Abstract
Maintaining reactive oxygen species (ROS) homeostasis plays a central role in plants, and is also critical for plant root development. Threshold levels of ROS act as signals for elongation and differentiation of root cells. The protein phosphatase LIKE SEX FOUR2 (LSF2) has been reported to regulate starch metabolism in Arabidopsis, but little is known about the mechanism how LSF2 affect ROS homeostasis. Here, we identified that LSF2 function as a component modulating ROS homeostasis in response to oxidative stress and, thus regulate root development. Compared with wild type Arabidopsis, lsf2-1 mutant exhibited reduced rates of superoxide generation and higher levels of hydrogen peroxide upon oxidative stress treatments. The activities of several antioxidant enzymes, including superoxide dismutase, catalase, and ascorbate peroxidase, were also affected in lsf2-1 mutant under these oxidative stress conditions. Consequently, lsf2-1 mutant exhibited the reduced root growth but less inhibition of root hair formation compared to wild type Arabidopsis plants. Importantly, protein phosphatase LSF2 interacted with mitogen-activated protein kinase 8 (MPK8), a known component of ROS homeostasis pathways in the cytoplasm. These findings indicated the novel function of LSF2 that controls ROS homeostasis to regulate root development.
Plants absorb water and nutrients from the soil through their roots and root hairs, which sense abiotic and biotic stress during plant life cycle. Reactive oxygen species (ROS) play critical roles in root growth and root hair development in plants1. The most common ROS are hydroxyl radical (·OH), superoxide radicals (O2•−), and hydrogen peroxide (H2O2). Compared with other types of ROS, H2O2 is more stable, but O2•− is spontaneously or enzymatically dismutated to H2O2. ·OH in plants is directly responsible for cell wall loosening and cell expansion in the root elongation zone2. In Arabidopsis thaliana roots, O2•− localizes predominantly to the apoplast of cell elongation zone, and H2O2 is present mainly in the differentiation zone and cell walls of root hairs3. Accumulation of H2O2 inhibits root growth but promotes the growth and formation of adventitious roots4. Studies in which ROS levels were modified by various treatments have demonstrated that O2•− and H2O2 are involved in root growth and root hair development.
Low levels of ROS serve as regulatory signals for the cell, but high levels of ROS, representing byproducts of plant aerobic metabolism produced in response to environmental stresses, are undesirable or toxic5,6. When Arabidopsis leaves were exposed to the herbicide paraquat (methyl viologen, MV), H2O2 accumulation increased7. Salicylic acid (SA) is an oxidative signal inducer that is required for the development of systemic acquired resistance (SAR) against various pathogens through its interaction with NPR3 and NPR48. Plants contain several ROS scavenging enzymes, including superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT). The SOD family of metalloenzymes, including iron SOD (Fe-SOD), manganese SOD (Mn-SOD), and copper-zinc SOD (Cu/Zn-SOD), specifically catalyze the dismutation of O2•− to H2O2 and molecular oxygen (O2)9. APX is a scavenging peroxidase of H2O2 that uses ascorbate as a reductant and has a high affinity for H2O2. CAT is the key enzyme that scavenges H2O2, with a high reaction rate but low affinity for H2O210. ROS act as either damaging or signaling molecules in plants depending on their levels, which in turn depend on the rates of production and scavenging of ROS.
The precise balance of ROS plays an important role in regulating plant growth and development. Recent studies suggest that ROS homeostasis regulates the transition from cell proliferation to cell differentiation in Arabidopsis roots. Arabidopsis UPBEAT1 (UPB1) controls the balance between O2•− and H2O2 by repressing peroxidases; upb1 mutant plants contain higher O2•− levels and lower H2O2 levels in the root tip compared with the wild type plants, suggesting that ROS homeostasis is altered in these plants, leading to changes in root growth and development11. Lack of RHD2/AtrbohC in the Arabidopsis rhd2 mutant causes it to have reduced ROS levels and atypical tubulin formation, affecting cell division of the root meristem12. PHYTOCHROME AND FLOWERING TIME1 (PFT1), which primarily controls the expression of class III peroxidase genes (such as UPBEAT) in Arabidopsis, regulates the expression of genes encoding redox-active proteins13. In the pft1 mutant, the balance between O2•− and H2O2 is altered, resulting in defective root hair differentiation. Over-expressing of PLANT FERREDOXIN-LIKE PROTEIN (PFLP) in Arabidopsis increases ROS production and significantly promotes root hair growth14. Taken together, these studies support the important role of ROS homeostasis in regulating root growth and development.
In Arabidopsis, protein phosphatases play key roles in regulating cellular activities and often affect ROS homeostasis, especially in response to environmental stimuli15,16,17. There are two known glucan phosphatases in plants: STARCH EXCESS4 (SEX4) and LIKE SEX FOUR2 (LSF2). SEX4 is a major phosphatase that binds to starch granules, and is regulated by redox18. It is a disulfide bridge in the SEX4 phosphatase domain that acts as a redox-dependent switch to regulate SEX4 activity19. LSF2, which is located in the chloroplast and cytoplasm, specifically hydrolyzes phosphate groups from the C3-position and is involved in transitory starch metabolism20. However, no study has proved that LSF2 activity is controlled by redox in plants21. In this present study, for the first time, we identify that LSF2 plays a role in ROS homeostasis to regulate root growth and root hair development in Arabidopsis.
Results
ROS status is balanced under exogenous oxidative stresses in lsf2–1 mutant
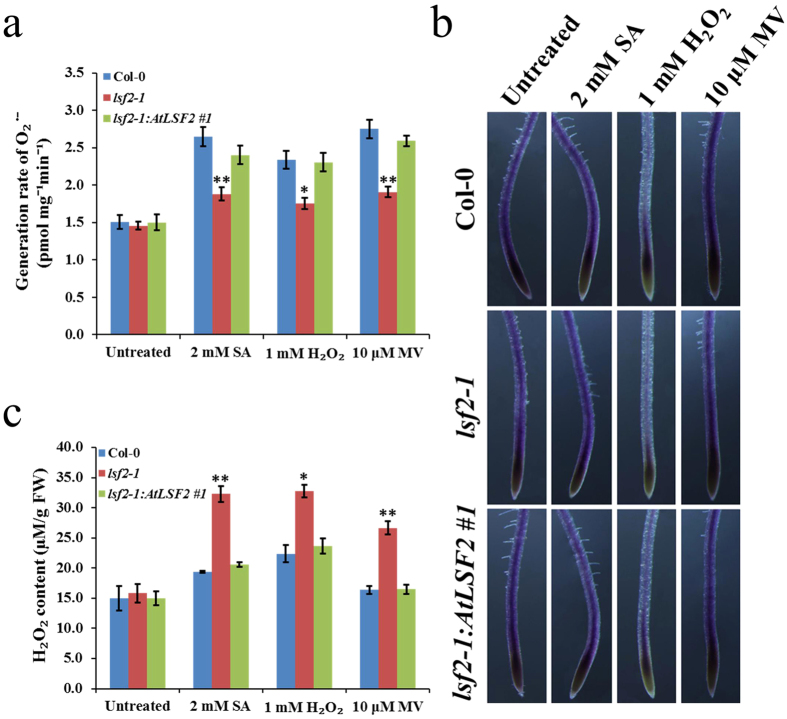
In order to determine the role of LSF2 in maintenance of ROS levels, we have isolated a homozygous T-DNA inserted mutant lsf2-1 allele (SAIL line 595_F04 of AT3G10940 from ABRC; Supplementary Fig. S1a–c) and four independent homozygous lsf2-1:AtLSF2 complementary transgenic lines (Supplementary Fig. S2a,b). As it is already known that O2•− is rapidly converted to H2O2 spontaneously or via dismutation by SOD enzymes. We used XTT as a probe to photometrically measure the generation rate of O2•−, as XTT is reduced to soluble formazan by O2•−. Under normal growth conditions, there were no statistical significant differences in O2•− generation between lsf2-1 and Col-0 (Fig. 1a, untreated). When treated with oxidative stress upon exposure to H2O2, the generation rate of O2•− was obviously lower in lsf2-1 than in Col-0 plants, and these differences were more significant after treatment with exogenous SA or MV (Fig. 1a). We also used NBT as a histochemical probe for O2•−, as it forms an insoluble blue/violet formazan product in the presence of O2•−. Strong blue/violet staining (revealing O2•−) was detected in the elongation zone under abiotic stress treatment, which also extended to the meristematic zone and differentiation zone (Fig. 1b). However, no significant differences were observed between the staining patterns of Col-0, lsf2-1, and lsf2-1:AtLSF2 #1 roots. These results suggest that null mutation of LSF2 in Arabidopsis results in more suppressed generation of O2•− in response to exogenous oxidative stresses in leaves.
Figure 1. Generation rate of O2•− and H2O2 content in plants.
The generation rate of O2•− (a) or endogenous H2O2 content (c) was examined after pre-treated with SA, H2O2 or MV (mean values ± SE, n = 3; student’s t-test, *P < 0.05; **P < 0.01). (b) NBT stained main roots that were pre-treated with SA, H2O2 or MV.
The accumulation of endogenous H2O2 in plants is an early response to environmental stimuli, such as pathogen attack and exogenous application of SA or MV22. We measured the contents of endogenous H2O2 using a peroxidase enzyme assay. No significant differences in H2O2 levels were detected between lsf2-1 and Col-0 plants under normal growth conditions (Fig. 1c, untreated). However, compared with Col-0 plants, lsf2-1 mutant plants produced higher levels of H2O2 under oxidative stress conditions (Fig. 1c). These results indicate that endogenous H2O2 accumulates much more rapidly in lsf2-1 than in Col-0 plants under oxidative stress conditions, suggesting that LSF2 is involved in regulating endogenous H2O2 levels exposed to environmental stress. Analysis of LSF2 expression patterns under these exogenous oxidative stresses (SA, H2O2 and MV) in Col-0 plants, the transcriptional expression of LSF2 was strongly induced in leaves but slightly down-regulated in roots (Supplementary Fig. S1d). Therefore, we hypothesized that LSF2 might regulate ROS homeostasis (to a certain extent) by maintaining the balance between O2•− levels and H2O2 levels in Arabidopsis leaves.
Oxidative stress affects antioxidant enzyme activities in lsf2-1 mutant
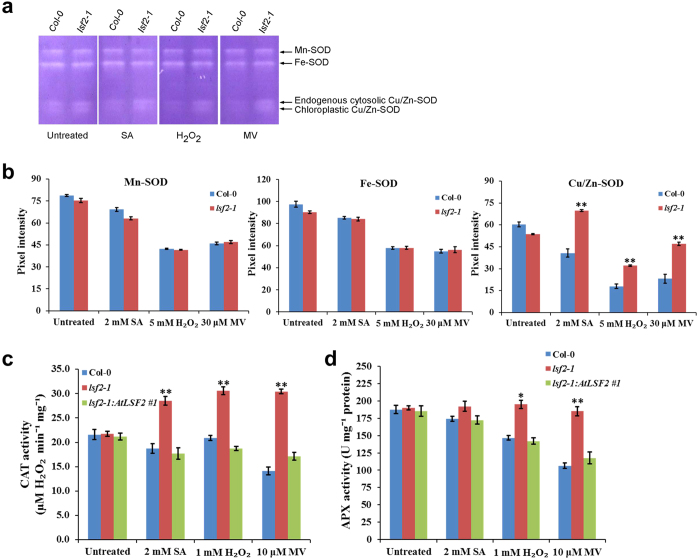
By determining the levels of ROS in the lsf2-1 mutant and wild type Col-0 plants, we found that the species of O2•− and H2O2 were altered in the lsf2-1 mutant under external treatment of several oxidative stress inducers. Therefore, we planned to investigate the activities of several enzymes involved in ROS homeostasis such as SOD, APX and CAT in lsf2-1 mutant under oxidative stress. In plants, SOD represents the first line of defense against excess ROS by catalyzing the dismutation of O2•− to H2O223. Plants contain four types of SODs: Fe-SOD is present in plastids, Mn-SOD is mainly located in the mitochondria, and Cu/Zn-SOD isoforms are normally present in the cytosol and chloroplasts. Here, we investigated SOD activities through NBT staining following non-denaturing polyacrylamide gel electrophoresis, and found that there were no obvious differences in the four SOD profiles (as an indicator of SOD activities) between lsf2-1 mutant and Col-0 plants under normal growth conditions (Fig. 2a, untreated). When the seedlings were exposed to inducers of oxidative stress such as SA, H2O2, or MV, no change in Mn-SOD or Fe-SOD activity was observed in Col-0 and lsf2-1 mutant plants; but the activities of endogenous cytosol Cu/Zn-SOD and chloroplast Cu/Zn-SOD were less inhibited in lsf2-1 compared with Col-0 under oxidative stress conditions (Fig. 2a,b), suggesting that the activities of Cu/Zn-SODs were more strongly inhibited in the presence of Arabidopsis LSF2 under oxidative stress.
Figure 2. The activities of ROS antioxidant enzymes in plants.
(a) SOD activity was examined by NBT staining after non-denaturing PAGE. (b) Pixel intensity of SODs was calculated using a plot profile. CAT activity (c) and APX activity (d) were examined after pre-treatment with SA, H2O2 or MV (n = 3; student’s t-test, *P < 0.05; **P < 0.01).
CAT activity is measured by monitoring the extinction of H2O2, whereas APX activity is measured by monitoring ascorbate oxidization. Since endogenous H2O2 levels increased much more strongly in lsf2-1 mutant than in Col-0 plants under oxidative stress conditions, we analyzed the activities of CAT and APX in Col-0, lsf2-1, and lsf2-1:AtLSF2 #1 plants. Under normal growth conditions, no obvious differences in the activities of these enzymes were detected in lsf2-1 mutant compared with Col-0 plants (Fig. 2c,d, untreated). When the seedlings were treated with SA, H2O2, or MV, reduced CAT and APX activity were observed in Col-0, but more significant increases in CAT activity were observed in lsf2-1 mutant (Fig. 2c). However, APX activity was not altered in lsf2-1 in response to the various oxidative stress conditions (Fig. 2d). When seedlings were under oxidative stress, CAT and APX activity were inhibited in the presence of LSF2, whereas CAT activity increased in the absence of LSF2, and APX activity was not altered in the absence of this protein. Therefore, CAT, not APX, is mainly responsible for H2O2 scavenging in the absence of Arabidopsis LSF2 under oxidative stress conditions.
Root development is affected by oxidative stresses in lsf2-1 mutant
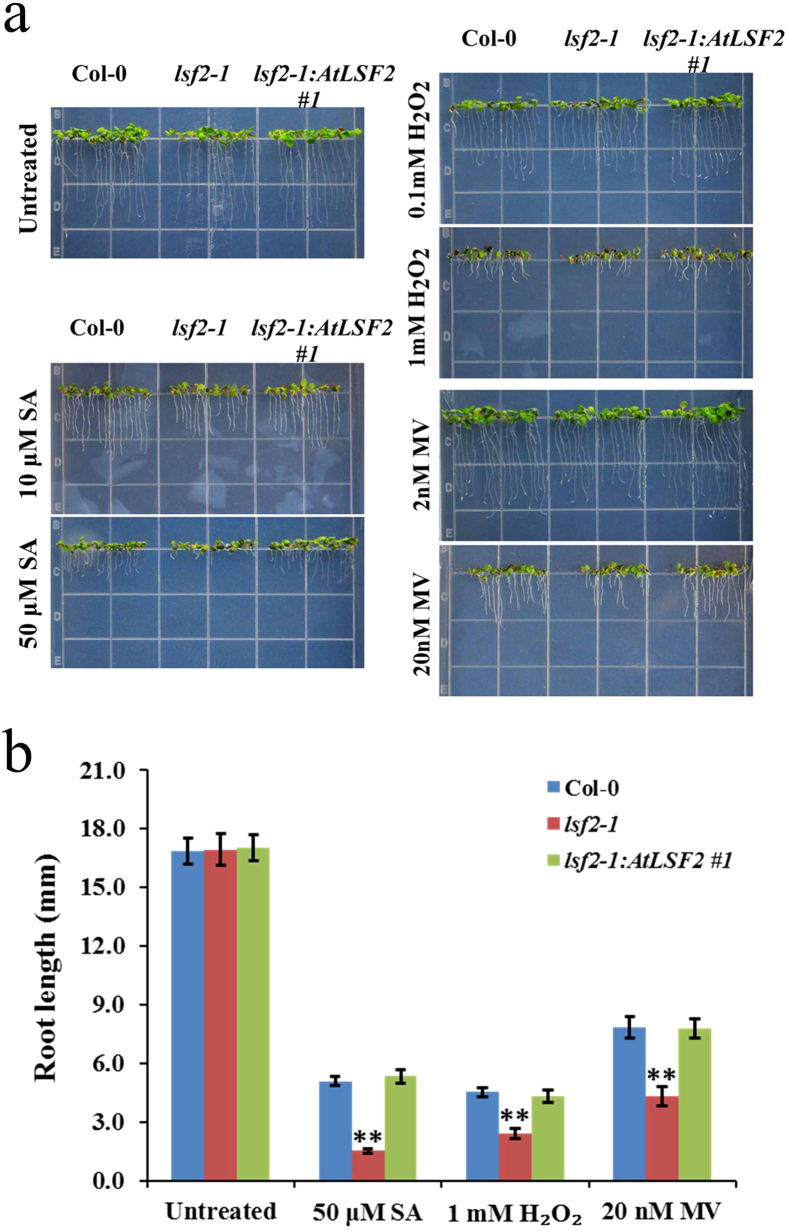
Based on the altered activities of ROS scavenging enzymes such as SOD, APX and CAT in lsf2-1 and wild type Col-0 plants under several oxidative stress conditions, it seems that LSF2 might be involved in differential regulation of these above mentioned enzymes, which could be the reason for the altered levels of ROS. Previous studies have suggested that ROS level also regulates the root length and root hair development1,24. Therefore, we have performed the detail phenotypic assays to investigate the root growth and development under various oxidative stress conditions. Under normal growth conditions, the phenotype of lsf2-1 mutant was not significantly different from that of Col-0. However, when treated with exogenous oxidative stress via exposure to SA, H2O2, or MV, lsf2-1 mutant plants grew poorly compared with Col-0 plants (Fig. 3a,b). Specifically, lsf2-1 roots were shorter than Col-0 roots on medium containing 10 μM SA, 0.1 M H2O2, or 2 nM MV (Fig. 3a). The differences in root length between Col-0 and lsf2-1 became more pronounced when grown on medium containing higher concentrations of these agents, such as 50 μM SA, 1 mM H2O2, or 20 nM MV (Fig. 3a,b). These findings indicate that the root growth of lsf2-1 is more strongly inhibited than that of Col-0 under oxidative stress conditions, suggesting that lsf2-1 mutant is hypersensitive to oxidative stress.
Figure 3. Phenotypic analysis of root growth in lsf2-1 mutant and wild type plants under oxidative stress conditions.
(a) Seedlings were grown under oxidative stress. (b) The assay of root length of seedlings, which were grown on MS medium containing 50 μM SA, 1 mM H2O2, or 20 nM MV (n = 20; student’s t-test, **P < 0.01).
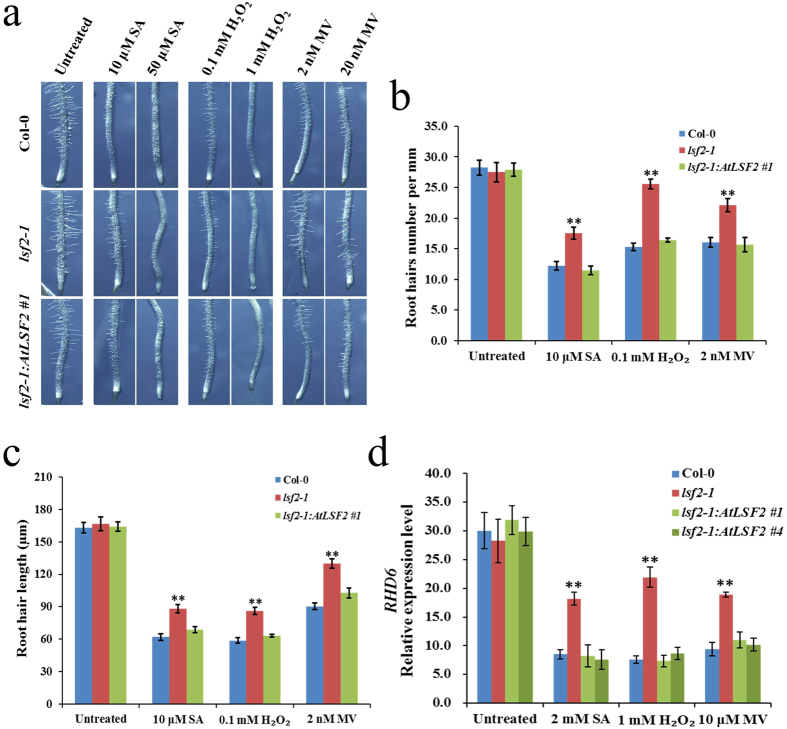
No significant differences were found in root hair length or root hair number between lsf2-1 mutant and Col-0 plants under normal growth conditions (Fig. 4a, untreated). However, when treated with oxidative stress via exposure to high concentrations of oxidative stress-inducing agents (50 μM SA, 1 mM H2O2, or 20 nM MV), root hair formation was almost completely inhibited in both Col-0 and lsf2-1 mutant (Fig. 4a). When grown on lower concentrations of these agents (such as 10 μM SA, 0.1 mM H2O2, or 2 nM MV), the root hair density of lsf2-1 was higher than those of Col-0 (Fig. 4b), and the root hairs length of lsf2-1 were much longer than those of Col-0 (Fig. 4c). We also examined the expression of ROOT HAIR DEFECTIVE6 (RHD6), which positively regulates root hair development25. The transcriptional expression of RHD6 was very significantly higher in lsf2-1 than in Col-0 under oxidative stress conditions (Fig. 4d). Together, these results indicate that deficiency of LSF2 in Arabidopsis results in less inhibition of root hair development, as well as less inhibition of RHD6 expression, under oxidative stress conditions compared with normal growth condition (Fig. 4d, untreated).
Figure 4. Phenotypic analysis of root hair development in lsf2-1 mutant and wild type plants under oxidative stress conditions.
Morphology of root hairs (a) of seedlings was grown under oxidative stress. Average number of root hairs per mm (b) and root hair length (c) were determined in seedlings which were grown on MS medium containing 10 μM SA, 0.1 mM H2O2, or 2 nM MV (n = 20; student’s t-test, **P < 0.01). (d) The relative expression of RHD6 under oxidative stress was detected by qPCR (n = 3; student’s t-test, **P < 0.01).
Arabidopsis LSF2 interacts with MPK8 in vivo
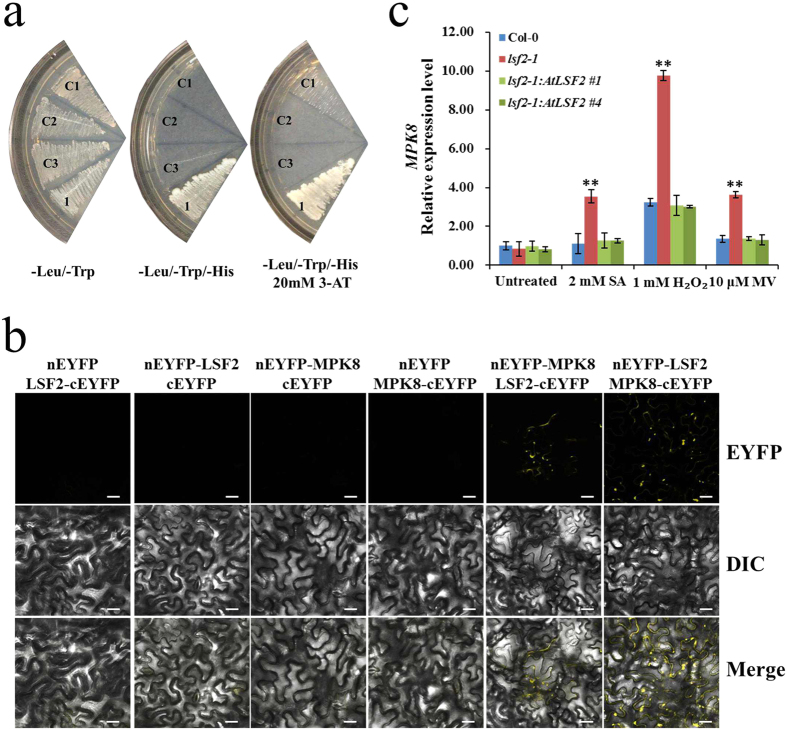
Previously, several MAP kinases have been shown to be involved in both biotic and abiotic stresses and affect the ROS homeostasis26. Also dual specificity phosphatases (DSPs) are known to regulate and target several MAP kinases in different abiotic stress condition, such as oxidative stress. To determine if LSF2, a dual specificity protein phosphatase, is also targeting any MAP kinase, we have performed a comprehensive yeast two-hybrid analysis with several MAP kinases (data not shown). Using yeast two-hybrid assays, we found that LSF2 interacted with MPK8 (AT1G18150) (Fig. 5a). To confirm this finding, we purified both full-length LSF2 fused with His6 tag and full-length MPK8 fused with glutathione-S-transferase (GST) from E. coli extracts, and then examined the possibility of LSF2 and MPK8 to interact in vitro via a pull down assay and western blotting with anti-GST antibody. The results showed that MPK8 interacted with LSF2 in vitro (Supplementary Fig. S3). To further examine the interaction between LSF2 and MPK8 in vivo, we performed a bimolecular fluorescence complementation (BiFC) assay. Agrobacterium tumefacien strain EHA105 containing expression binary vectors for nEYFP-LSF2 together with MPK8-cEYFP, or LSF2-cEYFP together with nEYFP-MPK8, were co-infiltrated into N. benthamiana leaves, respectively. Coexpression of nEYFP-LSF2 and MPK8-cEYFP (or LSF2-cEYFP and nEYFP-MPK8) resulted in strong fluorescent signals in the cytoplasm due to reconstitution of the fluorescent marker, EYFP (Fig. 5b). No fluorescent signals were observed when nEYFP-LSF2 or LSF2-cEYFP was coexpressed with cEYFP or nEYFP empty vector, which served as negative controls. Interestingly, the transcriptional expression level of MPK8, not respiratory burst oxidase homolog D (RbohD), was enhanced under these oxidative stress conditions in leaves of lsf2-1 mutant (Fig. 5c; Supplementary Fig. S4).
Figure 5. Interaction analysis of LSF2 and MPK8 by Y2H and BiFC methodologies.
(a) Interaction between LSF2 and MPK8 was assayed by Y2H, C1: AD + BD; C2: AD-LSF2 + BD; C3: AD + BD-MPK8; 1: AD-LSF2 + BD-MPK8. (b) Fluorescence was observed due to complementation of fusion protein LSF2-cEYFP with nEYFP-MPK8, or nEYFP-LSF2 with MPK8-cEYFP, Bars = 20 μm. (c) The relative expression of MPK8 under oxidative stress was detected by qPCR (n = 3; student’s t-test, **P < 0.01).
Discussion
Protein phosphatases (PPs) play important roles in plants15. To date, only a few PPs have been characterized in Arabidopsis and other flowering plants. AtRP1 (PPDK regulatory protein) not only exhibits functional properties of protein phosphatases, but it also displays functional properties of protein kinases, as it regulates the activity of pyruvate orthophosphate dikinase (PPDK) via phosphorylation or dephosphorylation of its threonine residue27. Interestingly, the plastid-localized enzyme NADP-THIOREDOXIN REDUCTASE C (NTRC) regulates starch synthesis in chloroplasts and amyloplasts in response to either light or sucrose, and it is also involved in maintaining redox homeostasis in plastids of both photosynthetic and nonphotosynthetic tissues, thereby affecting the formation of lateral roots in Arabidopsis28,29. LSF2 is a dual specificity protein phosphatase that displays high activity toward para-nitrophenyl phosphate (pNPP) (Supplementary Fig. S5), and it dephosphorylates glucan to provide access for amylases that release maltose and glucose from starch in Arabidopsis30. When the effect of LSF2 null-mutation compared at morphological level, a severity of curly dwarf symptom in lsf2-1 leaves was increased under SA treatment (Supplementary Fig. S6). This suggests the possibility that LSF2 could improve the tolerance to oxidative stresses in plant, which was correlated with the up-regulated expression of LSF2 in wild type leaves under oxidative stress conditions. Hence, the function of LSF2 is not only projected in the root development but LSF2 could also be involved in improving plant tolerance to oxidative stresses. The present results suggest that LSF2 also functions in maintaining cellular ROS homeostasis in plants.
Many environmental and genetic factors influence root growth and root hair formation, such as light, calcium, free radicals, and ethylene31. Changes in redox are involved in controlling plant growth and development, and ROS production is associated with root development32. NADPH oxidases and peroxidase have often been shown to be responsible for the production of endogenous O2•− and H2O2 in plants. When H2O2 levels are reduced in plant, increased production of O2•− is an important mechanism that maintains ROS homeostasis11. Redox regulation of AGPase is severely affected in leaves and roots of the Arabidopsis ntrc mutant, and introducing fully functional chloroplasts into this mutant by complementation is both necessary and sufficient for sustaining root growth and lateral root formation, with similar rates to those of the wild type28. The induction of REDOX RESPONSIVE TRANSCRIPTION FACTOR1 (RRTF1) in plants results in ROS accumulation via ROS-generating biotic signals33. Therefore, the balance between O2•− and H2O2 to maintain ROS homeostasis might be involved in root development in plants. Accordingly, in the present study, we investigated the production of O2•− and H2O2 and the activities of several antioxidant enzymes in response to oxidative stresses, and we examined the associated effect of the absence of functional LSF2 on root development in Arabidopsis. Under oxidative stress conditions, the generation rate of O2•− was obviously lower, and the level of endogenous H2O2 was higher, in the lsf2-1 mutant. These changes are consistent with the changes in Cu/Zn-SODs, CAT, and APX activity observed in the lsf2-1 mutant, which led to more inhibition of root growth and less inhibition of root hair development under oxidative stress conditions compared to Col-0 plants. Meanwhile, the expression of some of the marker genes involved in the regulation of root elongation, such as SCN1, CPC and PIN3, were analyzed between Col-0 and lsf2-1 mutant, but no significant difference was found (Supplementary Fig. S7). Furthermore, the expressions of marker genes involved in the regulation of root hairs also were determined by qPCR, including RHD6 gene. RHD6 regulates the development of root hair-forming cells, and Arabidopsis rhd6 mutants have fewer root hairs than the wild type25. A recent report showed that ROS production is induced by vanadate treatment and that RHD6 is involved in vanadate-induced root hair initiation34. In the present study, the lack of functional LSF2 resulted in less reduced expression of RHD6 under oxidative stress in Arabidopsis, which is consistent with the reduced inhibition of root hair development in the lsf2-1 mutant compared with Col-0 plants. Collectively, the results demonstrate that LSF2 affects root development through modulating ROS homeostasis in Arabidopsis.
Maintaining steady ROS levels appears to require a network involving at least 152 genes in Arabidopsis35. PFT1 mainly regulates ROS-related gene expression to control the formation of root hairs via ROS homeostasis. Lower levels of H2O2 are correlated with the reduced expression of POX, and higher levels of O2•− are correlated with the increased expression of NADPH13. A recent study demonstrated that Mitogen-activated protein kinase kinase kinase 1 (MEKK1) is involved in cellular redox control and integrating ROS homeostasis with plant development and hormone signaling, and MPK4 is the downstream target of MEKK1 in plants36,37. Ca2+ influx and Ca2+/Calmodulins (CaMs) regulate ROS homeostasis and the activities of all antioxidant enzymes. MPK6 mediates Ca2+ influx across the plasma membranes of root cells to control H2O2-induced root elongation in Arabidopsis38. Arabidopsis MPK8 interacts with CaM3, CaM4, and CaM7 in the cytoplasm, and is activated by CaMs and mitogen-activated protein kinase kinase 3 (MKK3) to help maintain ROS homeostasis39. After mechanical wounding, ROS accumulation increases in the wounding sites of the mpk8 mutant, whereas ROS accumulation decreases in the tissues of MPK8-GFP plants. Furthermore, MPK8 has a conserved phosphorylation motif-TDY and is dual phosphorylated in Arabidopsis pollen at Thr and Tyr residues in the TDY motif40. LSF2, a dual specificity protein phosphatase, dephosphorylates phospho-Ser, -Thr, and -Tyr in proteinaceous substrates. We propose that LSF2 might also inactivate MPK8 in Arabidopsis through dephosphorylation. Our results also show that production of ROS in lsf2-1 mutant is independent of RbohD, which functions as an essential ROS regulator. However, the expression of MPK8 is induced under these oxidative stress conditions in leaves of lsf2-1 mutant, and LSF2 interacts with MPK8 in the cytoplasm, suggesting that LSF2 regulates ROS homeostasis through interaction with MPK8 in response to oxidative stress in Arabidopsis.
Materials and Methods
Plant materials
All plant materials used in this study were in the ecotype Columbia-0 (Col-0) Arabidopsis thaliana background. The lsf2-1 allele is the SAIL line 595_F04 of AT3G10940 and was obtained from the Arabidopsis Biological Resource Center (ABRC). To produce the construct used for functional complementation of the lsf2-1 mutant, the genomic DNA fragment of LSF2 (gLSF2) including the 5′promoter region, coding region, 3′UTR, and following 1282 bp sequence was amplified with the primer pair gLSF2_Fw and gLSF2_Rv (Supplementary Table S1). The resulting fragment was cloned into entry vector pENTR-TOPO (Invitrogen), followed by cloning into binary vector pBA002a. The construct pBA002a-gLSF2 was transformed into the EHA105 strain of Agrobacterium tumefaciens, followed by transformation into lsf2-1 mutant plants by the floral dip method41. Transgenic plants harboring gLSF2 were selected on MS medium plates containing 50 μg/ml BASTA. T1 seedlings expressing gLSF2 were grown in soil, and homozygous plants of the four independent complementary lines (lsf2-1:AtLSF2) were identified after three rounds of BASTA application. The insertion site of gLSF2 was determined by genome walking kit (Takara) with three specific primers (SP1, SP2 and SP3; Supplementary Table S1).
Arabidopsis seeds were surface-sterilized, and imbibed for 2 days at 4 °C in darkness for stratification, then germinated on MS medium solidified with 0.8% agar in Petri plates. Plants were grown in growth chamber under controlled conditions, 22 °C day/18 °C night with a relative humidity of 60% under long-day conditions (16-h light illumination of 150 μmol·m−2·sec−1, and 8-h dark cycle).
RNA extraction and qPCR analysis
Total RNA was extracted from Arabidopsis using an RNeasy Plant Mini kit (Qiagen). Reverse transcription was performed with 2 μg each of total RNA and oligo (dT)18 primers using the SuperScript II RT kit (Invitrogen). The cDNA was mixed with SYBR premix Ex Taq (TaKaRa) and gene-specific primers, and amplified in a Bio-Rad CFX96 real-time system. Expression of ACTIN2 was used as a control for cDNA levels. The primer sequences used for qPCR are listed in Supplementary Table S1.
Determination of O2 •− generation and endogenous H2O2 contents
Pre-treating 2-week-old Col-0, lsf2-1, and lsf2-1:AtLSF2 #1 plants with 2 mM SA, 1 mM H2O2, or 10 μM MV for 30 min, O2•− generation of seedlings was measured with the XTT assay, as described by Liszkay2, and endogenous H2O2 contents were quantified using a peroxidase enzyme assay according to the method of Youn-Il Park42. In addition, nitro blue tetrazolium (NBT) staining also was performed as described previously43. When NBT reacts with O2•−, the color shifts from pale yellow to dark blue, which is used to monitor the generation of superoxide radicals in a histo-chemical staining procedure. The roots were observed under a stereoscopic microscope (Olympus SZX16, Olympus Japan Co., Tokyo, Japan).
Antioxidant enzyme activity assays: superoxide dismutases (SODs), catalase (CAT), and ascorbate peroxidase (APX)
Pre-treating 2-week-old Col-0 and lsf2-1 plants with exogenous 2 mM SA for 3 h, 5 mM H2O2 for 30 min, or 30 μM MV for 2 h, SOD activity was detected using NBT staining following electrophoresis as described44. Separation of SOD isoforms was performed with each 100 μg protein sample by non-denaturing polyacrylamide gel electrophoresis (PAGE) at 4 °C using a 12% resolving gel and a 5% stacking gel. While, pre-treating 2-week-old Col-0, lsf2-1, and lsf2-1:AtLSF2 #1 plants with exogenous 2 mM SA, 1 mM H2O2, or 10 μM MV for 30 min, CAT activity was determined based on the decrease in absorbance at 240 nm through the extinction of H2O245, and APX activity was determined based on the decrease in absorbance at 290 nm due to ascorbate oxidation46.
Root growth and root hair development assays
Col-0, lsf2-1, and lsf2-1:AtLSF2 #1 seeds were surface sterilized and germinated in Petri plates containing MS medium. Exogenous oxidative treatments were performed by adding different concentrations of SA, H2O2, or MV (as noted in the figure) to the MS medium. Four- to five-day-old seedlings were transferred individually to defined MS medium. The initial position of the seedling root apex was marked, and the increase in root length was measured after an 8-day incubation. Root growth was also photo-recorded with a digital camera (Nikon D90). The length and density of root hairs were assayed under a stereoscopic microscope (Olympus SZX16). Root growth was calculated with Image J software based on the differences from photographs of the respective time points and plates.
Yeast two-hybrid (Y2H) assay
The Matchmaker GAL4-based two-hybrid system (Clontech) was used to perform yeast two-hybrid assays. The cDNAs encoding full-length LSF2 were cloned into the pGAD424 vector (Clontech) to generate activation domain (AD) constructs. The cDNA encoding full-length MPK8 was cloned into the pGBT9 vector (Clontech) to generate binding domain (BD) constructs. All constructs and empty vector controls were transformed into yeast stain AH109 using the modified lithium acetate method. Co-transformed yeast transformants were selected on plates containing SD/-Leu/-Trp medium and screened on selective medium containing SD/-Leu/-Trp/-His with 20 mM 3-amino-1,2,4,-triazole (3-AT) to test protein interactions, and empty vectors pGBT9 and pGAD424 were used as negative controls.
Expression of recombinant proteins and pull-down assay
Full-length LSF2 cDNA was cloned into pET-28a (+) (Novagen) to generate the protein expression vector for biosynthesis of the fusion protein LSF2-His6. The cDNA encoding full-length MPK8 was inserted into pGEX to generate the translational fusion of GST-MPK8. All recombinant vectors were transformed into E. coli BL21 cells. Isopropyl-β-D-thiogalactoside (IPTG) was used to induce the expression of fusion proteins. GST fusion proteins were purified on glutathione SepharoseTM R10-Flammable (GE Health), and His6 fusion protein was purified on Ni2+-nitrilotriacetate (Ni2+ -NTA) resins (Qiagen). Prey protein LSF2-His6 and bait fusion protein GST-MPK8 were then added to 1 ml of binding buffer (50 mM Tris-HCl pH 7.5, 100 mM NaCl, 0.5% Triton X-100, 0.5 mM β-mercaptoethanol, and 2% proteinase inhibiter cocktail) and incubated with amylose resin beads at 25 °C for 1 h. After incubation, the beads were washed six times with fresh binding buffer. Pull-down proteins were separated by SDS-polyacrylamide gel electrophoresis at on 7.5% resolving gel and detected by western blotting using anti-GST antibody (Santa Cruz Biotechnology).
Bimolecular Fluorescence Complementation (BiFC) assay
The vectors pBA-cEYFP(175-end)-N1 (pBA3032) and pBA-nEYFP (1-174)-C1 (pBA3036) were used as BiFC Gateway vectors, which respectively generate cDNA-cEYFP and nEYFP-cDNA47. The cDNA encoding full-length LSF2 or MPK8 was cloned into the BiFC Gateway vectors, respectively, to test interactions of the proteins in vivo. Recombinant plasmids encoding cEYFP and nEYFP fusions were transformed into competent Agrobacterium tumefaciens (strain EHA105) cells. The EHA105 transformants with recombinant plasmid were suspended in 10 mM MgCl2 and 150 μM acetosyringone. The mixtures were incubated at 25 °C for at least 3 h without shaking and co-infiltrated into Nicotiana benthamiana leaves. Microscopic observations of infiltrated plants were performed after 2–3 days. The unfused protein cEYFP or nEYFP was used as a negative control.
Additional Information
How to cite this article: Zhao, P. et al. The LIKE SEX FOUR2 regulates root development by modulating reactive oxygen species homeostasis in Arabidopsis. Sci. Rep. 6, 28683; doi: 10.1038/srep28683 (2016).
Supplementary Material
Acknowledgments
We are grateful to Prof. Sheng Luan for initiation of this study and comments on the manuscript and to Dr. Fugeng Zhao for technical assistance and to Prof. Dabing Zhang for comments on the manuscript. This work was supported by grants from the National Natural Science Foundation of China (30870219), and the Program for Changjiang Scholars and Innovative Research Team in University from the Ministry of Education of China (IRT_14R27, IRT1020), and State key laboratory of Plant Genomics, China to Jian Ye. Girdhar K. Pandey is thankful to Department of Biotechnology (DBT), Govt. of India for research work related to this work.
Footnotes
Author Contributions L.N.S., P.Z., G.-H.L. and Y.-H.Y. designed the experiments. P.Z. performed most of the experiments and drafted the manuscript. L.N.S. performed experiments of in vitro pull down and LSF2 activity. P.Z., L.N.S., C.-Y.T. and Y.Z. performed the Y2H experiment. P.Z. and J.Y. performed BiFC experiments. P.Z., G.-H.L., G.K.P. and Y.-H.Y. analyzed the data. G.K.P., G.-H.L., J.S., W.L., Z.H. and J.Q. revised the manuscript.
References
- Causin H. F. et al. The control of root growth by reactive oxygen species in Salix nigra Marsh. seedlings. Plant Sci. 183, 197–205, doi: 10.1016/j.plantsci.2011.08.012 (2012, February). [DOI] [PubMed] [Google Scholar]
- Liszkay A., van der Zalm E. & Schopfer P. Production of reactive oxygen intermediates (O2−, H2O2, and ·OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 136, 3114–3123, doi: 10.1104/pp.104.044784 (2004, October). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunand C., Crevecoeur M. & Penel C. Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol. 174, 332–341, doi: 10.1111/j.1469-8137.2007.01995.x (2007, January). [DOI] [PubMed] [Google Scholar]
- Li S. W., Xue L. G., Xu S. J., Feng H. Y. & An L. Z. Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Regulation 52, 173–180, doi: 10.1007/s10725-007-9188-9 (2007, June). [DOI] [Google Scholar]
- Swanson S. & Gilroy S. ROS in plant development. Physiol Plant 138, 384–392, doi: 10.1111/j.1399-3054.2009.01313.x (2010, April). [DOI] [PubMed] [Google Scholar]
- Choudhury S., Panda P., Sahoo L. & Panda S. K. Reactive oxygen species signaling in plants under abiotic stress. Plant Signal Behav . 8, e23681, doi: 10.4161/psb.23681 (2013, April). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustaka J., Tanou G., Adamakis I. D., Eleftheriou E. P. & Moustakas M. Leaf age-dependent photoprotective and antioxidative response mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 16, 13989–14006, doi: 10.3390/ijms160613989 (2015, June). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z. Q. et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486, 228–232, doi: 10.1038/nature11162 (2012, May). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkar R., Kapoor A. & Zhu J. K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell 18, 2051–2065, doi: 10.1105/tpc.106.041673 (2006, August). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orendi G., Zimmermann P., Baar C. & Zentgraf U. Loss of stress-induced expression of catalase3 during leaf senescence in Arabidopsis thaliana is restricted to oxidative stress. Plant Sci. 161, 301–314, doi: 10.1016/S0168-9452(01)00409-5 (2001, July). [DOI] [PubMed] [Google Scholar]
- Tsukagoshi H., Busch W. & Benfey P. N. Transcriptional regulation of ROS controls transition from proliferation to differentiation in the root. Cell 143, 606–616, doi: 10.1016/j.cell.2010.10.020 (2010, November). [DOI] [PubMed] [Google Scholar]
- Livanos P., Galatis B., Quader H. & Apostolakos P. Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana. Cytoskeleton (Hoboken) 69, 1–21, doi: 10.1002/cm.20538 (2011, January). [DOI] [PubMed] [Google Scholar]
- Sundaravelpandian K., Chandrika N. N. P. & Schmidt W. PFT1, a transcriptional Mediator complex subunit, controls root hair differentiation through reactive oxygen species (ROS) distribution in Arabidopsis. New Phytol. 197, 151–161, doi: 10.1111/nph.12000 (2013, January). [DOI] [PubMed] [Google Scholar]
- Shin L. J. et al. Ectopic ferredoxin I protein promotes root hair growth through induction of reactive oxygen species in Arabidopsis thaliana. J Plant Physiol 168, 434–440, doi: 10.1016/j.jplph.2010.08.002 (2011, March). [DOI] [PubMed] [Google Scholar]
- Schliebner I., Pribil M., Zuhlke J., Dietzmann A. & Leister D. A Survey of Chloroplast Protein Kinases and Phosphatases in Arabidopsis thaliana. Curr. Genomics 9, 184–190, doi: 10.2174/138920208784340740 (2008, May). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. & Luan S. Redox control of protein tyrosine phosphatases and mitogen-activated protein kinases in plants. Plant Physiol. 132, 1149–1152, doi: 10.1104/pp.103.020792 (2003, July). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C. Exploring the role of protein phosphorylation in plants: from signalling to metabolism. Biochem Soc. Trans. 35, 28–32, doi: 10.1042/BST0350028 (2007, February). [DOI] [PubMed] [Google Scholar]
- Sokolov L. N., Dominguez-Solis J. R., Allary A. L., Buchanan B. B. & Luan S. A redox-regulated chloroplast protein phosphatase binds to starch diurnally and functions in its accumulation. Proc. Natl. Acad. Sci. USA 103, 9732–9737, doi: 10.1073/pnas.0603329103 (2006, June). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver D. M. et al. Insight into the redox regulation of the phosphoglucan phosphatase SEX4 involved in starch degradation. FEBS J. 280, 538–548, doi: 10.1111/j.1742-4658.2012.08546.x (2013, January). [DOI] [PubMed] [Google Scholar]
- Santelia D. et al. The phosphoglucan phosphatase like sex Four2 dephosphorylates starch at the C3-position in Arabidopsis. Plant Cell 23, 4096–4111, doi: 10.1105/tpc.111.092155 (2011, November). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D., Trost P. & Sparla F. New insights into redox control of starch degradation. Curr. Opin. Plant. Biol. 25, 1–9, doi: 10.1016/j.pbi.2015.04.003 (2015, June). [DOI] [PubMed] [Google Scholar]
- Scarpeci T. E., Zanor M. I., Carrillo N., Mueller-Roeber B. & Valle E. M. Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol. Biol. 66, 361–378, doi: 10.1007/s11103-007-9274-4 (2008, March). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C. et al. Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J. 10, 1723–1732 (1991, July). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi H. Control of root growth and development by reactive oxygen species. Curr. Opin. Plant Biol. 29, 57–63, doi: 10.1016/j.pbi.2015 (2016, February). [DOI] [PubMed] [Google Scholar]
- Menand B. et al. An ancient mechanism controls the development of cells with a rooting function in land plants. Science 316, 1477–1480, doi: 10.1126/science.1142618 (2007, June). [DOI] [PubMed] [Google Scholar]
- Jalmi S. K. & Sinha A. K. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front. Plant Sci. 6, 769, doi: 10.3389/fpls.2015.00769 (2015, September). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastain C. J. et al. The pyruvate, orthophosphate dikinase regulatory proteins of Arabidopsis possess a novel, unprecedented Ser/Thr protein kinase primary structure. Plant J. 53, 854–863, doi: 10.1111/j.1365-313X.2007.03366.x (2008, March). [DOI] [PubMed] [Google Scholar]
- Kirchsteiger K., Ferrandez J., Pascual M. B., Gonzalez M. & Cejudo F. J. NADPH thioredoxin reductase C is localized in plastids of photosynthetic and nonphotosynthetic tissues and is involved in lateral root formation in Arabidopsis. Plant Cell 24, 1534–1548, doi: 10.1105/tpc.111.092304 (2012, April). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalska J., Zauber H., Buchanan B. B., Cejudo F. J. & Geigenberger P. NTRC links built-in thioredoxin to light and sucrose in regulating starch synthesis in chloroplasts and amyloplasts. Proc. Natl. Acad. Sci. USA 106, 9908–9913, doi: 10.1073/pnas.0903559106 (2009, June). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meekins D. A. et al. Mechanistic insights into glucan phosphatase activity against polyglucan substrates. J. Biol. Chem. 290, 23361–23370, doi: 10.1074/jbc.M115.658203 (2015, September). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422, 442–446, doi: 10.1038/nature01485 (2003, March). [DOI] [PubMed] [Google Scholar]
- Kocsy G. et al. Redox control of plant growth and development. Plant Sci. 211, 77–91, doi: 10.1016/j.plantsci.2013.07.004 (2013, October). [DOI] [PubMed] [Google Scholar]
- Matsuo M. et al. High Redox Responsive Transcription Factor1 levels result in accumulation of reactive oxygen species in Arabidopsis thaliana shoots and roots. Mol. Plant 8, 1253–1273, doi: 10.1016/j.molp.2015.03.011 (2015, August). [DOI] [PubMed] [Google Scholar]
- Lin C. Y. et al. Pathways involved in vanadate-induced root hair formation in Arabidopsis. Physiol. Plant 153, 137–148, doi: 10.1111/ppl.12229 (2015, January). [DOI] [PubMed] [Google Scholar]
- Mittler R., Vanderauwera S., Gollery M. & Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498, doi: 10.1016/j.tplants.2004.08.009 (2004, October). [DOI] [PubMed] [Google Scholar]
- Nakagami H., Soukupova H., Schikora A., Zarsky V. & Hirt H. A Mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J. Biol. Chem. 281, 38697–38704, doi: 10.1074/jbc.M605293200 (2006, December). [DOI] [PubMed] [Google Scholar]
- Wang F., Jing W. & Zhang W. The mitogen-activated protein kinase cascade MKK1-MPK4 mediates salt signaling in rice. Plant Sci. 227, 181–189, doi: 10.1016/j.plantsci.2014.08.007 (2014, October). [DOI] [PubMed] [Google Scholar]
- Han S., Fang L., Ren X., Wang W. & Jiang J. MPK6 controls H2O2-induced root elongation by mediating Ca2+ influx across the plasma membrane of root cells in Arabidopsis seedlings. New Phytol. 205, 695–706, doi: 10.1111/nph.12990 (2015, January). [DOI] [PubMed] [Google Scholar]
- Takahashi F., Mizoguchi T., Yoshida R., Ichimura K. & Shinozaki K. Calmodulin-dependent activation of MAP kinase for ROS homeostasis in Arabidopsis. Mol. Cell 41, 649–660, doi: 10.1016/j.molcel.2011.02.029 (2011, March). [DOI] [PubMed] [Google Scholar]
- Mayank P. et al. Characterization of the phosphoproteome of mature Arabidopsis pollen. Plant J. 72, 89–101, doi: 10.1111/j.1365-313X.2012.05061.x (2012, October). [DOI] [PubMed] [Google Scholar]
- Zhang X., Henriques R., Lin S. S., Niu Q. W. & Chua N. H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 1, 641–646, doi: 10.1038/nprot.2006.97 (2006, June). [DOI] [PubMed] [Google Scholar]
- Choi S. M. et al. Chloroplast Cu/Zn-superoxide dismutase is a highly sensitive site in cucumber leaves chilled in the light. Planta 216, 315–324, doi: 10.1007/s00425-002-0852-z (2002, December). [DOI] [PubMed] [Google Scholar]
- Sutherland M. W. & Learmonth B. A. The tetrazolium dyes MTS and XTT provide new quantitative assays for superoxide and superoxide dismutase. Free Radic. Res. 27, 283–289 (1997, September). [DOI] [PubMed] [Google Scholar]
- Sgherri C., Quartacci M. F. & Navari-Izzo F. Early production of activated oxygen species in root apoplast of wheat following copper excess. J. Plant Physiol. 164, 1152–1160, doi: 10.1016/j.jplph.2006.05.020 (2007, September). [DOI] [PubMed] [Google Scholar]
- Rao M. V., Paliyath G. & Ormrod D. P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 110, 125–136, doi: 10.1104/pp.110.1.125 (1996, January). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H. et al. An oxidative stress response to polycyclic aromatic hydrocarbon exposure is rapid and complex in Arabidopsis thaliana. Plant Sci. 176, 375–382, doi: 10.1016/j.plantsci.2008.12.002 (2009, March). [DOI] [Google Scholar]
- Park B. S., Seo J. S. & Chua N. H. Nitrogen Limitation Adaptation recruits PHOSPHATE2 to target the phosphate transporter PT2 for degradation during the regulation of Arabidopsis phosphate homeostasis. Plant Cell 26, 454–464, doi: 10.1105/tpc.113.120311 (2014, January). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.