Abstract
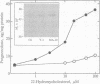
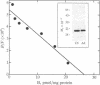
The C6-2B glioma cell line, rich in mitochondrial receptors that bind with high affinity to benzodiazepines, imidazopyridines, and isoquinolinecarboxamides (previously called peripheral-type benzodiazepine receptors), was investigated as a model to study the significance of the polypeptide diazepam binding inhibitor (DBI) and the putative DBI processing products on mitochondrial receptor-regulated steroidogenesis. DBI and its naturally occurring fragments have been found to be present in high concentrations in C6-2B glioma cells, to compete against specific isoquinolinecarboxamide or 4'-chlorodiazepam binding to mitochondrial recognition sites with high affinity, and to stimulate mitochondrial pregnenolone formation. These data suggest that this cell type may express both the receptor and the putative agonist ligand to regulate steroidogenesis. Therefore, we propose to term this mitochondrial receptor MDR (mitochondrial DBI receptor) to indicate its responsiveness to DBI in steroid biosynthesis. In the present work, we show that mitochondria of C6-2B cells convert (22R)-22-hydroxycholesterol to pregnenolone by a mechanism blocked by aminoglutethimide. Immunoblotting confirmed the presence of relatively high levels of cytochrome P-450 cholesterol side-chain-cleavage enzyme in C6-2B cell mitochondria. Furthermore, isoquinolinecarboxamide binding sites associated with the 18-kDa mitochondrial polypeptide subunit of the MDR are abundant in C6-2B glioma cell mitochondria (Bmax approximately 30 pmol/mg protein) and are coupled to the regulation of steroid biosynthesis. Occupancy of MDRs with nanomolar concentrations of the naturally occurring polypeptide, DBI, as well as its naturally occurring processing product tetratriacontaneuropeptide [DBI-(17-50)] increases pregnenolone formation. Clonazepam and octadecaneuropeptide [DBI-(33-50)], which exhibit a higher affinity for gamma-aminobutyric acid type A receptors but a low affinity for MDR, were ineffective in stimulating pregnenolone synthesis. These findings provide evidence that C6-2B cells exhibit a significant steroidogenic activity which resembles that found in peripheral endocrine organs and they suggest that MDRs and DBI are involved in the regulation of glial cell steroidogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkovich A., McPhie P., Campagnone M., Guidotti A., Hensley P. A natural processing product of rat diazepam binding inhibitor, triakontatetraneuropeptide (diazepam binding inhibitor 17-50) contains an alpha-helix, which allows discrimination between benzodiazepine binding site subtypes. Mol Pharmacol. 1990 Feb;37(2):164–172. [PubMed] [Google Scholar]
- Besman M. J., Yanagibashi K., Lee T. D., Kawamura M., Hall P. F., Shively J. E. Identification of des-(Gly-Ile)-endozepine as an effector of corticotropin-dependent adrenal steroidogenesis: stimulation of cholesterol delivery is mediated by the peripheral benzodiazepine receptor. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4897–4901. doi: 10.1073/pnas.86.13.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovolin P., Schlichting J., Miyata M., Ferrarese C., Guidotti A., Alho H. Distribution and characterization of diazepam binding inhibitor (DBI) in peripheral tissues of rat. Regul Pept. 1990 Jul 30;29(2-3):267–281. doi: 10.1016/0167-0115(90)90089-f. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A. Diazepam binding inhibitor (DBI): a peptide with multiple biological actions. Life Sci. 1991;49(5):325–344. doi: 10.1016/0024-3205(91)90440-m. [DOI] [PubMed] [Google Scholar]
- GRAHAM R. C., Jr, LUNDHOLM U., KARNOVSKY M. J. CYTOCHEMICAL DEMONSTRATION OF PEROXIDASE ACTIVITY WITH 3-AMINO-9-ETHYLCARBAZOLE. J Histochem Cytochem. 1965 Feb;13:150–152. doi: 10.1177/13.2.150. [DOI] [PubMed] [Google Scholar]
- Gee K. W., Bolger M. B., Brinton R. E., Coirini H., McEwen B. S. Steroid modulation of the chloride ionophore in rat brain: structure-activity requirements, regional dependence and mechanism of action. J Pharmacol Exp Ther. 1988 Aug;246(2):803–812. [PubMed] [Google Scholar]
- Gower D. B. Modifiers of steroid-hormone metabolism: a review of their chemistry, biochemistry and clinical applications. J Steroid Biochem. 1974 Aug;5(5):501–523. doi: 10.1016/0022-4731(74)90051-x. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Glaister D., Seeburg P. H., Guidotti A., Costa E. Cloning and expression of cDNA for human diazepam binding inhibitor, a natural ligand of an allosteric regulatory site of the gamma-aminobutyric acid type A receptor. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7547–7551. doi: 10.1073/pnas.83.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarneri P., Papadopoulos V., Pan B., Costa E. Regulation of pregnenolone synthesis in C6-2B glioma cells by 4'-chlorodiazepam. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5118–5122. doi: 10.1073/pnas.89.11.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A., Forchetti C. M., Corda M. G., Konkel D., Bennett C. D., Costa E. Isolation, characterization, and purification to homogeneity of an endogenous polypeptide with agonistic action on benzodiazepine receptors. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3531–3535. doi: 10.1073/pnas.80.11.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. F. Cellular organization for steroidogenesis. Int Rev Cytol. 1984;86:53–95. doi: 10.1016/s0074-7696(08)60177-1. [DOI] [PubMed] [Google Scholar]
- Hu Z. Y., Bourreau E., Jung-Testas I., Robel P., Baulieu E. E. Neurosteroids: oligodendrocyte mitochondria convert cholesterol to pregnenolone. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8215–8219. doi: 10.1073/pnas.84.23.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung-Testas I., Hu Z. Y., Baulieu E. E., Robel P. Neurosteroids: biosynthesis of pregnenolone and progesterone in primary cultures of rat glial cells. Endocrinology. 1989 Oct;125(4):2083–2091. doi: 10.1210/endo-125-4-2083. [DOI] [PubMed] [Google Scholar]
- Krueger K. E., Papadopoulos V. Peripheral-type benzodiazepine receptors mediate translocation of cholesterol from outer to inner mitochondrial membranes in adrenocortical cells. J Biol Chem. 1990 Sep 5;265(25):15015–15022. [PubMed] [Google Scholar]
- Le Goascogne C., Robel P., Gouézou M., Sananès N., Baulieu E. E., Waterman M. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987 Sep 4;237(4819):1212–1215. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- Majewska M. D., Harrison N. L., Schwartz R. D., Barker J. L., Paul S. M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986 May 23;232(4753):1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Massotti M., Slobodyansky E., Konkel D., Costa E., Guidotti A. Regulation of diazepam binding inhibitor in rat adrenal gland by adrenocorticotropin. Endocrinology. 1991 Aug;129(2):591–596. doi: 10.1210/endo-129-2-591. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., De Kloet E. R., Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986 Oct;66(4):1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- Mienville J. M., Vicini S. Pregnenolone sulfate antagonizes GABAA receptor-mediated currents via a reduction of channel opening frequency. Brain Res. 1989 Jun 5;489(1):190–194. doi: 10.1016/0006-8993(89)90024-3. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V., Berkovich A., Krueger K. E., Costa E., Guidotti A. Diazepam binding inhibitor and its processing products stimulate mitochondrial steroid biosynthesis via an interaction with mitochondrial benzodiazepine receptors. Endocrinology. 1991 Sep;129(3):1481–1488. doi: 10.1210/endo-129-3-1481. [DOI] [PubMed] [Google Scholar]
- Papadopoulos V., Mukhin A. G., Costa E., Krueger K. E. The peripheral-type benzodiazepine receptor is functionally linked to Leydig cell steroidogenesis. J Biol Chem. 1990 Mar 5;265(7):3772–3779. [PubMed] [Google Scholar]
- Puia G., Santi M. R., Vicini S., Pritchett D. B., Purdy R. H., Paul S. M., Seeburg P. H., Costa E. Neurosteroids act on recombinant human GABAA receptors. Neuron. 1990 May;4(5):759–765. doi: 10.1016/0896-6273(90)90202-q. [DOI] [PubMed] [Google Scholar]
- Slobodyansky E., Guidotti A., Wambebe C., Berkovich A., Costa E. Isolation and characterization of a rat brain triakontatetraneuropeptide, a posttranslational product of diazepam binding inhibitor: specific action at the Ro 5-4864 recognition site. J Neurochem. 1989 Oct;53(4):1276–1284. doi: 10.1111/j.1471-4159.1989.tb07425.x. [DOI] [PubMed] [Google Scholar]
- Sprengel R., Werner P., Seeburg P. H., Mukhin A. G., Santi M. R., Grayson D. R., Guidotti A., Krueger K. E. Molecular cloning and expression of cDNA encoding a peripheral-type benzodiazepine receptor. J Biol Chem. 1989 Dec 5;264(34):20415–20421. [PubMed] [Google Scholar]
- Wu F. S., Gibbs T. T., Farb D. H. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991 Sep;40(3):333–336. [PubMed] [Google Scholar]
- Yanagibashi K., Ohno Y., Nakamichi N., Matsui T., Hayashida K., Takamura M., Yamada K., Tou S., Kawamura M. Peripheral-type benzodiazepine receptors are involved in the regulation of cholesterol side chain cleavage in adrenocortical mitochondria. J Biochem. 1989 Dec;106(6):1026–1029. doi: 10.1093/oxfordjournals.jbchem.a122958. [DOI] [PubMed] [Google Scholar]