: 10.1016/j.neubiorev.2015.05.016 (2015). [DOI] [PubMed] [Google Scholar]
- van der Knaap L. J., Oldehinkel A. J., Verhulst F. C., van Oort F. V. & Riese H. Glucocorticoid receptor gene methylation and HPA-axis regulation in adolescents. The TRAILS study. Psychoneuroendocrinology 58, 46–50, doi: 10.1016/j.psyneuen.2015.04.012 (2015). [DOI] [PubMed] [Google Scholar]
- Perroud N. et al. The Tutsi genocide and transgenerational transmission of maternal stress: epigenetics and biology of the HPA axis. World J Biol Psychiatry 15, 334–345, doi: 10.3109/15622975.2013.866693 (2014). [DOI] [PubMed] [Google Scholar]
- Yehuda R. et al. Lower methylation of glucocorticoid receptor gene promoter 1F in peripheral blood of veterans with posttraumatic stress disorder. Biol Psychiatry 77, 356–364, doi: 10.1016/j.biopsych.2014.02.006 (2015). [DOI] [PubMed] [Google Scholar]
- Oberlander T. F. et al. Prenatal exposure to maternal depression, neonatal methylation of human. Epigenetics: Official Journal of the DNA Methylation Society 3, 97–106 (2008). [DOI] [PubMed] [Google Scholar]
- Hompes T. et al. Investigating the influence of maternal cortisol and emotional state during pregnancy on the DNA methylation status of the glucocorticoid receptor gene (NR3C1) promoter region in cord blood. Journal of Psychiatric Research 47, 880–891, doi: 10.1016/j.jpsychires.2013.03.009 (2013). [DOI] [PubMed] [Google Scholar]
- Wright R. J. Perinatal stress and early life programming of lung structure and function. Biological Psychology 84, 46–56, doi: 10.1016/j.biopsycho.2010.01.007 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave V. et al. Calcineurin/Nfat signaling is required for perinatal lung maturation and function. The Journal of Clinical Investigation 116, 2597–2609, doi: 10.1172/JCI27331 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii R. et al. Identification of neuromedin U as the cognate ligand of the orphan G protein-coupled receptor FM-3. The Journal of Biological Chemistry 275, 21068–21074, doi: 10.1074/jbc.M001546200 (2000). [DOI] [PubMed] [Google Scholar]
- Moriyama M. et al. The neuropeptide neuromedin U promotes IL-6 production from macrophages and endotoxin shock. Biochemical and Biophysical Research Communications 341, 1149–1154, doi: 10.1016/j.bbrc.2006.01.075 (2006). [DOI] [PubMed] [Google Scholar]
- Hanada R. et al. A role for neuromedin U in stress response. Biochemical and Biophysical Research Communications 289, 225–228, doi: 10.1006/bbrc.2001.5945 (2001). [DOI] [PubMed] [Google Scholar]
- Klenerova V. & Sida P. Changes in beta-adrenergic receptors in the neurohypophysis and intermediate lobe of rat hypophysis exposed to stress. Physiological research/Academia Scientiarum Bohemoslovaca 43, 289–292 (1994). [PubMed] [Google Scholar]
- Brighton P. J., Szekeres P. G., Wise A. & Willars G. B. Signaling and ligand binding by recombinant neuromedin U receptors: evidence for dual coupling to Galphaq/11 and Galphai and an irreversible ligand-receptor interaction. Mol Pharmacol 66, 1544–1556, doi: 10.1124/mol.104.002337 (2004). [DOI] [PubMed] [Google Scholar]
- Mucenski M. L. et al. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. The Journal of Biological Chemistry 278, 40231–40238, doi: 10.1074/jbc.M305892200 (2003). [DOI] [PubMed] [Google Scholar]
- Li C., Xiao J., Hormi K., Borok Z. & Minoo P. Wnt5a participates in distal lung morphogenesis. Developmental Biology 248, 68–81 (2002). [DOI] [PubMed] [Google Scholar]
- Li C. et al. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol 287, 86–97, doi: 10.1016/j.ydbio.2005.08.035 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.