Abstract
Sulfur participates in many important mechanisms and pathways of plant development. The most common source of sulfur in soil –SO42−– is absorbed into root tissue and distributed into aerial part through vasculature system, where it is reduced into sulfite and finally sulfide within the subcellular organs such as chloroplasts and mitochondria and used for cysteine and methionine biosynthesis. MicroRNAs are involved in many regulation pathways by repressing the expression of their target genes. MiR395 family in Arabidopsis thaliana has been reported to be an important regulator involved in sulfate transport and assimilation, and a high-affinity sulphate transporter and three ATP sulfurylases (ATPS) were the target genes of AthmiR395 (Arabidopsis thaliana miR395). We have cloned a miR395 gene from rice (Oryza sativa) and studied its function in plant nutritional response. Our results indicated that in rice, transcript level of OsamiR395 (Oryza sativa miR395) increased under sulfate deficiency conditions, and the two predicted target genes of miR395 were down-regulated under the same conditions. Overexpression of OsamiR395h in tobacco impaired its sulfate homeostasis, and sulfate distribution was also slightly impacted among leaves of different ages. One sulfate transporter (SULTR) gene NtaSULTR2 was identified to be the target of miR395 in Nicotiana tobacum, which belongs to low affinity sulfate transporter group. Both miR395 and NtaSULTR2 respond to sulfate starvation in tobacco.
As a rudimental and essential element, sulfur is one of the six macronutrients required for plant growth and participates in many important physiological and biochemical processes. In nature, sulfur exists in both inorganic and organic forms, and sulfate (SO42−) is the most common inorganic source of sulfur plants acquire from soil.
The sulfate absorption and assimilation pathway in plants is a complex system. In the very beginning, sulfate is absorbed into root tissue. Except for a small amount of sulfate stored in vacuole of root cells, the majority of them are distributed into aerial part through vasculature system. Upon transfer into subcellular organs such as chloroplasts and mitochondria in cells of aerial part, the sulfate is reduced into sulfite, then sulfide used for the synthesis of cysteine and methionine, two amino acids that play a pivotal role in sulfate assimilation pathway1, and essential for supporting many important redox reactions in plants. The reduced form of the cysteine could function as an electron donor and its oxidized form could act as an electron acceptor.
Given the important role sulfur plays in plant growth and development, its deficiency (−S) would cause severe problems to plants, resulting in decreased plant yields and quality2. To genetically improve plant sulfate uptake and utilization under −S conditions, it is essential to fully understand the functions of the genes encoding sulfate transporters and other important components involved in sulfate assimilation pathways2.
Over the course of the past 20 years, essential genes involved in sulfate uptake, distribution and assimilation pathways have been identified and well-studied in different plant species. Shst 1, Shst 2 and Shst 3 were the first sulfate transporter genes cloned from Stylosanthes hamate responsible for initial sulfate uptake and internal transport3. In Arabidopsis, since the cloning of the first sulfate transporters, AST56 and AST68 two decades ago4, at least 12 Arabidopsis sulfate transporters belonging to five different groups have been identified5. These include two high-affinity sulfate transporters SULTR1;1 and SULTR1;2 responsible for uptake of sulfate from soil6,7 low-affinity sulfate transporters SULTR2;1 and SULTR2;2 responsible for internal transport of sulfate from root to shoot7, SULTR3;5, the function partner of the SULTR2;1 that facilitates the influx of sulfate8, and SULTR4;1 and SULTR4;2 involved in distribution of sulfate between Arabidopsis vacuoles and symplastic9. The ORYsa;Sultr1;1 and ORYsa;Sultr4;1 are the first two sulfate transporters cloned from rice in early 2000 s10, followed by the identification of additional 12 sulfate transporters11.
ATP sulfurylase (ATPS) catalyzes the synthesis of the essential metabolic intermediate, adenosine 5′-phosphosulfate (APS), and this step is the branch point of the sulfate assimilation pathway followed by the synthesis subpathways of either cysteine or other sulfated compounds. ATPS has been extensively studied for the past decade because of its important role in the sulfate assimilation pathway12,13,14,15. SULTR or ATPS gene families would be the ideal targets for genetic modification to increase the efficiency of plant sulfate uptake and assimilation under −S conditions. It is therefore important to understand how they are regulated in plants.
MicroRNAs (miRNAs) are short non-coding RNAs with only 20–24 nt, regulating many metabolisms in the post-transcriptional level by repressing translation of their target genes. In plants, with the help of RISC (RNA inducing silence complex), mature miRNA could form near-perfect pairs with its complementary sequences of the mRNA target, followed by cleavage of the base-pairing region and degradation of the transcripts16. Among thousands of identified miRNAs, miR395 family in Arabidopsis has previously been reported to be an important regulator involved in sulfate transport and assimilation17,18,19. The targets of AthmiR395 (Arabidopsis thaliana miR395) are sulfate transporter genes and ATPS, such as high-affinity sulfate transporter gene, AthSULTR2:1 and ATP sulfurylase genes, AthATPS1, 3, and 419,20,21,22.
The divergence of monocot and dicot plants occurred at 200 million years ago23, but the miRNA-mediated gene regulation mechanism has an even longer history, which is more than 425 million years24. These facts suggest that monocot and dicot plants should have a similar miRNA-mediated gene regulation mechanism and conserved miRNA families sharing the same gene ancestors and regulating the same biological events. Research for the past two decades has led to the identification of 21 miRNA families including many well-studied ones such as miR156 and miR399 that seem to be highly conserved between monocots and dicots25. MiR395 is also on the list, but experimental support is still lacking.
Sequences of mature miR395 are highly conserved between model plant, Arabidopsis and crop species. Understanding the role miR395 plays in important food crops would allow development of novel biotechnology approaches to genetically engineer these plants for ameliorated nutrient uptake and utilization, improving plant growth, yield and agricultural productivity. We have cloned pri-OsamiR395h (Oryza sativa miR395) from rice (Oryza sativa) and studied its function in plant nutritional response. Our results showed that transcript level of OsamiR395 increased under −S condition accompanied with down regulation of its two predicted target genes. Overexpression of pri-OsamiR395h in tobacco (Nicotiana tobacum) impaired its sulfate homeostasis. Sulfate distribution was also slightly impacted between leaves of different ages in transgenic plants. One potential target gene of miR395 named NtaSULTR2 was identified in tobacco (Nicotiana tobacum), which encodes a sulfate transporter. The expression of both endogenous NtamiR395 (Nicotiana tobacum miR395) and NtaSULTR2 was significantly induced under low sulfate conditions in tobacco leaf tissues, but the expression level of NtaSULTR2 was inversely correlated to that of NtamiR395 under different sulfate conditions in root tissues. These results indicate that OsamiR395 responds to −S by inducing degradation of two target genes, and pri-OsamiR395h can function in dicot plant tobacco and impact its sulfate transportation and distribution. As the first target gene of miR395 identified in tobacco, NtaSULTR2 encodes a sulfate transporter belonging to the low-affinity group.
Results
Sulfate regulates the expression of OsamiR395 and its target genes
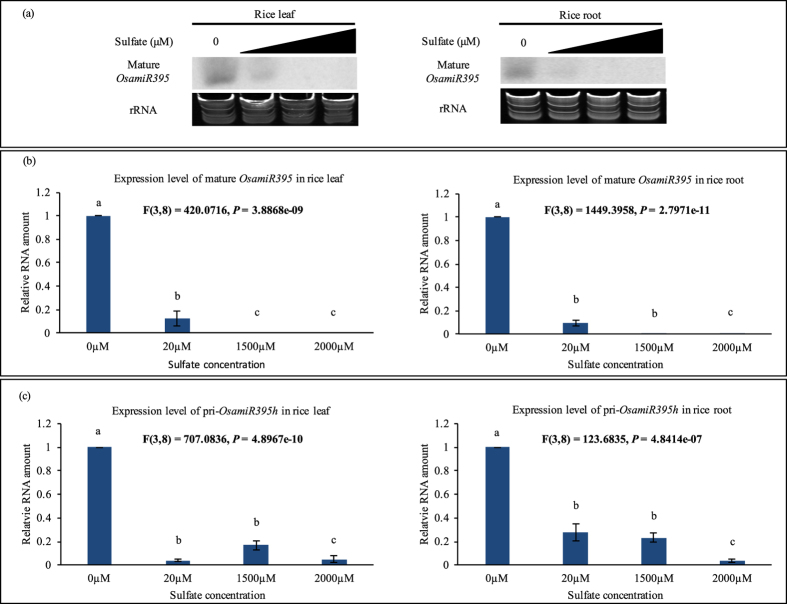
According to previous research and miRNA database (http://mirbase.org), 24 family members belonging to four clusters comprise OsamiR395 family26. The sequence of mature OsamiR395 is highly conserved while the pre-microRNA sequences are divergent. It has previously been demonstrated in Arabidopsis that mature AthmiR395 transcript accumulates under sulfur-limited conditions18,19,27. To investigate whether OsamiR395 also responds to low sulfate conditions as its counterpart in Arabidopsis, transcript level of OsamiR395 in two weeks old rice plants grown in N6 solid medium supplemented with different concentrations of sulfate was analyzed. Both northern blotting and stem-loop RT-PCR results showed that the transcripts of mature OsamiR395 accumulated under low sulfate conditions (0 and 20 μM SO42−), but declined significantly under sulfate-adequate conditions (1500 and 2000 μM SO42, Fig. 1a,b).
Figure 1. Sulfate deficiency induces accumulation of OsamiR395 in rice.
(a) Small RNA northern blotting analysis of mature OsamiR395 under different sulfate concentrations. Total RNA samples were prepared from leaf and root tissues of two weeks old rice grown in N6 medium with 0, 20, 1500 or 2000 μM (NH4+)2SO4 and used for small RNA northern blotting analysis. Antisense oligonucleotides of OsamiR395 was labeled with γ-[32P]ATP and used as probe to detect the transcript level of mature OsamiR395. rRNA was used as a loading control. (b) Stem-loop real-time PCR analysis of mature OsamiR395 under different sulfate concentrations. Total RNA samples were prepared as in (a) and used for stem-loop real-time PCR analysis. OsaSIZ1 was used as a reference gene. Data are presented as means of three technique replicates, error bars represent SD (n = 3). (c) Real-time PCR analysis of rice pri-OsamiR395h under different sulfate concentrations. Total RNA samples were prepared as in (a) and used for real-time PCR analysis. OsaSIZ1 was used as a reference gene. Data are presented as means of three technique replicates, error bars represent SD (n = 3). The statistically significant difference between groups was determined by one-way ANOVA (F(dfbetween, dfwithin) = F ration, p = p-value, where df = degrees of freedom). Means not sharing the same letter are statistically significantly different (P < 0.05).
In plant nucleus, miRNA gene is first transcribed into a long pri-miRNA, which is then processed into pre-miRNA and finally mature miRNA that is later translocated by HASTY into cytoplasm and induces the degradation of its target gene(s). To further understand whether OsamiR395 is regulated at the transcription level or post-transcription level, real-time PCR experiment was conducted to investigate the transcript level of pri-OsamiR395h in two weeks old rice plants grown in N6 solid medium supplemented with 0, 20, 1500 or 2000 μM SO42−. Real-time PCR results showed that excess sulfate could repress the accumulation of pri-OsamiR395h transcript (Fig. 1c). Conversely, the transcription level of pri-OsamiR395h increased significantly under sulfate deficient conditions (0 and 20 μM SO42−, Fig. 1c). Transcript levels of pri- and mature OsamiR395 exhibit the same trend under sulfate starvation stress, indicating that OsamiR395 expression is transcriptionally regulated by sulfate. Sulfate starvation stress induces the expression of pri-OsamiR395h, leading to the production of more mature OsamiR395 transcripts.
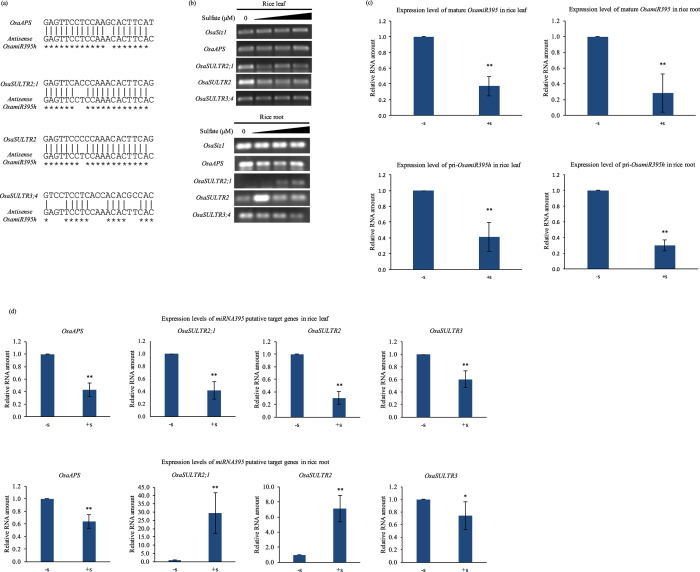
Computational analysis of the rice genome sequences leads to the identification of four putative targets of OsamiR395, including one ATPS and three sulfate transporter genes, OsaSULTR2;1, OsaSULTR2 and OsaSULTR3;4 (Fig. 2a)17,27. RT-PCR results indicated that OsaATPS did not exhibit any responses in both roots and leaves under −S stress. OsaSULTR3;4 did not respond to sulfate treatment in leaves either, but was down-regulated in roots with the increasing sulfate concentrations, exhibiting similar expression pattern as OsamiR395 (Fig. 2b). OsaSULTR2;1 and OsaSULTR2 genes were both down-regulated in leaves with the increasing sulfate concentrations (Fig. 2b), similar to the expression pattern of OsmiR395 in response to sulfate treatment (Fig. 1). On the contrary, they were both up-regulated in roots in response to increasing sulfate concentrations (Fig. 2b). It should be noted that OsaSULTR2 exhibited the highest induction under 20 μM sulfate, suggesting that other regulation machineries may also participate in the regulation of the OsaSULTR2 gene under this particular condition. These results support the hypothesis that OsaSULTR2;1 and OsaSULTR2 are the putative target genes of, and regulated by OsamiR395 in rice roots. In rice leaves, however, OsamiR395-mediated transcript cleavage of the OsaSULTR2;1 and OsaSULTR2 genes may not be able to take place due to their non-overlapping tissue-specific expression. Instead, there may exist some other mechanisms regulating the expression of OsaSULTR2;1 and OsaSULTR2. This is also likely the case for OsaSULTR3;4 in roots. Similar phenomena have previously been observed in Arabidopsis18,19. It should be noted that there are multiple mismatches in the OsamiR395 target sequence of the OsaSULTR3;4 (Fig. 2a). This raises the question of whether or not OsaSULTR3;4 is indeed the true target of OsamiR395.
Figure 2. Predicted target OsaSULTR1 and OsaSULTR2 exhibit opposite expression patterns to that of the OsamiR395 in rice root.
(a) Target sites of the four putative OsamiR395 target genes in rice. The target sites were compared with the complementary sequence of mature OsamiR395h. Asterisks indicate the identical sequences. (b) RT-PCR analysis of expression levels of the OsamiR395 putative targets. Total RNA samples used for RT-PCR were extracted from leaf and root tissues of two weeks old rice grown in N6 medium with 0, 20, 1500 or 2000 μM (NH4+)2SO4 and used for RT-PCR analysis. OsaSIZ1 was used as a reference gene. Experiment was repeated three times. (c) Stem-loop real-time RT-PCR analysis of mature OsamiR395 and real-time RT-PCR analysis of pri-OsamiR395h. Total RNA samples were prepared from leaf and root tissues of two weeks old rice grown in regular N6 medium (+S) or N6 medium without SO4+ (−S) and used for RT-PCR analysis. OsaSIZ1 was used as a reference gene. (d) Real-time RT-PCR analysis was also conducted to determine the expression levels of the OsamiR395 putative targets in rice leaves and roots. Total RNA samples were prepared as in (c) and used for real-time RT-PCR analysis. OsaSIZ1 was used as a reference gene. For (c,d), data are presented as means of two independent biological replicates and three technical replicates, error bars represent SD (n = 6). Asterisks indicate the significant differences between expression levels under −S and +S conditions. P < 0.05 is marked as*, P < 0.01 is marked as**.
To confirm the results of semi-quantitative RT-PCR, real-time RT-PCR was conducted to determine the expression levels of OsamiR395 and its putative targets in rice under −S condition (N6 medium without sulfate) and +S condition (regular N6 medium). Real-time PCR results were consistent with that of the semi-quantitative RT-PCR. In both leaves and roots, pri- and mature OsamiR395 were up-regulated under −S condition (Fig. 2c). Among the four putative target genes, only OsaSULTR2;1 and OsaSULTR2 were significantly down-regulated in rice roots under −S condition, exhibiting opposite trend of expression to OsamiR395 (Fig. 2d), in agreement with the results obtained by semi-quantitative RT-PCR and supporting the notion that OsaSULTR2;1 and OsaSULTR2 are the putative targets of OsamiR395 in rice roots.
Expression of the OsamiR395 and its target genes is spatiotemporally regulated
Besides the response of OsamiR395 and its targets to sulfate starvation stress, we also investigated the expression patterns of OsamiR395 and its target genes in different developmental stages and tissues. To this end, we particularly focused on the primary miRNA level for one of the rice OsamiR395 genes, OsamiR395h and the expression of its putative target genes in both roots and leaves at different developmental stages under normal growth conditions. The RT-PCR results showed that the expression of pri-OsamiR395h was strongly induced only in the roots of the four weeks old plants, but otherwise remained very low in both roots and leaves in any other developmental stages (Fig. 3).
Figure 3. Expression level of pri-OsamiR395h and its target genes in rice leaf and root tissues at different developmental stages.
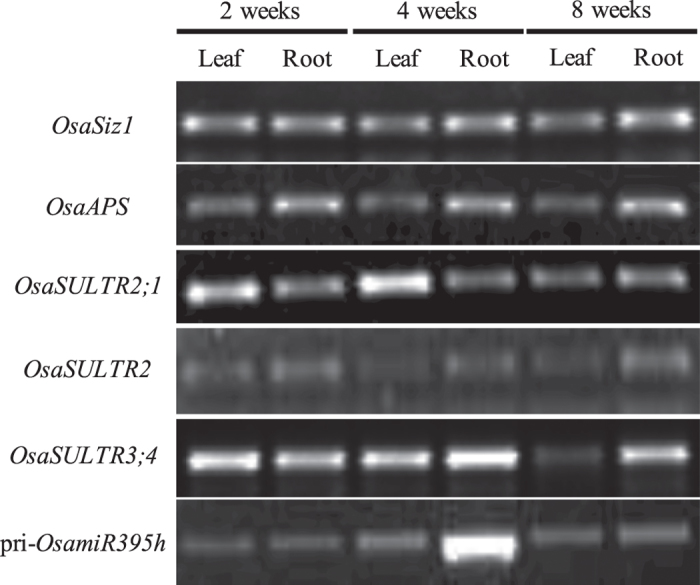
Total RNA samples were prepared from leaf and root tissues of rice harvested at indicated time points and used for RT-PCR analysis. OsaSIZ1 was used as a reference gene. Experiment was repeated three times.
The expression of the ATPS again was quite stable in both tissues throughout the rice development, but an elevated expression level in roots was observed compared to that in leaves (Fig. 3). The expression levels of the three sulfate transporter genes were variable, but none of them was inversely correlated with that of the OsamiR395h (Fig. 3).
Heterologous expression of pri-OsamiR395h in Nicotiana tabacum
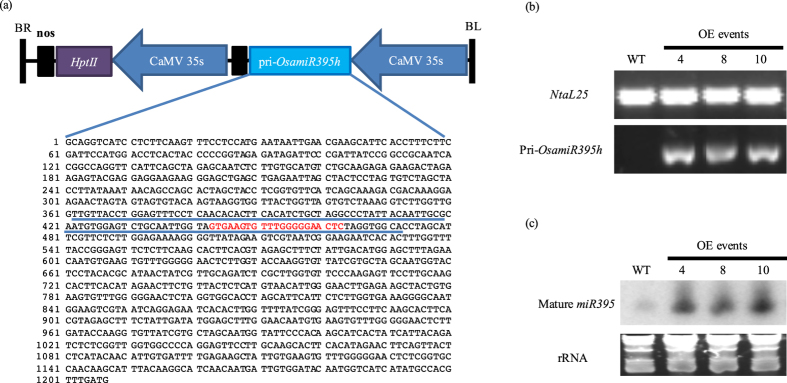
To further study the role OsamiR395 plays in sulfate transportation and distribution, we generated a chimeric DNA construct containing the pri-OsamiR395h sequence driven by the CaMV35S promoter (Fig. 4a). This construct was then introduced into tobacco (Nicotiana tabacum) to produce a total of 10 independent transgenic events. RT-PCR analysis suggested rice pri-OsamiR395h was successfully expressed in tobacco (Fig. 4b), and small RNA northern blotting result suggested rice pri-OsamiR395h was successfully processed into mature miRNA (Fig. 4c). The detection of tobacco endogenous mature NtamiR395 in northern blotting indicated that mature NtamiR395 shares a highly conserved sequence with its rice homolog. Three independent transgenic events were selected for further analysis.
Figure 4. Heterologous expression of pri-OsamiR395h in Nicotiana tabacum.
(a) The Schematic diagram of rice pri-OsamiR395h overexpression construct. Rice pri-OsamiR395h sequence containing stem-loop structure of OsamiR395h was cloned from rice genomic DNA and put under the control of the CaMV35S promoter. The hptII gene driven by CaMV35S promoter was used as selectable maker. The pre-OsamiR395h sequence was underlined. Sequence emphasized with red color indicates the mature miR395h. LB, Left border; RB, right border. (b) RT-PCR analysis of pri-OsamiR395h expression in wild type and three transgenic tobacco lines. Total RNA samples were prepared from two weeks old wild type and transgenic tobacco plants grown in MS medium. NtaL25 was used as reference gene. (c) Small RNA northern blotting analysis of mature miR395 transcripts in wild type and three transgenic tobacco lines. Total RNA samples were prepared from two weeks old wild type and transgenic tobacco plants grown in MS medium. rRNA was used as loading control. WT: wild type plant. OE: overexpression line.
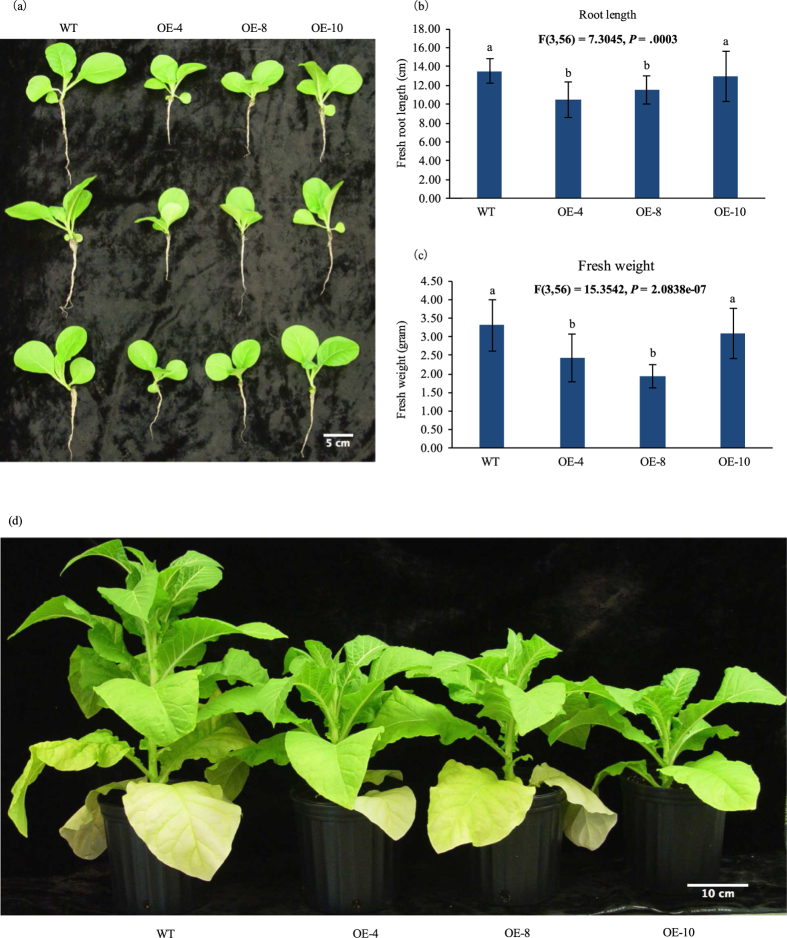
Overexpression of the rice pri-OsamiR395h impairs sulfate homeostasis and leads to retarded plant growth in transgenic tobacco
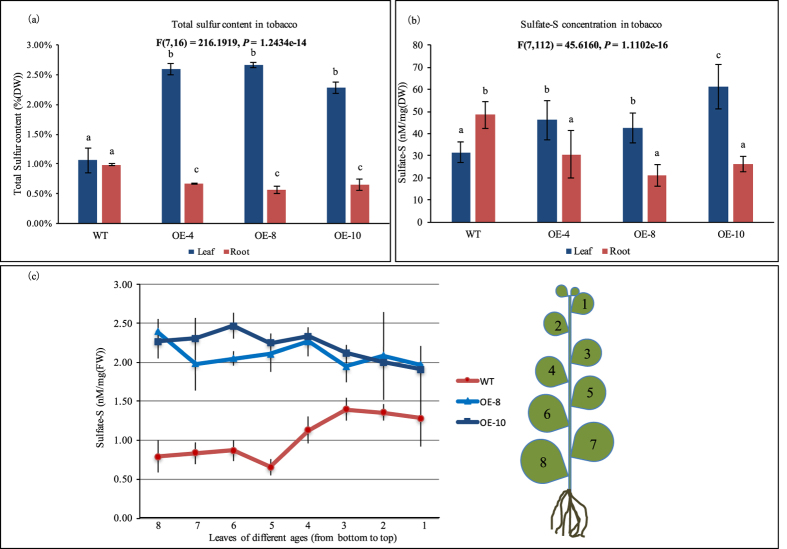
It has previously been shown that overexpression of AthmiR395 in Arabidopsis impairs its sulfate distribution and assimilation19. To evaluate the impact of the OsamiR395 in tobacco sulfate metabolism and plant development, we first measured the total sulfur contents in transgenic tobacco plants and wild type (WT) controls. Not surprisingly, the total leaf sulfur content of all the transgenic lines was 2.16 to 2.50 times higher than that in WT controls. On the contrary, the root sulfur content in transgenic lines was 32% to 42% less than that in WT controls (Fig. 5a).
Figure 5. Overexpression of pri-OsamiR395h impacts tobacco sulfate transportation and distribution.
(a) Statistical analysis of total sulfur in leaf and root tissues. Samples were harvested from four weeks old wild type and three transgenic tobacco lines. Data are presented as means of three biological replicates contains mixed samples from five biological replications, error bars represent SD (n = 3). (b) Statistical analysis of sulfate-S concentrations in leaf and root tissues. Samples were harvested from four weeks old wild type plants and three transgenic tobacco lines. Data are presented as means of fifteen biological replicates, error bars represent SD (n = 15). The statistically significant difference between groups was determined by one-way ANOVA (F(dfbetween, dfwithin) = F ration, p = p-value, where df = degrees of freedom). Means not sharing the same letter are statistically significantly different (P < 0.05). (c) Statistical analysis of sulfate concentration in tobacco leaves of different ages. Leaves of 12 weeks old wild type and three transgenic tobacco lines were harvested in the positions as indicated in the figure. Data shown are an average of three biological replicates, error bars represent SD (n = 3). DW: dry weight. FW: fresh weight. WT: wild type.
Next, we determined the sulfate-S (sulfate-sulfur) concentration in WT and transgenic plants. Again, the difference in sulfate-S concentrations between transgenics and WT controls was similar to that of the total sulfur contents. In transgenic leaf tissues, the sulfate-S concentration was 1.35 to 1.96 times higher than that in WT leaves, whereas in roots, transgenics had 38% to 57% less sulfate than WT controls (Fig. 5b). This result indicated that the high-level of miR395 accumulation in transgenic plants impacts the uptake and transportation of sulfur and sulfate.
Similar to a previous report in Arabidopsis that overexpression of AthmiR395 represses the expression of sulfate transporter gene AthSULTR2;1 and causes impaired sulfate distributions between leaves of different ages19, we also observed that leaf sulfate distribution patterns are different between transgenic tobacco plants and WT controls (Fig. 5c). Because sulfate or sulfur compounds could be transported from old to young leaves under normal or sulfate-adequate conditions28, sulfate accumulation in young leaves should be higher than that in old ones as observed in WT control plants (Fig. 5c). Contrary to this, transgenic tobacco plants accumulate fewer sulfates in younger leaves than in older ones (Fig. 5c), indicating that sulfate delivery pathway is impaired in transgenics, which is most likely one of the consequences caused by repressed expression of sulfate transporter genes. Furthermore, compared with WT controls, transgenic tobacco exhibited retarded growth (Fig. 6a,d). As shown in Fig. 6b,c, one-month-old transgenic plants displayed shorter root and less fresh weight than wild type controls, a similar phenotype observed in transgenic Arabidopsis overexpressing AthmiR39519. The slow-growth phenotype of transgenic plants suggests that the expression of ATPS may also have been strongly repressed in transgenics, resulting in interrupted sulfate assimilation pathway and consequently retardation in plant growth because of the shortage of cysteine and other sulfate metabolic products.
Figure 6. Overexpression of pri-OsamiR395h leads to retarded growth of transgenic tobacco.
Wild type and transgenic tobacco were grown in soil under 16 h light/8 h dark in greenhouse. Photos were taken (a) four weeks and (d) seven weeks after seed germination. Representative plants were shown. (b) Root length and (c) fresh weight of wild type and transgenic tobacco were measured. Data are presented as means of fifteen biological replicates, error bars represent SD (n = 15). The statistically significant difference between groups was determined by one-way ANOVA (F(dfbetween, dfwithin) = F ration, p = p-value, where df = degrees of freedom). Means not sharing the same letter are statistically significantly different (P < 0.05). WT: wild type plant. OE: overexpression line.
Identification of miR395 target gene in tobacco
To understand how the excess miR395 impacts tobacco sulfate homeostasis at the molecular level, we sought to identify putative new target genes of miR395 using two approaches29. We first used the DNA sequences of the Arabidopsis SULTR2;1 and ATPS genes to blastn against the Nicotiana tabacum EST sequences. All the DNA sequences with high similarity (identity of more than 70%) were used to do alignment with complementary sequence of the mature OsamiR395h. The following criteria were used to determine the predicted target sequences with minor modifications: (1) No more than four mismatches between OsamiR395h and its predicted target genes; (2) No more than two constitutive mismatches between OsamiR395h and its predicted target genes; (3) No mismatches between position 10 and 11; (4) No gaps between OsamiR395h and its predicted target genes29. Besides, we also designed primers based on the AthmiR395 target genes (AthSULTR2;1 and AthATPS1, 3, 4) to amplify and identify the putative homologous genes in tobacco.
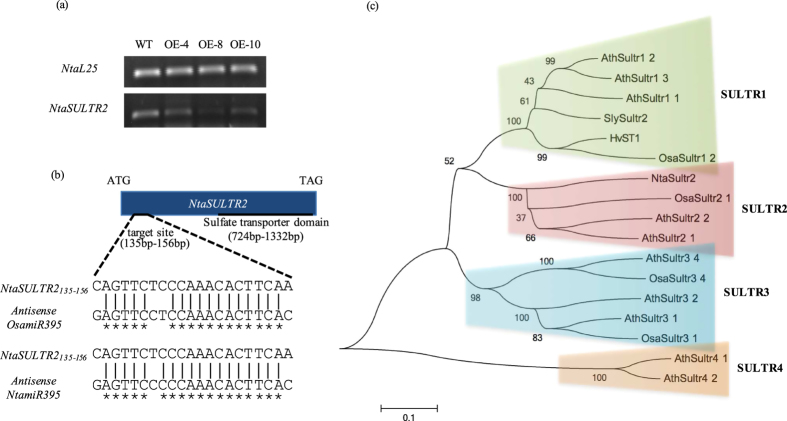
Using these approaches, we identified a novel gene named NtaSUTLR2 to be a putative target of OsamiR395h (Fig. 7). Semi-quantitative RT-PCR analysis revealed that NtaSULTR2 was significantly down-regulated in transgenic tobacco (Fig. 7a). We cloned the full-length cDNA sequence of NtaSULTR2 using RACE (Rapid Amplification of cDNA Ends) method, and identified the target site of miR395 that is located between 135 bp and 156 bp of its coding region. There are four mismatches and three mismatches between NtaSULTR2 target sequence and mature OsamiR395 and NtamiR395, separately (Fig. 7b), indicating that NtaSUTLR2 should be efficiently regulated by miR395 because of their near perfect complementary sequence.
Figure 7. Identification of a sulfate transporter gene, NtaSULTR2, the target of miR395 in tobacco.
(a) RT-PCR analysis of NtaSULTR2 expression in tobacco. Total RNA samples were prepared from two weeks old wild type and transgenic tobacco and used for RT-PCR analysis. NtaL25 was used as a reference gene. Experiment was repeated three times. (b) General structure of tobacco gene NtSULTR2. NtaSULTR2 with a length of 1335 bp contains a sulfate transporter domain between 724 bp to 1332 bp, and a miR395 target site between 135 bp to 156 bp. The target site was compared with the complementary sequence of mature OsamIR395h and NtamiR395. Asterisks indicate the identical sequences. (c) phylogenetic analysis of NtaSULTR2 protein. Protein sequences of NtaSULTR2 and 16 sulfate transporters of rice and Arabidopsis were used to establish phylogenetic tree with MEGA6. In this phylogenetic tree, NtaSULTR2 protein is classified into the second group of sulfate transporter subfamily together with AthSULTR2;1, AthSULTR2;2 and OsaSULTR2;1.
We further characterized NtaSULTR2 by generating a phylogenetic tree using protein sequence of NtaSULTR2 and other sixteen well-studied sulfate transporters from rice and Arabidopsis using MEGA6. In this phylogenetic tree, NtaSULTR2 protein is classified into the second group of sulfate transporter subfamily together with AthSULTR2;1, AthSULTR2;2 and OsaSULTR2;1 proteins (Fig. 7c). The three sulfate transporters from Arabidopsis and rice are low-affinity sulfate transporters and involved in the inter-organ delivery of sulfate in vascular to transport sulfate from root to leaf, and distribution of sulfate between leaves4,7,8.
Taken together, our results indicate that overexpression of OsamiR395h in tobacco represses sulfate transporter NtaSULTR2, which may play an important role in sulfate transportation and distribution, thus interrupting sulfate homeostasis and distribution in transgenics.
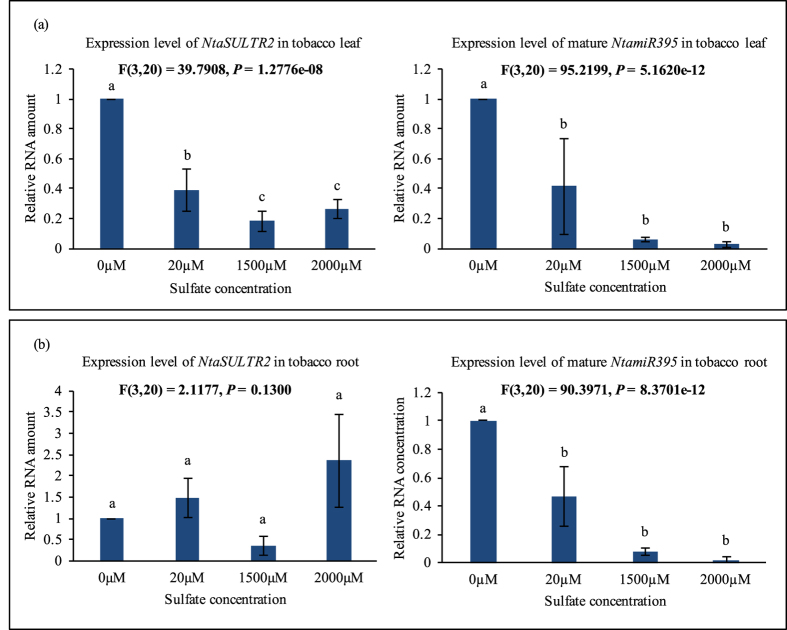
Sulfate regulates tobacco NtamiR395 and NtaSULTR2
To confirm that NtaSULTR2 is the target of miR395 in tobacco, we investigated the expression level of both NtaSULTR2 and mature NtamiR395 under different sulfate concentrations.
In leaf tissues, the transcription of the mature NtamiR395 was gradually up-regulated, contrary to the gradually reduced sulfate concentration. However, NtaSULTR2 did not exhibit an opposite, but a similar expression pattern to NtamiR395 with its lowest transcript level being under 1500 μM, but not 2000 μM (NH4+)2SO4 (Fig. 8a).
Figure 8. NtamiR395 and NtaSULTR2 exhibit opposite expression patterns in tobacco roots.
Real-time PCR analysis of expressions of NtaSULTR2 and mature NtamiR395 under different sulfate concentrations. Total RNA samples were prepared from (a) leaf tissue and (b) root tissue of four weeks old tobacco grown in MS medium with 0, 20, 1500 or 2000 μM (NH4+)2SO4. NtaL25 was used as a reference gene. Data are presented as means of three technical replicates and two biological replicates, error bars represent SD (n = 6). The statistically significant difference between groups was determined by one-way ANOVA (F(dfbetween, dfwithin) = F ration, p = p-value, where df = degrees of freedom). Means not sharing the same letter are statistically significantly different (P < 0.05).
In root tissues, the situation was different. The transcript level of the mature NtamiR395 increased in response to sulfate depletion, similar to that observed in leaves, whereas NtaSULTR2 exhibited a roughly opposite, but more complex expression pattern (Fig. 8b). Compared to sulfate depletion conditions with 0 μM (NH4+)2SO4 supply, NtaSULTR2 was up-regulated under both 20 μM and 2000 μM (NH4+)2SO4, but down-regulated under 1500 μM (NH4+)2SO4. The results indicate that NtaSULTR2 might be regulated by NtamiR395 in roots but not in leaf tissues. These results correspond to the previous studies in Arabidopsis and rice showing that the expression level of AthSULTR2 is opposite to that of AthmiR395 in some, but not all plant tissues most likely due to the fact that the spatial expression pattern of AthmiR395 does not overlap with that of AthSULTR2;118,19,30, which could probably also explain the similar observation in tobacco from this study.
MiR395 mediates the cleavage of NtaSULTR2 mRNA
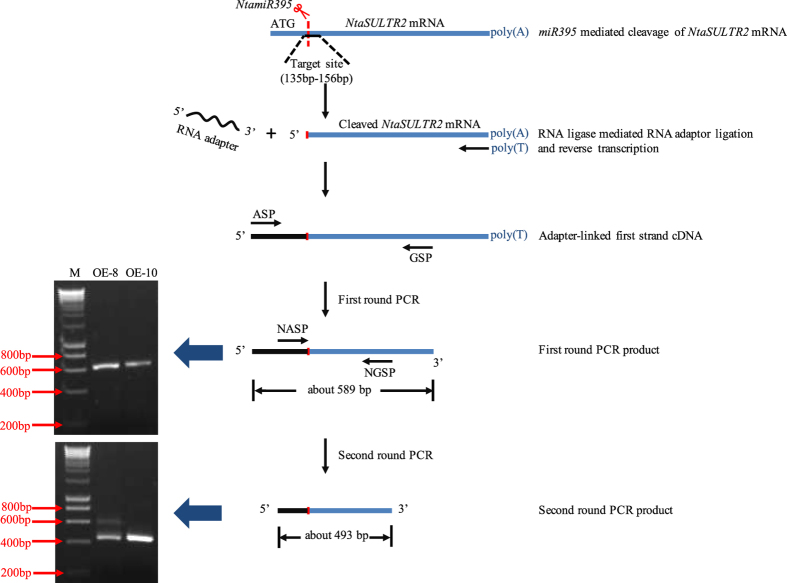
To further confirm that NtaSULTR2 is the true target of miR395, we conducted RLM-RACE (T4 RNA Ligase Mediated Rapid Amplification of cDNA Ends) to verify that NtaSULTR2 transcripts are cleaved by miR395. We used RNA from the miR395-overexpressing transgenic tobacco plants to facilitate the detection of cleaved NtaSULTR2 mRNA.
We used the forward primer ASP (Adapter Specific Primer) and the reverse primer GSP (Gene Specific Primer) to conduct the first round PCR after the adapter-linked first strand cDNA ends were generated. The RNA adapter has a length of 44 bp, and the reverse GSP is localized 545 bp downstream of the predicted miR395 target site in the NtaSULTR2 mRNA, so the product of the first round PCR should have a length of about 589 bp. As shown in Fig. 9, the first round PCR with transgenic tobacco cDNA indeed generated a clear band of about 600 bp.
Figure 9. Confirmation of miR395-mediated cleavage of NtSULTR2 mRNA.
RLM-RACE (T4-RNA ligase mediated amplification of 5′ cDNA ends) was conducted to confirm the cleavage of NtSULTR2 mRNA. Total RNA samples were isolated from two weeks old transgenic tobacco. 44 bp RNA adapter was ligated to the purified RNA by using T4 RNA ligase. Adapter-linked RNA was then used to synthesize first strand cDNA, followed by amplification of 5′ ends using the forward primer ASP and the reverse primer GSP. The 589 bp product from the first round PCR was then used as template for the second round PCR using the forward nest primer NASP and the reverse nest primer NGSP, producing a 493 bp second round PCR product. M: DNA molecular weight marker. OE: overexpression line. Red lines indicate miR395 cutting site.
A second round PCR was then conducted using the first round PCR product as template and a new set of primers to confirm the authenticity of the PCR product. The forward primer NASP (Nest Adapter Specific Primer) is localized on the adapter from 14 bp to 44 bp, and the reverse primer NGSP (Nest Gene Specific Primer) is localized 463 bp downstream of the predicted miR395 target site in the NtaSULTR2 mRNA, so the product of the second round PCR should be about 493 bp. As shown in Fig. 9, the second round PCR indeed generated a clear main band of about 500 bp as expected. Cloning and sequencing of the PCR product further confirmed the predicted miR395 cleavage site in the NtaSULTR2 mRNA.
Discussion
Previous studies on Arabidopsis miR395 have indicated its involvement in sulfate starvation response by repressing the expression of genes in sulfate transportation and assimilation pathways.
Under −S condition, the accumulation of AthmiR395 is enhanced under low internal sulfate levels, and correlated to GSH pool, indicating that the regulation of AthmiR395 is mediated by internal sulfate level and redox signaling in Arabidopsis22,31. The increased AthmiR395 then represses the expression of AthATPS1, AthATPS3, AthATPS4 and AthSULTR2;118,22. Further study in Arabidopsis revealed a whole picture of how AthmiR395 is involved in plant response to sulfate starvation. When sulfate supply is limited, the induced AthmiR395 mediates the degradation of ATPS mRNA leading to the accumulation of sulfate in leaf tissues as a result of decelerated sulfate assimilation19. At the same time, the cleavage of AthSULTR2;1 mRNA in shoots by AthmiR395 results in blocked sulfate transport into new leaves from old ones19. Furthermore, the impaired sulfate homeostasis and reduced sulfate assimilation impact seed germination under ABA-treated condition32.
MiR395 is highly conserved across species, which strongly suggests that its function in regulating plant response to nutrition, particularly sulfate supply could also be conserved during evolution. Our results in rice indicate that indeed, the transcript of mature OsamiR395 increases under −S condition, and this change in expression might be regulated at the transcription level (Fig. 1). Computational prediction led to the identification of four putative target genes of OsamiR395 in rice. We confirmed that OsaSULTR2;1 and OsaSULTR2 are regulated by OsamiR395 in roots suggesting that they may be the OsamiR395 target genes.
Knowledge about the functions of rice sulfate transporters is limited. Phylogenetic analysis grouped the fourteen rice sulfate transporters together with their Arabidopsis counterparts11, suggesting that they may share similar function. OsaSULTR2;1 and OsaSULTR2 may be responsible for the root-to-shoot sulfate transportation and distribution of sulfate between leaves of different ages. Our results (Fig. 2b–d) showed that the expression patterns of rice sulfate transporter genes were different from their Arabidopsis homologs, both OsaSULTR2;1 and OsaSULTR2 were reduced in leaves with the increasing sulfate concentrations. We speculate that the two sulfate transporter genes and miR395 may be differentially expressed in different leaf tissues and thus, OsaSULTR2;1 and OsaSULTR2 may not be subjected to miR395 regulation. Instead, other regulatory machineries may participate in the control of their expression in response to sulfate levels. It is likely that when rice plants are subjected to sulfate starvation, there is a need for the two sulfate transporters to be active, driving the transportation of sulfate from old leaves to younger ones to ensure plant growth and development. However, with abundant sulfate supply in the environment, there is no need for sulfate distribution to young leaves, and therefore the expression of both OsaSULTR2;1 and OsaSULTR2 declines.
The miRNA-mediated gene regulation mechanism emerged about 425 million years ago, which is at a very early stage of plant phylogeny prior to the divergence of monocot and dicot plants33. This suggests that monocot and dicot plants should have a similar miRNA-mediated gene regulation mechanism, and some highly conserved miRNA families regulating the same biological process have evolved from the same gene ancestors. Indeed, research data in the past twenty years indicate that 21 miRNA families, such as miR156 and miR399, are conserved in sequence across monocots and dicots25. More specifically, Zhang et al. found that 9 miRNA families are highly conserved33, 10 miRNA families are moderately conserved and 16 miRNA families including miR395 are lowly conserved across plant species. In a later work, miR395 family was identified in the common ancestor of all embryophytes25. Besides the miRNA sequences, the genes involved in miRNA and siRNA biogenesis pathways are also conserved across species. In plants, Dicer-like (DCL) is a key protein in the miRNA genesis pathway. DCL interacting with HYPONASTIC LEAVES1 (HYL1) and C2H2-zinc finger protein SERRATE (SE) in D-bodies cleaves the pri-miRNA from the base to yield a pre-miRNA with stem-loop structure, and this pre-miRNA is sliced again to yield mature miRNA34,35,36,37. Phylogenetic analysis indicated that divergence of DCL1 gene associated with miRNA production from other DCLs could be traced to the time before the emergence of moss Physcomitrella patens36, indicating that DCLs may have the same origin and are conserved across vascular plants.
Based on previous findings, we hypothesize that miRNA biogenesis pathway in dicots could accept pri-miRNAs from monocots, and process it into mature miRNA with function. To verify our hypothesis, the full-length DNA sequence of pri-OsamiR395h was cloned from rice genome. The expression cassette of the CaMV35S-controlled rice pri-OsamiR395h was then prepared and introduced into tobacco genome. By performing small molecule northern blotting, we observed high transcript level of miR395 in transgenic tobacco under normal condition, indicating that rice pri-OsamiR395h could be successfully expressed and processed into mature miR395h in tobacco (Fig. 4). At the same time, we also observed low level of endogenous mature miR395 in WT tobacco, confirming that tobacco mature miR395 is highly conserved with its rice homolog. All of the three transgenic tobacco lines exhibited impaired sulfate homeostasis and distribution (Fig. 5). Furthermore, transgenic plant had retarded growth phonotype (Fig. 6). All the facts suggest that mature OsamiR395 functions in transgenic tobacco.
Data obtained from this research revealed that the sulfate-S contents in transgenic tobacco are higher in leaf tissue, but lower in root tissue than those in WT controls. An even more significant difference in total sulfur content was observed between WT controls and OsamiR395h overexpression plants (Fig. 5a,b). Besides, we also observed that sulfate distribution between leaves of different ages is impaired in transgenic tobacco plants (Fig. 5c).
To reveal the molecular mechanism underlying miR395-mediated plant sulfate metabolism, we studied genes impacted by excessive dose of miR395 in transgenic tobacco, and identified a novel sulfate transporter gene NtaSULTR2 belonging to the second group of sulfate transporter genes (Fig. 7). Based on the results of real-time PCR and RML-RACE, we verified that NtaSULTR2 is the target gene of miR395 (Figs 8 and 9). We believe that the repression of NtaSULTR2 gene in transgenic tobacco plants partially impaired the sulfate homeostasis. In Arabidopsis shoot tissue, sulfate transporter AthSULTR2;1 is localized in both xylem and phloem, particularly in phloem parenchyma cells surrounding sieve and companion cells, and involved in distribution of sulfur between leaves of different ages7,28. We conjecture that in tobacco shoot tissue, NtaSULTR2, likes its homologs in Arabidopsis, retrieves sulfate from mesophyll cells to xylem and phloem cells, and sulfate is transported from old leaves to young leaves. But in transgenic plants, the delivery of sulfate from old leaves to young leaves is impaired because of significantly repressed NtaSULTR2 gene (Fig. 5c).
Although no ATPS gene have been identified and cloned in tobacco, we believe that there must be one or more ATPS gene(s) repressed in transgenic tobacco, causing interrupted sulfate assimilation. The interruption of the sulfate assimilation pathway would cause a shortage in cysteine and other sulfate metabolic products, resulting in retarded plant growth and triggering plant sulfate starvation signaling, which would promote sulfate absorption and transport into leaf tissue, and consequently a much more sulfur accumulation in leaves of transgenics than in that of WT controls (Fig. 5a,b).
Materials and Methods
Plant materials and growth conditions
To investigate the expression levels of OsamiR395 and its targets in rice under different sulfate concentrations, rice seeds were surface sterilized and grown in N6 medium under 16h light/8h dark at 28 °C38. Sulfate salts of the N6 medium were replaced with chloride salts and supplemented with 0, 20, 1500 or 2000 μM (NH4+)2SO4. Sterilized rice seeds were also grown in regular N6 medium (+S) and N6 medium without SO4+ (−S) under 16 h light/8 h dark at 28 °C. Two weeks old plants were harvested for RNA isolation.
To investigate the expression patterns of OsamiR395 and its targets in different developmental stages and tissues of rice, rice seeds were grown in soil in a greenhouse. Root and leaf samples were collected two, four and eight weeks after germination.
To investigate the expression levels of pri-OsamiR395h, mature miR395 and NtaSULTR2 in tobacco, tobacco seeds were surface sterilized and grown in MS medium under 16h light/8h dark at 22 °C39. To prepare MS mediums with different sulfate concentrations, sulfate salts of the MS medium were replaced with chloride salts and supplemented with 0, 20, 1500 or 2000 μM (NH4+)2SO4. Two weeks old and four weeks old plants were harvested for RNA isolation.
To measure total sulfate content and sulfate-S concentration in tobacco, and to determine the growth rate of tobacco, tobacco were grown in soil in a greenhouse. Four weeks old and 12 weeks old plants were collected for analysis.
Genomic DNA and total RNA isolation, and cDNA synthesis
Plant genomic DNA was isolated following previously described method40.
Total RNA was isolated from 100 mg plant samples with Trizol reagent (Ambion, USA), and the genomic DNA is removed by using RNase-free DNase I (Invitrogen, USA). 2 μg total RNA was used to synthesize first strand cDNA with SuperScript III Reverse Transcriptase (Invitrogen, USA) according to manufacturer’s instructions. The first strand cDNA was used for semi quantitative RT-PCR and regular real-time PCR.
To determine the transcript level of mature miR395, the first-strand cDNA used for stem-loop real-time PCR was synthesized following the regular SuperScript III Reverse Transcriptase (Invitrogen, USA) mediated method, except that the oligo (dT)20 was replaced with miR395 specific reverse transcription primer. Primers were all listed in Supplementary Table S1.
Semi-quantitative RT-PCR, stem-loop and regular real-time PCR
To conduct semi-quantitative RT-PCR, first-strand cDNA samples were diluted to 0.25 times based on the concentration of the first-strand cDNA samples. The loading volume of the cDNA samples was adjusted basing on the transcript level of a reference gene.
To conduct stem-loop and regular real-time PCR, first-strand cDNA samples were diluted to 0.025 to 0.005 times based on the concentration of the first-strand cDNA samples. Both stem-loop and regular real-time PCR were performed using SYBR Green Supermix (Bio-Rad, USA) following manufacturer’s instructions, and iQ5 real-time detection system (Bio-Rad USA) was used to detect and analyze the real-time PCR result.
Stem-loop and regular real-time PCR results were determined by using ΔΔCt method. ΔCt was defined as Cttest − Ct0h, in which Cttest stands for threshold cycle of one gene after treatment, and Ct0h stands for threshold cycle of one gene before treatment. ΔΔCt was defined as ΔCtreference − ΔCttarget, in which ΔCtreference stands for ΔCt of the endogenous gene used as a reference, and ΔCttarget stands for ΔCt of target gene. Finally, related expression ratio was calculated as 2ΔΔCt.
Primers used for semi-quantitative RT-PCR, stem-loop real-time PCR and regular real-time PCR were all listed in Supplementary Table 1.
Small molecule Northern blotting
Small molecule northern blotting was performed following the method previously described with minor modification41. 10 μg total RNA denatured at 95 °C was separated in 12.5% urea-polyacrylamide gel and transferred to Hybond-N+ nylon membrane (Amersham, USA) in a Trans-Blot SD Semi-Dry Transfer Cell (Bio-Rad, USA). To prepare radiolabeled probe for detecting mature miR395, DNA oligonucleotide GAGTTCCCCCAAACACTTCAC was synthesized (http://www.idtdna.com/site) and labeled with γ-[32P]-ATP by using T4 polynucleotide kinase. RNA membrane was then hybridized with radiolabeled probe and detected on a phosphorimaging screen.
Plasmid construction, bacterial strains and plant transformation
The predicted pri-OsamiR395h was amplified from rice genomic DNA and cloned at downstream of CaMV35S (Cauliflower Mosaic Virus 35S) promoter of binary vector pZH01, resulting in CaMV35S/OsamiR395h-CaMV35S/hygromycin42. This chimeric gene expression construct was then mobilized into Agrobacterium tumefaciens strain LBA4404 by electroporation for tobacco transformation. The Escherichia coli strain used in this experiment was DH5α.
The primers used for plasmid construction were all listed in Supplementary Table 1.
Determination of total sulfur content and sulfate-sulfur concentration
For determination of total sulfur, plant samples were collected and dried for 48 h at 80 °C. Total sulfur contents in dry samples were determined as previously described43 sulfate-S concentration was determined following a previous method with minor modification44. 10 mg dry plant sample or 200 mg fresh plant sample was immersed in 1 ml 0.1 M HCl for 2 h at room temperature, followed by 20 min centrifugation at 12000 g. Clear supernatant liquid was then transferred to a 50 ml Erlenmeyer flask and made to 20 ml by water. One ml of barium chloride-gelatin reagent was added to the liquid. After 40 min (no more than 120 min), absorbance of the resulting cloudy liquid was determined at 454 nm by using a spectrometer.
Rapid amplification of cDNA ends
To obtain 5′ cDNA end and 3′ cDNA end of NtaSULTR2, total RNA was extracted from 100 mg two weeks old WT tobacco with Trizol reagent (Ambion, USA) and treated with RNase-free DNase I (Invitrogen, USA) to remove genomic DNA. 1 μg total RNA was then used to amplify 5′ end and 3′ end cDNA of NtaSULTR2 with SMARTer RACE 5′/3′ commercial kit (Clontech, USA) following the manufacture’s instruction. Then, the 5′ end and 3′ end cDNA fragments were sequenced. Sequence information was used to design primers for cloning of full-length NtaSULTR2 cDNA.
The primers used for RACE and for cloning of full length NtaSULTR2 cDNA were all listed in Supplementary Table S1.
T4-RNA ligase mediated amplification of 5′ cDNA ends
To verify miR395 cleavage site within NtaSULTR2, T4-RNA ligase mediated amplification of 5′ cDNA ends was conducted following a previously described method45. Briefly, total RNA was isolated from 100 mg plant sample using Trizol reagent (Ambion, USA), followed by purification of RNA with RNeasy mini kit (Qiagen, Germany). RNA adapter was ligated to the purified RNA by using T4 RNA ligase (New England Biolabs, USA). Based on the fact that miRNA-mediated mRNA cleavage will generate 5′-monophosphate ends on the 3′ end cleavage product of the target mRNAs, it is possible to ligate RNA oligonucleotide adapter to the 5′ terminus of the 3′ end cleavage product by using T4 RNA ligase, whereas such RNA oligonucleotide adapter would not be ligated to mRNAs with conventional 5′ cap45. Adapter-linked RNA was then used to synthesize first strand cDNA with SuperScript II Reverse Transcriptase (Invitrogen, USA), followed by amplification of 5′ ends using the forward primer ASP and the reverse primer GSP. The product from the first round PCR was then used as template for the second round PCR with the forward nest primer NASP and the reverse nest primer NGSP. PCR product was cloned for sequencing.
The primer sequences used for RML-RACE were all listed in Supplementary Table 1.
Phylogenetic analysis of sulfate transporters
Phylogenetic tree of NtaSULTR2 and other sulfate transporter genes in rice and Arabidopsis inferred using the Neighbor-Joining method46. The optimal tree with the sum of branch length = 3.89795523 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Poisson correction method47 and are in the units of the number of amino acid substitutions per site. The analysis involved 17 amino acid sequences. All positions containing gaps and missing data were eliminated. There was a total of 347 positions in the final dataset. Evolutionary analyses were conducted in MEGA6 48. WT: wild type plant. OE: overexpression line.
Statistical analysis
Student’s t test was used to test the difference between the means from two groups. P < 0.05 was considered to be statistically significant and marked as *P < 0.01 was considered to be statistically highly significant and marked as**.
One-way ANOVA (F(dfbetween, dfwithin) = F ration, p = p-value, where df = degrees of freedom) with post hoc comparisons using the Tukey HSD test was used to determine the statistically significant difference between the means from three or more groups. Means not sharing the same letter are statistically significantly different (P < 0.05).
Additional Information
Accession codes: AthSULTR2;1: NM_121056.2, AthATPS1: NM_113189.4, AthATPS3: U06275.1, AthATPS4: AT5G43780, OsaSULTR2;1: NM_001055792, OsaSULTR2: NM_001055793, OsaSULTR3;4: Os06g0143700, OsaATPS: NM_001057769, OsaSiz1: Os05g0125000, NtaL25: L18908, NtaSULTR2: KT373983.
How to cite this article: Yuan, N. et al. Heterologous expression of a rice miR395 gene in Nicotiana tabacum impairs sulfate homeostasis. Sci. Rep. 6, 28791; doi: 10.1038/srep28791 (2016).
Supplementary Material
Acknowledgments
This work was supported by the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service grant no. SC-1700450. This is Technical Contribution no. 6290 of the Clemson University Experiment Station.
Footnotes
Author Contributions N.Y., Z.L. and H.L. designed the study. N.Y., D.L., S.Y. and Q.H. developed the methodology, performed the analysis, and collected the data. N.Y. and H.L. wrote the manuscript.
References
- Takahashi H., Kopriva S., Giordano M., Saito K. & Hell R. Sulfur assimilation in photosynthetic organisms: molecular functions and regulations of transporters and assimilatory enzymes. Annu Rev Plant Biol 62, 157–184, doi: 10.1146/annurev-arplant-042110-103921 (2011). [DOI] [PubMed] [Google Scholar]
- Hawkesford M. J. Plant responses to sulphur deficiency and the genetic manipulation of sulphate transporters to improve S-utilization efficiency. J Exp Bot 51, 131–138 (2000). [PubMed] [Google Scholar]
- Smith F. W., Ealing P. M., Hawkesford M. J. & Clarkson D. T. Plant members of a family of sulfate transporters reveal functional subtypes. Proc Natl Acad Sci USA 92, 9373–9377 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H. et al. Regulation of sulfur assimilation in higher plants: a sulfate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana. Proc Natl Acad Sci USA 94, 11102–11107 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriva S. Regulation of sulfate assimilation in Arabidopsis and beyond. Ann Bot 97, 479–495, doi: 10.1093/aob/mcl006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibagaki N. et al. Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J 29, 475–486 (2002). [DOI] [PubMed] [Google Scholar]
- Takahashi H. et al. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J 23, 171–182 (2000). [DOI] [PubMed] [Google Scholar]
- Kataoka T., Hayashi N., Yamaya T. & Takahashi H. Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiology 136, 4198–4204, doi: 10.1104/pp.104.045625 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T. et al. Vacuolar sulfate transporters are essential determinants controlling internal distribution of sulfate in Arabidopsis. Plant Cell 16, 2693–2704, doi: 10.1105/tpc.104.023960 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin R. M., Rae A. L., Carroll B. J. & Smith F. W. Cloning and characterization of two genes encoding sulfate transporters from rice (Oryza sativa L.). Plant and soil 257, 113–123 (2003). [Google Scholar]
- Kumar S., Asif M. H., Chakrabarty D., Tripathi R. D. & Trivedi P. K. Differential expression and alternative splicing of rice sulphate transporter family members regulate sulphur status during plant growth, development and stress conditions. Functional & integrative genomics 11, 259–273, doi: 10.1007/s10142-010-0207-y (2011). [DOI] [PubMed] [Google Scholar]
- Klonus D., Hofgen R., Willmitzer L. & Riesmeier J. W. Isolation and characterization of two cDNA clones encoding ATP-sulfurylases from potato by complementation of a yeast mutant. Plant J 6, 105–112 (1994). [DOI] [PubMed] [Google Scholar]
- Lunn J. E., Droux M., Martin J. & Douce R. Localization of ATP sulfurylase and O-acetylserine (thiol) lyase in spinach leaves. Plant Physiology 94, 1345–1352 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron N. J., Durnford D. G. & Kopriva S. Sulfate assimilation in eukaryotes: fusions, relocations and lateral transfers. BMC Evol Biol 8, 39, doi: 10.1186/1471-2148-8-39 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotte C. & Leustek T. Differential subcellular localization and expression of ATP sulfurylase and 5′-adenylylsulfate reductase during ontogenesis of arabidopsis leaves indicates that cytosolic and plastid forms of ATP sulfurylase may have specialized functions. Plant Physiology 124, 715–724, doi: 10.1104/Pp.124.2.715 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004). [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades M. W., Bartel D. P. & Bartel B. MicroRNAs and their regulatory roles in plants. Annu Rev Plant Biol 57, 19–53, doi: 10.1146/Annurev.Arplant.57.032905.105218 (2006). [DOI] [PubMed] [Google Scholar]
- Kawashima C. G. et al. Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J 57, 313–321, doi: 10.1111/j.1365-313X.2008.03690.x (2009). [DOI] [PubMed] [Google Scholar]
- Liang G., Yang F. & Yu D. MicroRNA395 mediates regulation of sulfate accumulation and allocation in Arabidopsis thaliana. The Plant journal: for cell and molecular biology 62, 1046–1057, doi: 10.1111/j.1365-313X.2010.04216.x (2010). [DOI] [PubMed] [Google Scholar]
- Adai A. et al. Computational prediction of miRNAs in Arabidopsis thaliana. Genome research 15, 78–91, doi: 10.1101/gr.2908205 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet E., Wuyts J., Rouze P. & Van de Peer Y. Detection of 91 potential conserved plant microRNAs in Arabidopsis thaliana and Oryza sativa identifies important target genes. Proc Natl Acad Sci USA 101, 11511–11516, doi: 10.1073/pnas.0404025101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadeeswaran G., Li Y. F. & Sunkar R. Redox signaling mediates the expression of a sulfate‐deprivation‐inducible microRNA395 in Arabidopsis. The Plant Journal (2014). [DOI] [PubMed] [Google Scholar]
- Wolfe K. H., Gouy M., Yang Y. W., Sharp P. M. & Li W. H. Date of the monocot-dicot divergence estimated from chloroplast DNA sequence data. Proc Natl Acad Sci USA 86, 6201–6205 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Pan X., Cobb G. P. & Anderson T. A. Plant microRNA: a small regulatory molecule with big impact. Dev Biol 289, 3–16, doi: 10.1016/j.ydbio.2005.10.036 (2006). [DOI] [PubMed] [Google Scholar]
- Cuperus J. T., Fahlgren N. & Carrington J. C. Evolution and functional diversification of MIRNA genes. Plant Cell 23, 431–442, doi: 10.1105/tpc.110.082784 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guddeti S. et al. Molecular evolution of the rice miR395 gene family. Cell Res 15, 631–638, doi: 10.1038/sj.cr.7290333 (2005). [DOI] [PubMed] [Google Scholar]
- Jones-Rhoades M. W. & Bartel D. P. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14, 787–799 (2004). [DOI] [PubMed] [Google Scholar]
- Takahashi H. Regulation of sulfate transport and assimilation in plants. International review of cell and molecular biology 281, 129–159, doi: 10.1016/S1937-6448(10)81004-4 (2010). [DOI] [PubMed] [Google Scholar]
- Frazier T. P., Xie F. L., Freistaedter A., Burklew C. E. & Zhang B. H. Identification and characterization of microRNAs and their target genes in tobacco (Nicotiana tabacum). Planta 232, 1289–1308, doi: 10.1007/S00425-010-1255-1 (2010). [DOI] [PubMed] [Google Scholar]
- Jeong D. H. et al. Massive analysis of rice small RNAs: mechanistic implications of regulated microRNAs and variants for differential target RNA cleavage. Plant Cell 23, 4185–4207, doi: 10.1105/tpc.111.089045 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthewman C. A. et al. miR395 is a general component of the sulfate assimilation regulatory network in Arabidopsis. FEBS letters 586, 3242–3248, doi: 10.1016/j.febslet.2012.06.044 (2012). [DOI] [PubMed] [Google Scholar]
- Kim J. Y. et al. Overexpression of microRNA395c or 395e affects differently the seed germination of Arabidopsis thaliana under stress conditions. Planta 232, 1447–1454, doi: 10.1007/s00425-010-1267-x (2010). [DOI] [PubMed] [Google Scholar]
- Zhang B., Pan X., Cannon C. H., Cobb G. P. & Anderson T. A. Conservation and divergence of plant microRNA genes. Plant J 46, 243–259, doi: 10.1111/j.1365-313X.2006.02697.x (2006). [DOI] [PubMed] [Google Scholar]
- Axtell M. J., Westholm J. O. & Lai E. C. Vive la difference: biogenesis and evolution of microRNAs in plants and animals. Genome Biol 12, 221, doi: 10.1186/gb-2011-12-4-221 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y., Takashi Y. & Watanabe Y. The interaction between DCL1 and HYL1 is important for efficient and precise processing of pri-miRNA in plant microRNA biogenesis. Rna 12, 206–212 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Feng Y. & Zhu Z. Dicer-like (DCL) proteins in plants. Functional & integrative genomics 9, 277–286, doi: 10.1007/s10142-009-0111-5 (2009). [DOI] [PubMed] [Google Scholar]
- Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell 136, 669–687, doi: 10.1016/j.cell.2009.01.046 (2009). [DOI] [PubMed] [Google Scholar]
- Chu C.-C. Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources. Scientia sinica 18, 659–668 (1975). [Google Scholar]
- Murashige T. & Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia plantarum 15, 473–497 (1962). [Google Scholar]
- Zhou M. et al. Constitutive expression of a miR319 gene alters plant development and enhances salt and drought tolerance in transgenic creeping bentgrass (Agrostis stolonifera L.). Plant Physiology, doi: 10.1104/pp.112.208702 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran N. Fast and Simple micro-RNA Northern Blots. Biochemistry Insights 2, 1–3 (2009). [Google Scholar]
- Xiao H. et al. Functional analysis of the rice AP3 homologue OsMADS16 by RNA interference. Plant Mol Biol 52, 957–966 (2003). [DOI] [PubMed] [Google Scholar]
- Plank C. O. Plant analysis reference procedures for the southern region of the United States. Southern cooperative series bulletin (USA) (1992).
- Tabatabai M. & Bremner J. A simple turbidimetric method of determining total sulfur in plant materials. Agronomy Journal 62, 805–806 (1970). [Google Scholar]
- Llave C., Franco-Zorrilla J. M., Solano R. & Barajas D. Target validation of plant microRNAs. Methods in molecular biology 732, 187–208, doi: 10.1007/978-1-61779-083-6_14 (2011). [DOI] [PubMed] [Google Scholar]
- Saitou N. & Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular biology and evolution 4, 406–425 (1987). [DOI] [PubMed] [Google Scholar]
- Zuckerkandl E. & Pauling L. Evolutionary divergence and convergence in proteins. Evolving genes and proteins 97, 97–166 (1965). [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A. & Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular biology and evolution 30, 2725–2729, doi: 10.1093/molbev/mst197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.