Abstract
To assess the potential for mating in several Fusarium species with no known sexual stage, we developed degenerate and semidegenerate oligonucleotide primers to identify conserved mating type (MAT) sequences in these fungi. The putative α and high-mobility-group (HMG) box sequences from Fusarium avenaceum, F. culmorum, F. poae, and F. semitectum were compared to similar sequences that were described previously for other members of the genus. The DNA sequences of the regions flanking the amplified MAT regions were obtained by inverse PCR. These data were used to develop diagnostic primers suitable for the clear amplification of conserved mating type sequences from any member of the genus Fusarium. By using these diagnostic primers, we identified mating types of 122 strains belonging to 22 species of Fusarium. The α box and the HMG box from the mating type genes are transcribed in F. avenaceum, F. culmorum, F. poae, and F. semitectum. The novelty of the PCR-based mating type identification system that we developed is that this method can be used on a wide range of Fusarium species, which have proven or expected teleomorphs in different ascomycetous genera, including Calonectria, Gibberella, and Nectria.
The genus Fusarium contains filamentous ascomycete fungi with a worldwide distribution. Fusarium species can parasitize cultivated plants (1) and/or produce mycotoxins that pose serious hazards to human and animal health (9, 18). Species of Fusarium can grow successfully on a variety of substrates, can tolerate diverse environmental conditions, and have high levels of intraspecific genetic and genotypic diversity (for examples, see references 8, 12, 17, and 26). Neither the origins of this diversity nor the mechanisms that maintain it are well understood.
Meiotic recombination can generate and maintain genotypic variation and result in the reassortment of genes that govern traits such as virulence or toxin production (7). The sexual spores (ascospores) produced by some Fusarium species also may function as infectious propagules (11, 19). Although several Fusarium species have a known sexual cycle, i.e., they mate in either a homothallic or heterothallic manner followed by subsequent meiosis and the production of ascospores, important pathogenic species, including Fusarium avenaceum, Fusarium cerealis, Fusarium culmorum, Fusarium equiseti, Fusarium poae, and Fusarium sporotrichioides, have no known sexual stage.
Assessing the potential for mating by toxigenic strains of Fusarium would increase our understanding of the genetic mechanisms that maintain intraspecific diversity and biological and evolutionary species integrity. The frequency of sexual reproduction is also an important parameter for the design of strategies to control plant pathogens, since these strategies are often different for clonally and sexually reproducing organisms. High levels of race-specific resistance can be developed in plant cultivars against clonally reproducing organisms, whereas horizontal resistance could be more effective against pathogens comprising genetically diverse populations as a result of mating and meiotic recombination (16).
The known teleomorphs of Fusarium species belong to the genera Calonectria, Gibberella, and Nectria (5). In heterothallic species, e.g., Gibberella fujikuroi, mating type is controlled by a single locus with two idiomorphic alleles, termed MAT-1 and MAT-2. These alleles contain a conserved α box domain and a high-mobility-group (HMG) box domain, respectively. Strains of Gibberella zeae (anamorph of Fusarium graminearum), a homothallic species, carry both the MAT-1 and MAT-2 idiomorphs, closely linked together (27). Strains of Fusarium oxysporum, a species complex with no known sexual stage, also contain transcribed MAT alleles (4, 13, 27). However, the MAT genes have not been studied in any other mitotic holomorph species within the genus Fusarium.
PCR amplification of MAT sequences from various Fusarium species belonging to the G. fujikuroi species complex has been utilized to standardize the mating type terminology for mating populations of this species complex (13) and to develop assays for identifying the presence of the MAT allele without sexual crosses (22, 25). However, the primers used in these previous studies were inadequate for the rest of the genus (13, 22), probably due to sequence divergence that may occur even in conserved MAT sequences of these fungi. The aims of the present study were (i) to demonstrate whether mating type sequences can be found in Fusarium species with no known sexual stage, (ii) to develop a PCR-based technique for the rapid identification of mating types in a wide range of Fusarium species with proven or expected Calonectria, Gibberella, and Nectria teleomorphs, and (iii) to demonstrate the transcription of mating type genes in selected “asexual” Fusarium species during their vegetative growth.
(A preliminary version of this work was presented at the 6th European Conference of Fungal Genetics [A. Moretti, Z. Kerényi, G. Mulé, C. Waalwijk, and L. Hornok, Abstr. 6th Eur. Conf. Fungal Genet., abstr. ECFG6, p. 394-395, 2002].)
MATERIALS AND METHODS
Strains and culture conditions.
Fungal strains involved in mating type identification were as follows (species and strain number [mating type identified by PCR]): Fusarium acuminatum subsp. acuminatum ITEM 791 (MAT-1), ITEM 1042 (MAT-2), ITEM 1137 (MAT-1), ITEM 1716 (MAT-1), ITEM 1892 (MAT-2), ITEM 1895 (MAT-2), ITEM 1896 (MAT-2), ITEM 1897 (MAT-1), ITEM 1899 (MAT-1), and ITEM 1901 (MAT-1); F. acuminatum subsp. armeniacum ITEM 795 (MAT-2), ITEM 796 (MAT-2), ITEM 797 (MAT-1), ITEM 800 (MAT-2), ITEM 988 (MAT-1), ITEM 992 (MAT-1), and ITEM 998 (MAT-1); F. avenaceum ABC-A2 (MAT-2), ABC-A3 (MAT-2), ABC-A10 (MAT-2), ITEM 158 (MAT-1), ITEM 858 (MAT-2), ITEM 859 (MAT-1), ITEM 1787 (MAT-2), ITEM 3397 (MAT-2), ITEM 3400 (MAT-1), ITEM 3406 (MAT-1), ITEM 3410 (MAT-2), and ITEM 3411 (MAT-2); Fusarium camptoceras ITEM 1116 (MAT-2) and ITEM 1128 (MAT-2); F. cerealis ITEM 661 (MAT-1), ITEM 663 (MAT-2), ITEM 664 (MAT-2), ITEM 665 (MAT-2), and ITEM 666 (MAT-2); Fusarium chlamydosporum ITEM 1806 (MAT-1), ITEM 1873 (MAT-1), and ITEM 2035 (MAT-2); Fusarium compactum ITEM 156 (MAT-1), ITEM 427 (MAT-2), ITEM 488 (MAT-2), ITEM 616 (MAT-2), ITEM 1288 (MAT-2), ITEM 1289 (MAT-1), ITEM 1867 (MAT-2), and ITEM 3695 (MAT-1); F. culmorum ABC-HUCU2 (MAT-2), ABC-HUCU3 (MAT-2), CBS 173-31 (MAT-2), CBS 251-52 (MAT-1), IPPV 72186 (MAT-1), IPPV 72305 (MAT-2), ITEM 627 (MAT-2), ITEM 628 (MAT-2), ITEM 741 (MAT-1), ITEM 3392 (MAT-1), ITEM 3393 (MAT-2), NRRL 23141 (MAT-1), NRRL 29138 (MAT-1), NRRL 29139 (MAT-2), NRRL 29140 (MAT-1), PRI 11F1 (MAT-2), PRI 19A1 (MAT-1), and SUF 995 (MAT-1); Fusarium decemcellulare (teleomorph, Calonectria rigidiuscula) PPI-F 29 (MAT-2); F. equiseti ITEM 192 (MAT-1), ITEM 358 (MAT-1), ITEM 359 (MAT-1), ITEM 372 (MAT-1), ITEM 679 (MAT-2), ITEM 1158 (MAT-2), ITEM 1736 (MAT-2), and ITEM 1856 (MAT-1); F. graminearum PRI 24F1 (MAT-1/MAT-2); Fusarium longipes ITEM 3202 (MAT-2); Fusarium merismoides CBS 798.70 (MAT-2); F. poae ABC-A15 (MAT-1), ABC-A18 (MAT-2), ABC-TAPO1 (MAT-1), ABC-TAPO7 (MAT-1), ABC-TAPO18 (MAT-1), ABC-TAPO21 (MAT-1), ABC-TAPO24 (MAT-1), and ABC-TAPO34 (MAT-2); Fusarium scirpi ITEM 1166 (MAT-1), ITEM 1717 (MAT-2), and ITEM 1718 (MAT-2); Fusarium semitectum ITEM 2562 (MAT-2), ITEM 3192 (MAT-1), ITEM 3193 (MAT-2), ITEM 3390 (MAT-2), ITEM 3391 (MAT-1), and ITEM 3394 (MAT-2); Fusarium solani (teleomorph, Nectria haematococca) ITEM 3320 (MAT-2) and PPI-9 (MAT-1); F. sporotrichioides ITEM 3592 (MAT-2), ITEM 3593 (MAT-2), ITEM 3594 (MAT-2), ITEM 3595 (MAT-2), ITEM 3596 (MAT-1), ITEM 3597 (MAT-2), ITEM 3598 (MAT-2), ITEM 3599 (MAT-2), ITEM 3600 (MAT-2), and ITEM 3601 (MAT-2); Fusarium torulosum ITEM 839 (MAT-1), ITEM 840 (MAT-2), ITEM 842 (MAT-2), ITEM 843 (MAT-2), ITEM 844 (MAT-1), and ITEM 845 (MAT-1); Fusarium tricinctum ABC-HF 011 (MAT-2), ITEM 2054 (MAT-2), ITEM 3405 (MAT-1), PPI-F 27 (MAT-2), and PPI-F 28 (MAT-2); Fusarium tumidum ITEM 2118 (MAT-2), ITEM 2119 (MAT-2), and NRRL 13394 (MAT-2); and Fusarium verticillioides (teleomorph, G. fujikuroi mating population A; synonymous organism, Gibberella moniliformis) FGSC 7600 (MAT-1) and FGSC 7603 (MAT-2). The strains were obtained from the following organizations: ABC, Agricultural Biotechnology Center, Gödöllõ, Hungary; CBS, Centraalbureau voor Schimmelcultures, Baarn, The Netherlands; FGSC, Fungal Genetic Stock Center, University of Kansas Medical Center, Kansas City; IPPV, Agricultural Research Centre, Institute of Plant Pathology, Vantaa, Finland; ITEM, Institute of Sciences of Food Production, CNR, Bari, Italy; NRRL, Northern Regional Research Laboratory, U.S. Department of Agriculture, Peoria, Ill.; PPI, Plant Protection Institute, HAS, Budapest, Hungary; PRI, Plant Research International, Wageningen, The Netherlands; SUF, Shinshu University, Ueda, Nagano-ken, Japan. All Fusarium strains used in this study were maintained as conidial suspensions in 16% glycerol at −70°C. For genomic DNA extractions, about 106 conidia were inoculated into 100 ml of complete medium (6) and grown for 2 days at 25°C with shaking (120 rpm). After that, the mycelium was filtered, washed with sterile water, and lyophilized. For reverse transcription-PCR (RT-PCR) experiments, plugs of mycelium were transferred to carrot agar, which is widely used to induce perithecia formation in crossing experiments (14), and incubated for 1 week at 25°C under a diurnal cycle of 12 h of light and 12 h of darkness.
Molecular techniques.
Common DNA and RNA manipulation techniques were performed as described by Sambrook et al. (21). PCRs were performed with reaction mixtures containing 1× PCR buffer (MBI Fermentas, Vilnius, Lithuania), 1.5 mM MgCl2, a 0.5 mM concentration of each deoxynucleoside triphosphate, a 0.25 μM concentration of each primer, 1 U of Taq polymerase (MBI Fermentas), and about 20 ng of fungal DNA. Initial denaturation was done at 95°C for 2 min, followed by 30 cycles consisting of 30 s at 94°C, 30 s at 55 to 60°C (depending on the melting temperature of the primers), and 0.5 to 5 min at 72°C (depending on the expected length of the amplicon), and a final elongation step at 72°C for 10 min. The amplification products were separated by electrophoresis in agarose gels, stained with ethidium bromide, and visualized with UV light. Amplicons were cloned into the pBluescript II KS (Stratagene, La Jolla, Calif.) or pGEM-T Easy (Promega, Madison, Wis.) plasmid vector, transformed into Escherichia coli DH5α cells (Clontech, Palo Alto, Calif.), and sequenced by the Sequencing Service of the Agricultural Biotechnology Center. DNA sequences were analyzed with the Lasergene (DNAStar Inc., Madison, Wis.) and Wisconsin (10) software packages, and BLAST searches were done with the GenBank database (2).
PCR amplification of conserved MAT boxes.
Conserved portions of the α or HMG box of the MAT-1 or MAT-2 idiomorph were amplified from different Fusarium species by using the degenerate Fα1 (CGNCCNCTNAAYGSNTTYATG) and Fα2 (ACYTTNGCNATNAGNGCCCAYTT) primers or the previously described NcHMG1 and NcHMG2 primers (3). Primers Fα1 and Fα2 were designed based on deduced amino acid sequence data for MAT-1 idiomorphs known from G. moniliformis (synonym, G. fujikuroi mating population A) (accession no. AF100925), G. zeae (accession no. AF318048), and F. oxysporum (accession no. AB011379) (27). The putative MAT-specific amplicons, identified on the basis of the expected lengths of the fragments, were cloned and sequenced. BLAST comparisons confirmed that these amplicons were indeed MAT-box homologs, i.e., α and HMG box homologues. Amplified fragments of the expected sizes were isolated from the gel, cloned, and sequenced.
Cloning entire MAT genes.
Inverse PCR was performed as previously described (23) by using Herculase Taq polymerase (Stratagene) according to the manufacturer's instructions. Amplicons were cloned and sequenced, and the appropriate contigs were assembled by using the SeqMan computer program (DNAStar). To clone intact MAT-1-1 and MAT-2 genes, we designed specific primer pairs (Table 1) based on the sequence information derived from inverse PCR. Using these primers, we amplified, cloned, and sequenced the entire MAT-1-1 and MAT-2 genes from F. avenaceum, F. culmorum, F. poae, and F. semitectum.
TABLE 1.
Primers used for amplification of entire MAT-1-1 and MAT-2 genes from F. avenaceum, F. culmorum, F. poae, and F. semitectum
| Primer | Nucleotide sequence (5′-3′) | Description |
|---|---|---|
| AVE-1-F | TCTTTAAATCCTCTCTCTCTGCCCA | F. avenaceum MAT-1-1 forward |
| AVE-1-R | ACCTTCTGACCAACCAGAAGCCT | F. avenaceum MAT-1-1 reverse |
| AVE-2-F | CACCCCAACAACCATTCGGAGTTT | F. avenaceum MAT-2 forward |
| AVE-2-R | CAATGGGATGGCAGGCTGTCCA | F. avenaceum MAT-2 reverse |
| CUL-1-F | AATTCATCTCTCCTGGCCTTTTGC | F. culmorum MAT-1-1 forward |
| CUL-1-R | ATTTCTCAGCCCTAGATCTCATTGC | F. culmorum MAT-1-1 reverse |
| CUL-2-F | TTCAGAACGCCAGCAGGACCAG | F. culmorum MAT-2 forward |
| CUL-2-R | GAGCGGGACGTTTGTGCCTACTTA | F. culmorum MAT-2 reverse |
| POA-1-F | GCCTCACACTTTTTTCCTTCTTC | F. poae MAT-1-1 forward |
| POA-1-R | CAGTAAACCGGAATCATCAACG | F. poae MAT-1-1 reverse |
| POA-2-F | ACGTACCATCTGACACTTGCTCG | F. poae MAT-2 forward |
| POA-2-R | AGTCGAGGAGGTCGTCAATCAAT | F. poae MAT-2 reverse |
| SEM-1-F | TCTCTTCTCTCATCTCAGGCTTTCA | F. semitectum MAT-1-1 forward |
| SEM-1-R | TCGCGTGCTACCCTAAACTTTT | F. semitectum MAT-1-1 reverse |
| SEM-2-F | CTCACAAAACGCCAACAGAACCAT | F. semitectum MAT-2 forward |
| SEM-2-R | TCCAGTCGATAACAACGCTCAAGA | F. semitectum MAT-2 reverse |
MAT box-specific PCR.
To identify the mating type of different Fusarium species, we designed diagnostic PCR primers (see Results). For amplification, we used the general PCR protocol described above, except for some minor modifications as follows: the concentration of MgCl2 was elevated to 2 to 2.5 mM, the number of cycles was increased to 35, and the elongation time was expanded to 30 s.
RT-PCR experiments.
Total RNAs were extracted from the mycelia grown on carrot agar plates by use of the TRI reagent (Sigma, St. Louis, Mo.) according to the manufacturer's instructions. The first-strand cDNA reaction (40 μl) contained 1× avian myeloblastosis virus buffer (Promega), a 0.5 mM concentration of each deoxynucleoside triphosphate, 1.7 μM oligo(dT)15 primer, 20 U of RNasin (Promega), 5 U of avian myeloblastosis virus reverse transcriptase (Promega), and about 5 μg of total RNA and was followed by an RQ1 DNase (Promega) treatment. The mixture was incubated at 42°C for 50 min and at 65°C for 10 min to inactivate the reverse transcriptase. Five microliters of the first-strand cDNA reaction was used as a template in a 25-μl standard PCR mixture. Amplifications were done with the same program as described above, except that the number of cycles was increased from 30 to 40.
Nucleotide sequence accession numbers.
The sequences of the amplified regions of F. avenaceum, F. culmorum, F. poae, and F. semitectum were deposited in the EMBL database under accession numbers AJ535625 to AJ535632.
RESULTS
Cloning of MAT genes from Fusarium species with no known sexual stage.
Regions flanking the α or HMG boxes were amplified by inverse PCR from F. avenaceum, F. culmorum, F. poae, and F. semitectum, and the resulting fragments were cloned and sequenced. Based on these sequences, new PCR primer pairs were designed for border regions of the MAT-1-1 and MAT-2 genes, and the DNA fragments generated by these primers were cloned and sequenced.
Putative MAT-specific fragments, MAT-1 and MAT-2 genes, and MAT-1-specific α box and MAT-2-specific HMG box sequences (Table 2) were identified by comparison with sequences available for the MAT idiomorphs of F. oxysporum, G. fujikuroi, and G. zeae (27). The MAT-1-1 gene identified in F. avenaceum ITEM 859 was 1,218 bp long and encoded a putative protein with an α-box motif. The sequences of MAT-1-1 genes from F. culmorum strain 19A1, F. poae TAPO21, and F. semitectum ITEM 3192 were 1,085, 1,203, and 1,129 bp long, respectively, and encoded putative proteins with conserved α-box domains. All of these MAT-1-1 gene sequences contained introns at conserved positions (20). No in-frame stop codons were found in these sequences.
TABLE 2.
Similarity of MAT sequences of Fusarium species with no known sexual stage to MAT sequences described previously for other members of the genera Fusarium and Gibberella
| MAT sequence | Accession no. | % Similarity for entire gene/% similarity for conserved boxes
|
||
|---|---|---|---|---|
| F. oxysporuma | G. fujikuroib | G. zeaec | ||
| F. avenaceum MAT-1-1 | AJ535625 | 63.1/64.9 | 51.1/75.7 | 55.2/75.5 |
| F. culmorum MAT-1-1 | AJ535626 | 48.7/58.9 | 52.7/75.7 | 97/97.3 |
| F. poae MAT-1-1 | AJ535627 | 52.5/66.7 | 55.3/76.6 | 81.3/85.3 |
| F. semitectum MAT-1-1 | AJ535628 | 49.8/61.3 | 60.5/75.2 | 70.7/78.8 |
| F. avenaceum MAT-2 | AJ535629 | 61.7/73.8 | 67.6/71.6 | 63.8/71.2 |
| F. culmorum MAT-2 | AJ535630 | 52.8/71.6 | 58.8/68.4 | 97.8/98.5 |
| F. poae MAT-2 | AJ535631 | 57.7/73.8 | 59.7/69.5 | 80.8/89.7 |
| F. semitectum MAT-2 | AJ535632 | 61.4/74.2 | 62.2/69.5 | 75.2/82.4 |
The MAT-2 gene from F. avenaceum ITEM 858 was 860 bp long and encoded a putative protein with an HMG box domain. Similar sequences from F. culmorum 11F1, F. poae TAPO34, and F. semitectum ITEM 3390 were 865, 859, and 856 bp long, respectively, and encoded proteins with conserved HMG domains. These MAT-2 gene sequences also contained introns at conserved positions (20). In-frame stop codons were not found in these sequences. Sequence similarities ranged from 49 to 99%, but the percentages of similarity between the MAT-specific box sequences were always higher than the values obtained from comparisons of entire MAT gene sequences. The Treebase database accession number for these comparisons is SN 1779.
Diagnostic PCR for mating type in Fusarium species.
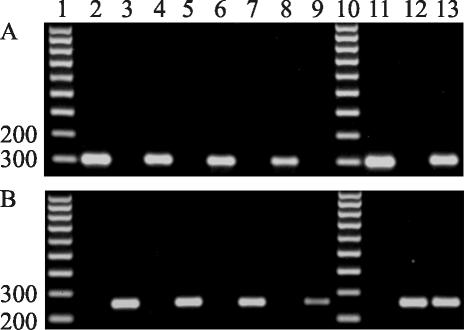
We designed new degenerate oligonucleotide primers, namely fusALPHAfor (CGCCCTCTKAAYGSCTTCATG), fusALPHArev (GGARTARACYTTAGCAATYAGGGC), fusHMGfor (CGACCTCCCAAY GCYTACAT), and fusHMGrev (TGGGCGGTACTGG TARTCRGG), and defined appropriate PCR conditions. The positions of these primers in the α and HMG box sequences of F. avenaceum (AJ535625 and AJ535629) are nucleotides 282 to 302, 456 to 479, 540 to 559, and 775 to 795, respectively. The sizes of the MAT-1- and MAT-2-specific fragments amplified from different species of Fusarium were 200 and 260 bp, respectively (Fig. 1).
FIG. 1.
PCR amplification of mating type-specific boxes from Fusarium species with no known sexual stage. (A) Amplification of MAT-1-specific α box by using the fusALPHAfor and fusALPHArev primers. (B) Amplification of MAT-2-specific HMG box by using the fusHMGfor and fusHMGrev primers. Lanes 1 and 10, 100-bp DNA ladder; lanes 2 to 9, F. avenaceum ITEM 859 (MAT-1) and ITEM 858 (MAT-2), F. culmorum 19A1 (MAT-1) and 11F1 (MAT-2), F. poae TAPO21 (MAT-1) and TAPO34 (MAT-2), and F. semitectum ITEM 3192 (MAT-1) and 3390 (MAT-2), respectively; lanes 11 to 13, F. verticillioides FGSC 7600 (MAT-1) and FGSC 7603 (MAT-2) and F. graminearum 24F1 (MAT1/2), respectively (used as controls).
The usability of the diagnostic PCR method for mating type identification was tested on 122 Fusarium strains representing 22 species from 9 sections. Both MAT-1 and MAT-2 individuals were identified among strains of F. acuminatum subsp. acuminatum, F. acuminatum subsp. armeniacum, F. avenaceum, F. cerealis, F. chlamydosporum, F. compactum, F. culmorum, F. equiseti, F. poae, F. scirpi, F. semitectum, F. solani, F. sporotrichioides, F. torulosum, and F. tricinctum. Only the MAT-2 mating type was found among strains of F. camptoceras, F. decemcellulare, F. longipes, F. merismoides, and F. tudmidum, but the number of isolates we could obtain for this assay was limited. The only strain for which both MAT-specific amplicons were identified belonged to G. zeae, a true homothallic member of the genus Gibberella that is known to harbor both MAT-1 and MAT-2 idiomorphs (27). The two opposing mating types of the reference strains of F. verticillioides were also clearly identified by using the semidegenerate primers developed in this work. The mating types of all of the strains are indicated in Materials and Methods.
Transcription of MAT genes in Fusarium species with no known sexual stage.
RT-PCR experiments primed with α box- or HMG box-specific primers, respectively, were performed to examine the expression of MAT genes in F. avenaceum, F. culmorum, F. poae, and F. semitectum. Electrophoretic separation of the RT-PCR products resulted in the appearance of one characteristic band at the appropriate size, i.e., an ∼150-bp and an ∼200-bp fragment in all samples (Fig. 2). The size differences observed between the amplicons obtained by RT-PCR and fragments generated from the genomic DNAs were due to the presence of an intron in the genomic copies of these MAT boxes. Northern blot analyses of these RT-PCR products, with the appropriate cloned MAT-1-1 or MAT-2 gene as a probe, confirmed the identities of the fragments. Thus, both the MAT-1-1 and MAT-2 genes were transcribed in all asexual Fusarium species involved in this experiment.
FIG. 2.
Expression of mating type genes in Fusarium species with no known sexual stage. Lanes 1 and 10, 100-bp DNA ladder; lanes 2 to 9, PCR amplification of MAT-1-specific α box from first-strand cDNA (even numbers) and genomic DNA (odd numbers) from F. avenaceum ITEM 859 (lanes 2 and 3), F. culmorum 19A1 (lanes 4 and 5), F. poae TAPO21 (lanes 6 and 7), and F. semitectum ITEM 3192 (lanes 8 and 9); lanes 11 to 18, PCR amplification of MAT-2-specific HMG box from first-strand cDNA (odd numbers) and genomic DNA (even numbers) from F. avenaceum ITEM 858 (lanes 11 and 12), F. culmorum 11F1 (lanes 13 and 14), F. poae TAPO34 (lanes 15 and 16), and F. semitectum ITEM 3390 (lanes 17 and 18).
DISCUSSION
For the present study, we developed a robust PCR-based method suitable for the identification of mating type in several Fusarium species with no known sexual stage. To achieve this, we designed PCR primers on the basis of known mating type sequences as well as conserved α and HMG box sequences of four asexual Fusarium species. All Fusarium species involved in this work were found to contain one or the other mating type idiomorph, with the exception of F. graminearum, which was used as a control. The MAT-1- and MAT-2-specific fragments that were amplified from these fungi showed substantial sequence similarities to conserved motifs of the MAT-1-1 and MAT-2 genes from F. oxysporum, G. fujikuroi, and G. zeae, suggesting that these partial sequences represent the mating type idiomorphs in these fungi. Degenerate MAT-specific primers designed by Arie et al. (3) or the G. fujikuroi-specific MAT primers developed in previous studies (13, 22) were unsuitable for generating unambiguous PCR fragments in such diverse Fusarium species (representatives of nine sections) due to sequence differences within the conserved MAT regions of these fungi.
Our findings clearly show that conserved MAT-specific sequences are present and expressed in Fusarium species with no known sexual stage. Since the strains of F. acuminatum, F. avenaceum, F. camptoceras, F. cerealis, F. chlamydosporum, F. compactum, F. culmorum, F. equiseti, F. longipes, F. merismoides, F. poae, F. semitectum, F. scirpi, F. sporotrichioides, F. torulosum, F. tricinctum, and F. tumidum all contained only a single MAT allele, presumably they are capable of heterothallic, but not homothallic, mating. These results are consistent with the hypothesis (24) that these fungi may have a cryptic sexual cycle, even though sexual structures have not been identified in field collections and there are no reports of successful forced parings among them in laboratory experiments.
Leslie and Klein (15) explained the absence of sexual reproduction in local populations of the G. fujikuroi species complex by the presence of mutations that concomitantly resulted in female sterility with an increased vegetative propagation capability. Selection for an increased number of asexual propagules can result in a selective accumulation of female sterile strains, which could become prevalent even in large geographic areas. Under such conditions, mating is limited by the absence of normal female fertile partners. The Fusarium species that we examined seem to have functional mating type genes, are aggressive pathogens, and can colonize a wide range of decaying substrates. Populations of these fungi could easily be dominated by successful female sterile clonal lineages that produce more asexual propagules and are therefore not under significant immediate selection pressure to participate in sexual reproduction. The female fertile strains could be such a small minority (<10% in some natural populations [15]) that they are likely to be infrequent, especially under epidemic conditions. Thus, their sexual structures may not be observed in nature simply because of their rarity. Alternatively, the purportedly asexual species also may require environmental conditions for sexual reproduction that are uncommon when disease epidemics occur or that are difficult or unusual conditions to mimic in the laboratory.
The PCR method that we developed for the mating type assessment of these Fusarium species facilitates the recognition of potentially compatible strains that could be used in crossing experiments to obtain teleomorphic structures. This approach could increase our knowledge of reproductive strategies in these fungi and allow a realistic evaluation of the potential for generating strains with new pathotypes and/or altered mycotoxin-producing abilities and could be used to assess disease control strategies that presume that limited genotypic variation and rearrangement occur within the pathogen population.
Acknowledgments
This research was supported in part by the Fifth Framework Program of the EU (QLK1-CT-1999-001380, DeTox-Fungi) and by a grant from OTKA (T 034735). Z.K. is the recipient of a Bolyai fellowship.
We are indebted to E. Barta for his advice on phylogenetic comparisons.
REFERENCES
- 1.Agrios, G. N. 1997. Plant pathology. Academic Press, San Diego, Calif.
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. P. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Arie, T., S. K. Christiansen, O. C. Yoder, and B. G. Turgeon. 1997. Efficient cloning of ascomycete mating-type genes by amplification of the conversed MAT HMG box. Fungal Genet. Biol. 21:118-130. [PubMed] [Google Scholar]
- 4.Arie, T., I. Kaneko, T. Yoshida, M. Noguchi, Y. Nomura, and I. Yamaguchi. 2000. Mating type genes from asexual phytopathogenic ascomycetes Fusarium oxysporum and Alternaria alternata. Mol. Plant-Microbe Interact. 13:1330-1339. [DOI] [PubMed] [Google Scholar]
- 5.Booth, C. 1984. Perfect states (teleomorphs) of Fusarium species, p. 446-452. In W. F. O. Marasas, P. E. Nelson, and T. A. Tousson (ed.), Toxigenic Fusarium species. Pennsylvania State University Press, University Park, Pa.
- 6.Correll, J. C., C. J. R. Klittich, and J. F. Leslie. 1987. Nitrate nonutilizing mutants of Fusarium oxysporum and their use in vegetative compatibility tests. Phytopathology 77:1640-1646. [Google Scholar]
- 7.Cumagun, C. J. R., R. L. Bowden, and T. Miedaner. 2002. Segregation of aggressiveness in a crossing population of Fusarium graminearum. J. Appl. Genet. 43A:39-44. [Google Scholar]
- 8.De Nijs, M., J. S. Larsen, W. Gams, F. M. Rombouts, K. Wernars, U. Thrane, and S. H. W. Notermans. 1997. Variations in random amplified polymorphic DNA patterns and secondary metabolite profiles within Fusarium species from cereals from various parts of The Netherlands. J. Food Microbiol. 14:449-457. [Google Scholar]
- 9.Desjardins, A. E., T. M. Hohn, and S. P. McCormick. 1993. Trichothecene biosynthesis in Fusarium species: chemistry, genetics, and significance. Microbiol. Rev. 57:595-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devereux, J., P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for VAX. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass, N. L., and G. A. Kuldau. 1992. Mating type and vegetative incompatibility in filamentous ascomycetes. Annu. Rev. Phytopathol. 30:201-224. [DOI] [PubMed] [Google Scholar]
- 12.Kerényi, Z., É. Táborhegyi, A. Pomázi, and L. Hornok. 1997. Variability amongst strains of Fusarium poae assessed by vegetative compatibility and RAPD polymorphism. Plant Pathol. 46:882-889. [Google Scholar]
- 13.Kerényi, Z., K. Zeller, L. Hornok, and J. F. Leslie. 1999. Molecular standardization of mating type terminology in the Gibberella fujikuroi species complex. Appl. Environ. Microbiol. 65:4071-4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klittich, C. J. R., and J. F. Leslie. 1988. Nitrate reduction mutants of Fusarium moniliforme (Gibberella fujikuroi). Genetics 118:417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leslie, J. F., and K. K. Klein. 1996. Female fertility and mating type effects on effective population size and evolution in filamentous fungi. Genetics 144:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McDonald, B. A., and J. M. McDermott. 1993. Population genetics of plant pathogenic fungi. BioScience 43:311-319. [Google Scholar]
- 17.Miedaner, T., and A. G. Schilling. 1996. Genetic variation of aggressiveness in individual field populations of Fusarium graminearum and Fusarium culmorum tested on young plants of winter rye. Eur. J. Plant Pathol. 102:823-830. [Google Scholar]
- 18.Nelson, P. E., A. E. Desjardins, and R. D. Plattner. 1993. Fumonisins, mycotoxins produced by Fusarium species: biology, chemistry, and significance. Annu. Rev. Phytopathol. 31:233-252. [DOI] [PubMed] [Google Scholar]
- 19.Obst, A., B. Günther, R. Beck, J. Lepschy, and H. Tischner. 2002. Weather conditions conducive to Gibberella zeae and Fusarium graminearum head blight of wheat. J. Appl. Genet. 43A:185-192. [Google Scholar]
- 20.Radford, A., and J. H. Parish. 1997. The genome and genes of Neurospora crassa. Fungal Genet. Biol. 21:258-266. [DOI] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Steenkamp, E. T., B. D. Wingfield, T. A. Coutinho, K. A. Zeller, M. J. Wingfield, W. F. O. Marasas, and J. F. Leslie. 2000. PCR-based identification of MAT-1 and MAT-2 in the Gibberella fujikuroi species complex. Appl. Environ. Microbiol. 66:4378-4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Triglia, T., M. G. Peterson, and D. J. Kemp. 1988. A procedure for in vitro amplification of DNA segments that lie outside the boundaries of known sequences. Nucleic Acids Res. 16:8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turgeon, B. G. 1998. Application of mating type gene technology to problems in fungal biology. Annu. Rev. Phytopathol. 36:115-137. [DOI] [PubMed] [Google Scholar]
- 25.Wallace, M. M., and S. F. Covert. 2000. Molecular mating type assay for Fusarium circinatum. Appl. Environ. Microbiol. 66:5506-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yli-Mattila, T., S. Paavanen-Huhtala, S. A. Bulat, I. A. Alekhina, and H. I. Nirenberg. 2002. Molecular, morphological and phylogenetic analysis of the Fusarium avenaceum/F. arthrosporioides/F. tricinctum species complex—a polyphasic approach. Mycol. Res. 106:655-669. [Google Scholar]
- 27.Yun, S. H., T. Arie, I. Kaneko, O. C. Yoder, and B. G. Turgeon. 2000. Molecular organization of mating type loci in heterothallic, homothallic, and asexual Gibberella/Fusarium species. Fungal Genet. Biol. 31:7-20. [DOI] [PubMed] [Google Scholar]