Abstract
Objective
(1) Describe current epidemiology of thyroid cancer in the United States; (2) evaluate hypothesized causes of the increased incidence of thyroid cancer; and (3) suggest next steps in research and clinical action.
Methods
Analysis of data from Surveillance, Epidemiology and End Results System and the National Center for Vital Statistics. Literature review of published English-language articles through December 31, 2013.
Results
The incidence of thyroid cancer has tripled over the past 30 years, whereas mortality is stable. The increase is mainly comprised of smaller tumors. These facts together suggest the major reason for the increased incidence is detection of subclinical, nonlethal disease. This has likely occurred through: health care system access, incidental detection on imaging, more frequent biopsy, greater volumes of and extent of surgery, and changes in pathology practices. Because larger-size tumors have increased in incidence also, it is possible that there is a concomitant true rise in thyroid cancer incidence. The only clearly identifiable contributor is radiation exposure, which has likely resulted in a few additional cases annually. The contribution of the following causes to the increasing incidence is unclear: iodine excess or insufficiency, diabetes and obesity, and molecular disruptions. The following mechanisms do not currently have strong evidence to support a link with the development of thyroid cancer: estrogen, dietary nitrate, and autoimmune thyroid disease.
Conclusion
Research should focus on illuminating which thyroid cancers need treatment. Patients should be advised of the benefits as well as harms that can occur with treatment of incidentally identified, small, asymptomatic thyroid cancers.
INTRODUCTION
Thyroid cancer has been much in the news lately because of reports of large increases in incidence. However, it is now recognized that not all pathologic entities labeled as cancer act similarly, nor do they all act aggressively. Also, it is increasingly recognized that measures of incidence, mortality, and survival may be affected by many epidemiologic factors and may not always be reflective of either true increases in disease, treatment successes, or screening efforts (1). A current example is found in prostate cancer, a disease for which 20 years ago screening and aggressive treatment were recommended. Screening now is no longer uniformly advised, after it was understood that the disease is present at autopsy in many asymptomatic men dying of other causes.
Although thyroid cancer is different from prostate cancer in that it is not detected through concerted screening programs, it does have a similar large subclinical reservoir and is often found at autopsy in asymptomatic people who died of other causes. This has made it challenging to interpret observed trends in the incidence and mortality of thyroid cancer in the U.S. Here, we outline the current incidence and mortality trends for differentiated thyroid cancer and examine the 2 main hypotheses about the cause of the increasing incidence: (1) the detection of a subclinical reservoir, and (2) a true increase in the disease due to known or new risk factors.
METHODS
Epidemiologic Data
Data on the incidence of thyroid cancer are from SEER 9 (Surveillance, Epidemiology and End Results) Program, supported by the National Cancer Institute, from 1975 to 2011. SEER 9 covers approximately 10% of the U.S. population and includes the longest contributing sites to the program: Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah. SEER is the best source of population-based data available in the U.S. for cancer incidence, histology, and initial treatment because it is gathered from high-quality registries chosen specifically to accurately represent the U.S. population and because it is extensively quality checked. Mortality data are from the Centers for Disease Control National Vital Statistics System.
For all incidence calculations, we included all incident cases of cancer with “thyroid” as the site of origin (The International Classification of Diseases for histology, Third Revision [2] [ICD-03], code 73.9). Rates were calculated for each year using SEERStat v.8.1.5 (Surveillance Research Program of the National Cancer Institute, Rockville, MD). All rates were age adjusted to the 2000 standard population.
Histology was categorized as follows: papillary (ICD-03 8050, 8052, 8130, 8260, 8340–8344, 8450–8452), follicular (ICD-03 8290, 8330–8332, 8335), and poorly differentiated – a combination of medullary (ICD-03 8345–8346, 8510) and anaplastic (ICD-03 8021). Histologic classification was accepted as reported by each registry, slides were not re-read by a central monitoring body.
Review of Contributors to Thyroid Cancer’s Increasing Incidence
An initial broad search of the literature was performed to identify commonly hypothesized reasons for the increasing incidence of thyroid cancer. Next, a detailed literature search of PubMed for English-language studies published prior to December 31, 2013 was performed for the topic areas deemed likely to represent potential causes of the increasing incidence. Two topic areas were not subjected to detailed search based on limited supporting evidence: bisphenol A and progesterone. Studies were selected for inclusion if they were clinical trials, large cohort studies, or considered likely to be influential to research or clinical practice.
RESULTS
Part I: Current Epidemiology of Thyroid Cancer in the U.S
In 2014, it was estimated that there would be 62,980 new cases of thyroid cancer in the U.S. Thyroid cancer accounts for about 3.8% of new cancers diagnosed each year in the U.S. and is the ninth most common in incidence after prostate, breast, lung, colon, melanoma of the skin, bladder, non-Hodgkin’s lymphoma, and renal cancer. Deaths due to thyroid cancer are uncommon. It was estimated that there would be an estimated 1,890 deaths due to thyroid cancer in the U.S. in 2014. The more common cancers, such as breast and lung, lead to deaths in the hundreds of thousands each year (3). Recent and historic trends are briefly outlined below for orientation, more detailed analyses are available elsewhere (4–6).
Overall Incidence Trends
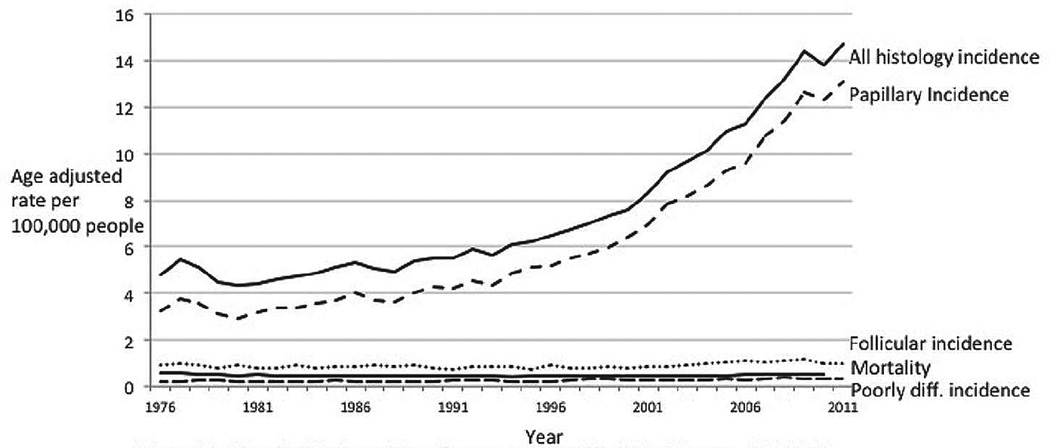
Thyroid cancer incidence was relatively stable until the 1990s, when it began to increase dramatically (Fig. 1). Overall thyroid cancer incidence increased from 4.9 per 100,000 population to 14.7 per 100,000 population in 2011. The rise has been due almost entirely to papillary histology (rising from 3.4 to 13.1 per 100,000), with a tiny contribution from follicular histology (rising from a range of 0.75 to 1.0 per 100,000 in 1975–1977 to 0.97 to 1.15 per 100,000 in 2009–2011).
Fig. 1.
Incidence by histology of thyroid cancer and mortality of thyroid cancer, 1975–2011. Data are from the Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2012 Sub (1973–2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission. Underlying mortality data for 1975–2010 are provided by NCHS (www.cdc.gov/nchs).
Trends in Incidence by Size
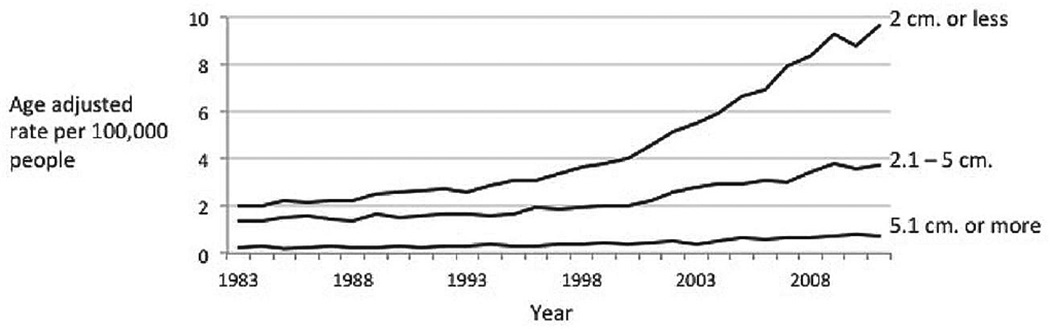
Examining incidence rates by size gives clues about potential causes of the increase. Thyroid cancers of all sizes increased between 1983 and 2011, the greatest contribution in both relative and absolute terms came from small cancers (Fig. 2). The incidence of the smallest cancers (2 cm or less) increased more than 4-fold, from 2 to 9.6 per 100,000 population. Cancers measuring 2.1 to 5 cm more than doubled in incidence, from 1.4 to 3.7 per 100,000 population. Cancers measuring greater than 5 cm are quite rare, but their rates tripled, from 0.2 to 0.7 per 100,000 population.
Fig. 2.
Differentiated thyroid cancer incidence trends by size, 1983–2011. Data are from the Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2012 Sub (1973–2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
Trends in Incidence by Age Group
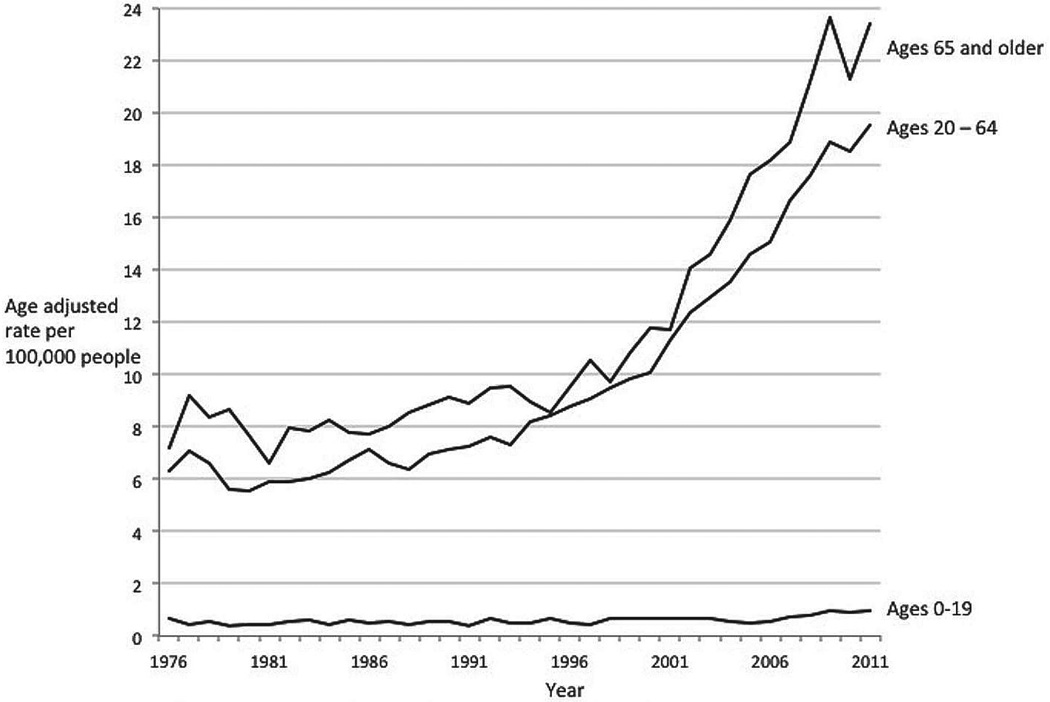
The incidence of thyroid cancer is highest among those over age 65 years. Since 1975, the incidence has risen dramatically in all age groups (Fig. 3). The largest burden of the increase in absolute terms has been in those aged 65 years and over; the incidence increased from 8 to 23.4 per 100,000 population in 2011. The greatest relative increase has been in those aged 20 to 64 years. The incidence rate went from 6.4 to 19.5 per 100,000 population in 2011, a 3.1-fold increase. Among those aged 0 to 19 years, there has been a similar relative change of 2.6-fold, but thyroid cancer in this group is very rare and has risen in absolute terms from only 0.4 to 1 per 100,000 population in 2011.
Fig. 3.
Thyroid cancer incidence trends by age group, 1975–2011. Data are from the Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2012 Sub (1973–2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2011 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
Current Trends in Mortality
Mortality from thyroid cancer hovers around 0.5 per 100,000 population. Between 1975 and 2010 (the most recent year for which data are available), the high was recorded in 1977, at 0.57 per 100,000 population (approximately 1,710 deaths per year nationwide), and the low was recorded in 1994, at 0.42 per 100,000 population (approximately 1,260 deaths per year nationwide). Between 2001 and 2010, the mortality rate varied between 0.45 and 0.52 per 100,000 population, never moving outside the range we have seen over the past 30 years. Because the mortality rate for thyroid cancer is so low, any fluctuations appear relatively large. Thus, statistical tests of trend reported by SEER do show a 0.9% increase in this time period, but examination of the underlying data suggest that the trend is not indicative of clinically or epidemiologically relevant changes overall, because the data have not varied outside the range we have observed over the past 30 years.
Trends in Molecular Profiles
Studies of archival thyroid cancer specimens show historical shifts in the landscape of molecular alterations (e.g., rearrangements in the BRAF, RAS, and RET/PTC genes). In a recent study analyzing trends in demographic, clinical, pathologic, and molecular characteristics of papillary thyroid cancer from 1974 to 2009, one group found 3 important trends in the mutational composition of papillary thyroid cancer over time: (1) the overall proportion of RAS point mutations increased significantly after 2000, due entirely to increases in the follicular variant of papillary thyroid cancer; (2) the proportion of BRAF mutations was stable over the time period; and (3) the proportion of RET/PTC rearrangements significantly decreased. They concluded that rising percentages of the follicular variant histology and RAS mutations after 2000 suggest (a) new etiologic factors and (b) that the increased incidence is not likely due to radiation because RET/PTC rearrangements decreased (7).
In another recent study, the rate of the BRAF V600E mutation was seen to rise over 15 years of archived thyroid specimens. The authors concluded that BRAF mutations in papillary thyroid cancer may be contributing to the increasing incidence of thyroid cancer (8). Collectively, these data are intriguing, but because they are potentially confounded by secular trends in the detection of subclinical cancers, more work will be required to determine whether molecular changes are a significant contributor to the increasing incidence of thyroid cancer.
Part II: Examination of Hypotheses for the Increasing Incidence
Hypothesis #1: The Increased Incidence of Thyroid Cancer is Due to Detection of Subclinical Disease
Evidence for and Against the Hypothesis
There is strong evidence to support the hypothesis that the increasing incidence of thyroid cancer is due to the detection of subclinical disease, termed ‘overdiagnosis’ in cancer epidemiology. First, papillary thyroid cancer is commonly found at autopsy in people who died of other causes. Estimates of the prevalence range from about 3 to about 35%, depending on the country of the study and the pathology methods used to examine the specimens (9–11). Among thyroid specimens removed for benign disease, up to 50% harbor small, incidental papillary thyroid cancers (12).
Another observation that supports the subclinical disease detection hypothesis is the fact that the majority of the increase has been observed in small cancers. The largest rate increases have been observed in cancers that are too small even for most experienced clinicians to feel during thyroid palpation (13). Small cancers are less likely to be causing symptoms at the time of their discovery and are in the size range commonly found in the autopsy studies described above.
Last, mortality rates have not changed substantially over the 30 years that thyroid cancer incidence has been rising. Some argue that this is because of improving treatments. For this to be the case, the changes in treatment would have had to exactly keep pace with the increasing incidence; if improvements in treatment occurred too fast, the mortality line would fall. If improvements in treatment occurred too slowly, the mortality line would rise. Although this is possible, it is not particularly plausible. This finding of stable mortality means that in a setting of increasing numbers of thyroid cancers being detected, only a small constant number are aggressive, lethal cancers.
Proposed Mechanisms of Detection of Subclinical Thyroid Cancer
There are several interrelated mechanisms through which subclinical thyroid cancers might be detected. These are outlined in Table 1 and detailed below.
Table 1.
Potential Contributors to the Increasing Incidence of Thyroid Cancer in the United States, by Category
| Contributor | Summary | Type of data |
|---|---|---|
| Associated with the increasing incidence of thyroid cancer | ||
| Health care system access (14–17) |
Thyroid cancer incidence across the U.S. varies by factors such as insurance, education rates, and poverty. Higher incidence is observed in U.S. counties with higher levels of access to health care. Incidence is higher in countries with more fee-for-service–based health systems. |
Population-based ecologic studies, health economics modeling |
| Increased use of imaging tests (18) |
Between 1985 and 2010, use of computed tomography scans increased on average 8% per year, magnetic resonance imaging 10% per year. Positron emission tomography scans increased 57% per year between 2004 and 2010. These imaging tests are common sources of incidental detection of thyroid nodules. |
Large closed health care system cohort |
| Changing thresholds to work up thyroid nodules (19–22) |
Historic articles from the 1970s suggested thyroid nodules should be solid and 2 cm in size before intervening – guidelines were not common in this era. Current professional society guidelines suggest nodules as small as 0.5 cm might be appropriate for workup. |
Historical documents, current guidelines |
| Increase in number and extent of surgery (23) |
The number of thyroid surgeries increased by about 39% between 1996 and 2006. In addition, recent professional society guidelines now almost uniformly recommend total thyroidectomy, rather than lobectomy, should be performed for papillary cancers of any size, leading to more incidentally detected carcinomas on pathology reports. |
Population-based studies, current guidelines |
| Increased scrutiny of pathology specimens (24–28) |
Pathology reporting guidelines have increased in complexity and completeness since at least the 1980s. Single-institution studies demonstrate changes in pathology specimen handling and diagnostic practices that increase cancer detection rates. |
Historical documents, single-institution cohort studies |
| Limited or unclear association with the increasing incidence of thyroid cancer | ||
| Radiation exposure (34–40) |
Average levels of ionizing radiation exposure in the U.S. have doubled since the 1970s, but this is almost entirely due to increased medical imaging rates. Only people exposed to radiation before age 20 (esp. before age 5) are at increased risk of getting thyroid cancer compared to the general population. Because relatively few computed tomography scans are performed in young children, the contribution of radiation-induced thyroid cancer to increasing thyroid cancer incidence is likely very small. |
Population-based cohort studies, radiation physics modeling, health care utilization statistics |
| Obesity and diabetes (41–48) |
Similar to the rise in thyroid cancer incidence, there has been a rise in obesity and diabetes mellitus over time. Although still controversial, some studies have shown a positive association between obesity and diabetes with thyroid cancer incidence. These studies are potentially confounded by secular trends of increased imaging rates overall, which will increase incidental detection of thyroid nodules. |
Cohort studies, meta- analyses |
| Iodine insufficiency or excess (49–54) |
Epidemiologic studies show that the proportions of histologic thyroid cancer by type are affected by rates of iodine intake. However, studies to assess whether iodine contributes to the increasing incidence overall have been conflicting and confounded by background radiation risks in volcanic/nuclear accident-exposed regions and secular trends in the use of sensitive imaging studies. |
Population-based cohort studies of high- and low-iodine regions, and before and after iodine supplementation |
| No demonstrated association with thyroid cancer | ||
| Estrogen (55–59) | Thyroid cancer cell lines show increased proliferation, migration, and invasion with estrogen stimulation. However, there are not convincing human studies linking estrogen stimulation with thyroid cancer incidence. |
Laboratory studies with thyroid cancer cell lines |
| Dietary nitrates (60–62) | Nitrate is a contaminant of drinking water in agricultural areas and is found at high levels in some vegetables, such as beets. Nitrate competes with uptake of iodide by the thyroid, thus potentially affecting thyroid function. Study results thus far have been contradictory. |
Population-based cohort studies |
| Autoimmune thyroid disease (63,64) |
Elevated levels of thyroid-stimulating hormone found in hypothyroid patients with autoimmune thyroid disease may stimulate follicular epithelial proliferation, promoting the development of papillary carcinoma. Needle biopsy and thyroidectomy specimen studies have yielded conflicting results, and existing studies are subject to confounding by indication. |
Single-institution cohort and case series |
Access to health care services has been shown to be a determinant of thyroid cancer incidence rates (14) by making it more likely that a subclinical cancer is found. A current example is South Korea. Between 1996 and 2010, the age-adjusted incidence of thyroid cancer in women residing in the Gwangju and Jeonnam provinces in South Korea increased from 10.6 to 111.3 per 100,000 population, and in men, the incidence increased from 1.9 to 27.0 per 100,000 population. Despite these increases, thyroid cancer mortality remained stable. The incidence change was mainly attributable to the detection of asymptomatic cancers (which increased from less than 5% to more than 50% of all cancers) and sub-centimeter cancers (the rate increased from 0 to 50 per 100,000 population) (15). The primary mechanism of detection has been physician-offered thyroid ultrasound screenings. A 2009 survey showed 13% of South Korean adults had undergone a screening thyroid ultrasound. The rate was higher among women, with the highest rate (26%) among women 50 to 59 years old (16).
Health care financing systems also affect thyroid cancer incidence rates. A 2012 study modeled thyroid cancer incidence based on the characteristics of national health systems. Adjusted for gross domestic product and education levels, the share of health care expenditure that was privately funded was a significant predictor of thyroid cancer incidence (for women, P = .02; men, P = .09). Health care systems with more fee for service, private models had higher incidence rates of thyroid cancer (17).
General increases in imaging rates have made thyroid findings more common. In a recent study of a large health care system (18), people underwent an average of 1.18 imaging studies per year, of which 35% were advanced diagnostic imaging studies: computed tomography (CT), magnetic resonance imaging (MRI), nuclear medicine, or ultrasound. Over the 15 years of the study, imaging rates for CT scanning increased 8% annually. MRI rates increased 10% annually, ultrasound 4%, and positron emission tomography scanning 57% (2004 to 2010 only). It is likely that increased rates of imaging have led to more thyroid findings.
Physician management of identified thyroid nodules has also changed. Guidelines were not common in the 1970s. One article from this era suggested that thyroid nodules smaller than 2 cm do not require intervention (19). Guidelines from 2009 and 2010 suggest that in some circumstances, nodules of any size might be appropriate to biopsy (20,21). Similarly, recommendations for thyroid surgery for small papillary cancers have also shifted. Until recently, at least one guideline still listed thyroid lobectomy as an appropriate treatment (22), but more recently, total thyroidectomy has been the standard recommendation for thyroid cancers of any size (20,21). More frequent biopsies and larger specimens provide more opportunities for pathologists to find cancer; thus, it is likely that these 2 phenomena are contributing to the increasing incidence of thyroid cancer.
Thyroidectomy volumes have increased as a result of changes in imaging rates and physician behavior. A study using the population-based Nationwide Inpatient Sample and National Survey of Ambulatory Surgery databases estimated that annual thyroid surgery volumes increased 39% over 10 years, from 66,864 in 1996 to 92,931 in 2006 (23). More thyroidectomies means more specimens to examine for cancer, another contributing factor to increased detection.
Pathologist management of thyroid specimens has also changed. Surgical pathology textbooks from the 1980s suggested 5 descriptive areas for a surgical pathology report of a thyroid specimen (24). The 2012 guidelines from the College of American Pathologists list a minimum of 14 descriptive areas; each thyroid specimen is examined much more closely compared to the past (25). Furthermore, whereas historically only representative sections of a thyroid specimen were examined by a pathologist, the thyroid specimen in its entirety is now typically examined, increasing the chances for identification of small cancers. Lastly, although there has been no official change in the definition of follicular or papillary thyroid cancer by the World Health Organization, pathologists now categorize more cases as ‘follicular variant of papillary carcinoma’ rather than follicular adenoma (26,27). Together with the increased numbers of thyroid specimens delivered to pathologists, these changes are likely a significant contributor to the increasing identification of thyroid cancer.
Hypothesis #2: The Increased Incidence of Thyroid Cancer is Due to a True Rise in Disease
Evidence for and Against the Hypothesis
A major argument against detection of subclinical disease as a cause of the increasing incidence of thyroid cancer is that the entire increase in thyroid cancer incidence has not been due to the detection of small cancers. Larger cancers and those diagnosed at a more advanced stage (i.e., with lymph node metastases) have also increased in incidence (5,28). The detection of tumors at increased stage may not represent a true increase in disease, because imaging and pathology studies have become more sensitive over time, leading to upstaging of newly diagnosed cancers. However, the detection of more large cancers based on primary size alone is potentially supportive of a true increase in the incidence of thyroid cancer, as large cancers were likely picked up just as well historically as they are now. It should be noted, however, that even large cancers may be picked up incidentally, making it possible that the increasing incidence of large cancers is not due to a true rise in disease. A recent paper reported that 38% of cancers larger than 4 cm had been found incidentally on imaging studies done for other reasons (29). Another group that examined cancers diagnosed at their institution by size and stage reported similar findings (30).
Some also suspect that mortality trends might not have changed yet due to long patient survival times with papillary thyroid cancer. For this to be the case, survival times would have to be extraordinarily long, as the increasing incidence has been present for nearly 20 years, and the mortality has been stable for more than 30 years. If this is the case, it is not yet observable in available data.
Some have suggested that the rise in papillary thyroid cancer alone suggests that increased detection is not occurring, because one would also expect increases across all specified histologies except anaplastic (28). However, the autopsy reservoir of other histologies besides papillary is small, so this argument is less compelling.
Last, a change of histologic classification for thyroid cancer might be proposed as a reason for the observed increase in incidence of papillary carcinoma. However, the SEER data available do not show a reciprocal decrease in the incidence of follicular thyroid carcinoma during the past decades, the most likely substitution for a diagnosis of papillary cancer (31,32).
Proposed Mechanisms Causing a True Increase in the Incidence of Thyroid Cancer
I. Mechanisms with limited or unclear association with increasing incidence
Exposure to ionizing radiation is the most extensively studied and clearly defined risk factor for thyroid cancer. Ionizing radiation causes DNA strand breaks and somatic mutations that are thought to be the cause of the cancers (33).
Radiation exposure only increases the risk of thyroid cancer when people are exposed as children, mostly under age 5 years (34). No increased risk of thyroid cancer over the baseline in the population has been observed in patients are over age 20 years at the time of exposure (35). Some wonder if there has been more exposure to radiation in recent decades. Background radiation from natural sources in the environment has been stable at 3 mSv since the 1970s. Ionizing radiation exposure to U.S. residents has doubled over the past 30 years that thyroid cancer rates have increased, but this is nearly entirely attributable to medical imaging (36). In the U.S., the number of CT scans performed annually (a significant source of radiation exposure) increased from 3 million in 1980 to 62 million in 2007 (37). Most CT scans are performed in patients over 55 years of age. By multiplying published utilization numbers (38) by mean excess lifetime thyroid cancer risk per scan (34), one can estimate that approximately 150 to 350 excess thyroid cancers annually are attributable to pediatric CT scans.
Radiation exposure attributable to sources other than advanced imaging is minimal. A bite-wing dental X-ray delivers 5 µGy (less than 1/1,000th of the dose of a CT scan); the estimated dose to the thyroid is essentially zero (39). Radiation exposure from airplane travel is very low: 2 to 6 µGy per prolonged flight (40). For comparison, the increased background radiation one would receive moving from Minneapolis to Denver for one year is 150 µGy (39).
Thus, while ionizing radiation exposure has increased significantly due to wider use of medical imaging, elevated thyroid cancer risk is confined to people exposed as children. Although the excess risk for children is significant, in absolute numbers it is quite small. By our estimates, pediatric CT scans probably account for less than 1% of the increased incidence in thyroid cancer.
Diabetes mellitus and obesity have also been proposed as a cause of the increasing incidence of thyroid cancer, but the data are conflicting (41). When a positive relationship has been demonstrated with diabetes, it was weakly correlative and therefore unlikely to explain the marked rise in thyroid cancer incidence (42). Proposed mechanisms are hypothetical: increased body mass index (BMI), elevated insulin, long-term elevation of thyroid-stimulating hormone, long-term exposure to high levels of glucose or triglycerides, and use of antidiabetic medications (43). One study which found no correlation between diabetes and thyroid cancer did find a positive association between diabetes and the likelihood of undergoing thyroid ultrasound and thyroid fine-needle aspiration (44). This suggests that a diagnosis of diabetes mellitus may be associated with increased screening for thyroid cancer rather than a true difference in cancer incidence.
The increasing incidence of thyroid cancer has paralleled a rising prevalence of obesity over the past 3 decades (41). A pooled analysis of prospective cohort studies found a combined hazard ratio of 1.53 for thyroid cancer in obese men and women (BMI ≥30 kg/m2) (45). A large meta-analysis confirmed an association between BMI and thyroid cancer risk (46). Proposed mechanisms include exposure to adipokines such as leptin and adiponectin, or thyroid-stimulating hormone and estrogens; insulin resistance and the insulin–insulin-like growth factor 1 axis; increased aromatase activity; chronic inflammation; or oxidative stress (43). However, a causal relationship between obesity and thyroid cancer has not been proven. The association may be attributable in part to increased diagnosis in obese persons, who utilize more health care and diagnostic services (47). A study of obesity-related genetic polymorphisms did not demonstrate an association with thyroid cancer risk (48). More prospective studies and studies designed to assess causality would be needed to establish a relationship between the risk of thyroid cancer and obesity-related markers of thyroid function/insulin resistance.
Iodine deficiency and excess are known to affect the proportions of thyroid cancer histologies found in studied populations (49,50). Animal studies support it as a possible factor in the increasing incidence of thyroid cancer (51,52). However, data in humans are not compelling.
Two epidemiologic studies in European and Pacific countries comparing iodine-sufficient and -insufficient areas have shown opposite effects of iodine intake on thyroid cancer rates (49,53). Iodine supplementation has coincided with increased thyroid cancer incidence in many countries, but these findings suffer from confounding due to the increasing application of sensitive imaging technologies, and recent epidemiologic studies of iodine and thyroid cancer risk are confounded by issues of local radiation exposure (51,52,54). Thus, although iodine does have an association with thyroid cancer, it is not possible to conclude that changes in iodine intake or supplementation are the reason for increasing rates of thyroid cancer.
II. Mechanisms not shown to be associated with thyroid cancer
Estrogen has historically been proposed as a mechanistic cause of thyroid cancer, given the higher prevalence of thyroid cancer among women. To date, positive research findings in this area are limited to cell line studies.
Laboratory studies using thyroid cancer cell lines show thyroid cancer proliferation is enhanced by estrogen stimulation through various mechanisms, the details of which are beyond the scope of this review. Additionally, estrogen stimulation may lead to an increased metastatic phenotype (55), possibly through induction of matrix metalloproteinase activity (56). Furthermore, in cell lines, estrogen may promote thyroid cancer cell migration and invasion and angiogenesis through vascular endothelial growth factor pathways (57).
Clinical studies linking estrogen to thyroid cancer are weaker. One case control study showed higher estrogen-DNA adducts in the urine of women with thyroid cancer compared to women without thyroid cancer (58). Another study showed thyroid cancers with estrogen receptor (ER)-α–positive, ER-β–negative, and positive androgen receptor expression are associated with a more aggressive disease course (59).
Because much of the work in this field is cell line studies, and strong clinical and epidemiologic studies in patients are lacking, there is not yet evidence to support estrogen as a risk factor for the development of thyroid cancer or its increasing incidence.
Dietary nitrate is a contaminant of drinking water in agricultural areas and is found at high levels in some vegetables, such as beets. Dietary nitrate is proposed to cause proliferative changes in follicular cells—including hypertrophy, hyperplasia, and neoplasia—through chronically elevated levels of thyroid-stimulating hormone. Nitrate competes with uptake of iodide by the thyroid, potentially affecting thyroid function by binding to the sodium-iodide symporter on the surface of thyroid follicles. This reduces the levels of the thyroid hormones triiodothyronine and thyroxin, leading to chronic elevations of thyroid-stimulating hormone. However, epidemiologic data are conflicting.
One study showed increased thyroid cancer risk in people with high dietary nitrate intake, but this was limited somewhat by confounding due to increasing use of sensitive imaging technology over the same time frame (60). Two other studies showed mixed results, with increased risk in men shown in one study and increased risk in both sexes in another, but there was not a steady, dose-dependent trend with increasing dietary nitrate intake (61,62). To show a relationship between dietary nitrate and thyroid cancer, larger-scale epidemiologic studies with more detail regarding confounders and other contributors to increased risk would be needed.
Autoimmune thyroid disease has been proposed as a cause of thyroid cancer; elevated levels of thyroid-stimulating hormone found in hypothyroid patients with autoimmune thyroid disease may stimulate follicular epithelial proliferation, promoting the development of papillary carcinoma (63).
Although limited by the lack of definitive pathology for correlation testing, population-based fine-needle aspiration studies have not shown a statistically significant correlation between Hashimoto’s thyroiditis and papillary thyroid cancer. Thyroidectomy studies, which in most cases report a statistically significant positive correlation, are subject to confounding by indication (i.e., people with a worrisome needle biopsy result are more likely to be taken to surgery than those without) (64). More prospective studies with longer follow-up would be needed to test the relationship between autoimmune thyroid disease and thyroid cancer.
DISCUSSION
The incidence of thyroid cancer has tripled over the past 30 years, with the majority of the rise occurring in the past 15 years. The overwhelming majority of the increase has been due to the increased detection of papillary cancer, a histologic type known to be commonly present at death without ever having caused symptoms. Tumors of all sizes have increased in incidence, but in absolute and relative terms the greatest increase has been observed in cancers less than 2 cm in size. Throughout, mortality has never moved substantially from about 0.5 per 100,000 population. These epidemiologic facts, taken together, strongly suggest that the main contributor to the increasing incidence of thyroid cancer is detection of subclinical disease. The mechanisms of detection of subclinical disease are incentives in the health care system, imaging practices, individual clinician behaviors around thyroid examination and testing, shifting surgical norms around thyroidectomy and neck dissection, and pathology management of thyroid glands once they are removed from the neck.
There is an argument against subclinical detection as the sole driver of the increasing incidence: large tumors are increasing in incidence as well, suggesting there might be new or increasing risk factors causing a true rise in the incidence. Radiation, the only well-established risk factor for thyroid cancer, may be adding a small number of new cases each year, but this is likely to number in the low hundreds out of all thyroid cancer cases diagnosed each year. Studies of other risk factors, such as obesity, diabetes, and iodine deficiency or excess show relationships that are correlative and fall short of establishing causality. Unfortunately, these studies also show confounding; for example, the association between thyroid cancer and diabetes/obesity may be secondary to these patients having more imaging and therefore more identification of subclinical disease.
Future research should focus on determining which cancers need which treatments, with the express intent of avoiding overtreatment of what amounts to generally indolent disease. Study protocols should include detailed data on mode of detection of the thyroid finding that led to surgery and all known and suspected risk factors, so potential causes for a real rise in incidence can be clearly elucidated.
Epidemiologic data describing detection of subclinical disease are useful on a population scale but do not help when in the exam room with one patient facing their own decision. Patients should be explicitly included in discussions of the management of their identified cancer and given the opportunity to understand both the benefits, as well as the potential harms, of treatment of an incidentally identified cancer. The development of decision-support tools for both physicians and patients will provide immediate benefit by making care decisions both more transparent and collaborative.
CONCLUSION
Research should focus on illuminating which thyroid cancers need treatment. Patients should be advised of the benefits as well as harms that can occur with treatment of incidentally identified, small, asymptomatic thyroid cancers.
The opinions represented in the AACE/ACE Disease State Clinical Review: The Increasing Incidence of Thyroid Cancer are the expressed opinions of the Endocrine Surgery Scientific Committee of the American Association of Clinical Endocrinologists. AACE/ACE Disease State Clinical Reviews are systematically developed documents written to assist health care professionals in medical decision making for specific clinical conditions, but are in no way a substitute for a medical professional’s independent judgment and should not be considered medical advice. Most of the content herein is based on literature reviews. In areas of uncertainty, professional judgment of the authors was applied.
This review article is a working document that reflects the state of the field at the time of publication. Because rapid changes in this area are expected, periodic revisions are inevitable. We encourage medical professionals to use this information in conjunction with, and not a replacement for, their best clinical judgment. The presented recommendations may not be appropriate in all situations. Any decision by practitioners to apply these guidelines must be made in light of local resources and individual patient circumstances.
Acknowledgments
Dr. Davies received support from the Department of Veterans Affairs; Dr. Haymart received support from the National Institutes of Health (1K07CA154595-02).
Abbreviations
- BMI
body mass index
- CT
computed tomography
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
DISCLOSURE
The authors have no multiplicity of interest to disclose.
REFERENCES
- 1.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 2.International Classification of Diseases for Oncology. Geneva: World Health Organization; 2000. [Google Scholar]
- 3.Cancer Fast Stats, National Cancer Institute. [Accessed April 9, 2014]; Available at: http://seer.cancer.gov/faststats/.
- 4.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 5.Chen AY, Jemal A, Ward EM. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 6.Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140:317–322. doi: 10.1001/jamaoto.2014.1. [DOI] [PubMed] [Google Scholar]
- 7.Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99:E276–E285. doi: 10.1210/jc.2013-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mathur A, Moses W, Rahbari R, et al. Higher rate of BRAF mutation in papillary thyroid cancer over time: a single-institution study. Cancer. 2011;117:4390–4395. doi: 10.1002/cncr.26072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Tello FJ, Martinez-Cabruja R, Fernandez-Martin J, Lasso-Oria C, Ballestin-Carcavilla C. Occult carcinoma of the thyroid. A systematic autopsy study from Spain of two series performed with two different methods. Cancer. 1993;71:4022–4029. doi: 10.1002/1097-0142(19930615)71:12<4022::aid-cncr2820711236>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Mortensen JD, Woolner LB, Bennett WA. Gross and microscopic findings in clinically normal thyroid glands. J Clin Endocrinol Metab. 1955;15:1270–1280. doi: 10.1210/jcem-15-10-1270. [DOI] [PubMed] [Google Scholar]
- 12.Pakdaman MN, Rochon L, Gologan O, et al. Incidence and histopathological behavior of papillary microcarcinomas: study of 429 cases. Otolaryngol Head Neck Surg. 2008;139:718–722. doi: 10.1016/j.otohns.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Wiest PW, Hartshorne MF, Inskip PD, et al. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med. 1998;17:487–496. doi: 10.7863/jum.1998.17.8.487. [DOI] [PubMed] [Google Scholar]
- 14.Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid. 2013;23:885–891. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kweon SS, Shin MH, Chung IJ, Kim YJ, Choi JS. Thyroid cancer is the most common cancer in women, based on the data from population-based cancer registries, South Korea. Jpn J Clin Oncol. 2013;43:1039–1046. doi: 10.1093/jjco/hyt102. [DOI] [PubMed] [Google Scholar]
- 16.Han MA, Choi KS, Lee HY, Kim Y, Jun JK, Park EC. Current status of thyroid cancer screening in Korea: results from a nationwide interview survey. Asian Pac J Cancer Prev. 2011;12:1657–1663. [PubMed] [Google Scholar]
- 17.Lee TJ, Kim S, Cho HJ, Lee JH. The incidence of thyroid cancer is affected by the characteristics of a healthcare system. J Korean Med Sci. 2012;27:1491–1498. doi: 10.3346/jkms.2012.27.12.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996–2010. JAMA. 2012;307:2400–2409. doi: 10.1001/jama.2012.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenspan FS. Thyroid nodules and thyroid cancer. West J Med. 1974;121:359–365. [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 21.Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. Endocr Pract. 2010;16:468–475. doi: 10.4158/EP.16.3.468. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Care Network, Guidelines for Thyroid Carcinoma. [Accessed April 22, 2015]; Available at: http://www.nccn.org. [Google Scholar]
- 23.Sun GH, DeMonner S, Davis MM. Epidemiological and economic trends in inpatient and outpatient thyroidectomy in the United States, 1996–2006. Thyroid. 2013;23:727–733. doi: 10.1089/thy.2012.0218. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt WA. Principles and Techniques of Surgical Pathology. Menlo Park, CA: Addison Wesley Publishing Company; 1983. [Google Scholar]
- 25.Ghossein R, Asa SL, Barnes L, et al. Protocol for the examination of specimens from patients with carcinomas of the thyroid gland. In: Edition #: Thyroid 3.0.0.2, editor. College of American Pathologists Protocols. College of American Pathologists; 2012. [Google Scholar]
- 26.Verkooijen HM, Fioretta G, Pache JC, et al. Diagnostic changes as a reason for the increase in papillary thyroid cancer incidence in Geneva, Switzerland. Cancer Causes Control. 2003;14:13–17. doi: 10.1023/a:1022593923603. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd RV, Erickson LA, Casey MB, et al. Observer variation in the diagnosis of follicular variant of papillary thyroid carcinoma. Am J Surg Pathol. 2004;28:1336–1340. doi: 10.1097/01.pas.0000135519.34847.f6. [DOI] [PubMed] [Google Scholar]
- 28.Enewold L, Zhu K, Ron E, et al. Rising thyroid cancer incidence in the United States by demographic and tumor characteristics, 1980–2005. Cancer Epidemiol Biomarkers Prev. 2009;18:784–791. doi: 10.1158/1055-9965.EPI-08-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malone MK, Zagzag J, Ogilvie JB, Patel KN, Heller KS. Thyroid cancers detected by imaging are not necessarily small or early stage. Thyroid. 2014;24:314–318. doi: 10.1089/thy.2012.0651. [DOI] [PubMed] [Google Scholar]
- 30.Yoo F, Chaikhoutdinov I, Mitzner R, Liao J, Goldenberg D. Characteristics of incidentally discovered thyroid cancer. JAMA Otolaryngol Head Neck Surg. 2013;139:1181–1186. doi: 10.1001/jamaoto.2013.5050. [DOI] [PubMed] [Google Scholar]
- 31.Aschebrook-Kilfoy B, Grogan RH, Ward MH, Kaplan E, Devesa SS. Follicular thyroid cancer incidence patterns in the United States, 1980–2009. Thyroid. 2013;23:1015–1021. doi: 10.1089/thy.2012.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu C, Zheng T, Kilfoy BA, et al. A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973–2004. Thyroid. 2009;19:1061–1066. doi: 10.1089/thy.2008.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikiforova MN, Stringer JR, Blough R, Medvedovic M, Fagin JA, Nikiforov YE. Proximity of chromosomal loci that participate in radiation-induced rearrangements in human cells. Science. 2000;290:138–141. doi: 10.1126/science.290.5489.138. [DOI] [PubMed] [Google Scholar]
- 34.Schonfeld SJ, Lee C, Berrington de González A. Medical exposure to radiation and thyroid cancer. Clin Oncol (R Coll Radiol) 2011;23:244–250. doi: 10.1016/j.clon.2011.01.159. [DOI] [PubMed] [Google Scholar]
- 35.Furukawa K, Preston D, Funamoto S, et al. Long-term trend of thyroid cancer risk among Japanese atomicbomb survivors: 60 years after exposure. Int J Cancer. 2013;132:1222–1226. doi: 10.1002/ijc.27749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Management NCRPa. NRCP Report No. 160, Ionizing Radiation Exposure of the Population of the United States. Bethesda, MD: NRCP; 2009. 2009. [DOI] [PubMed] [Google Scholar]
- 37.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 38.Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169:2071–2077. doi: 10.1001/archinternmed.2009.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ludlow JB, Davies-Ludlow LE, White SC. Patient risk related to common dental radiographic examinations: the impact of 2007 International Commission on Radiological Protection recommendations regarding dose calculation. J Am Dent Assoc. 2008;139:1237–1243. doi: 10.14219/jada.archive.2008.0339. [DOI] [PubMed] [Google Scholar]
- 40.Bottollier-Depois JF, Chau Q, Bouisset P, Kerlau G, Plawinski L, Lebaron-Jacobs L. Assessing exposure to cosmic radiation during long-haul flights. Radiat Res. 2000;153(5 Pt 1):526–532. doi: 10.1667/0033-7587(2000)153[0526:aetcrd]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 41.Schmid D, Behrens G, Jochem C, Keimling M, Leitzmann M. Physical activity, diabetes, and risk of thyroid cancer: a systematic review and meta-analysis. Eur J Epidemiol. 2013;28:945–958. doi: 10.1007/s10654-013-9865-0. [DOI] [PubMed] [Google Scholar]
- 42.Aschebrook-Kilfoy B, Sabra MM, Brenner A, et al. Diabetes and thyroid cancer risk in the National Institutes of Health-AARP Diet and Health Study. Thyroid. 2011;21:957–963. doi: 10.1089/thy.2010.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shih SR, Chiu WY, Chang TC, Tseng CH. Diabetes and thyroid cancer risk: literature review. Exp Diabetes Res. 2012;2012:578285. doi: 10.1155/2012/578285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tseng CH. Thyroid cancer risk is not increased in diabetic patients. PloS One. 2012;7:e53096. doi: 10.1371/journal.pone.0053096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitahara CM, Platz EA, Freeman LE, et al. Obesity and thyroid cancer risk among U.S. men and women: a pooled analysis of five prospective studies. Cancer Epidemiol Biomarkers Prev. 2011;20:464–472. doi: 10.1158/1055-9965.EPI-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao ZG, Guo XG, Ba CX, et al. Overweight, obesity and thyroid cancer risk: a meta-analysis of cohort studies. J Int Med Res. 2012;40:2041–2050. doi: 10.1177/030006051204000601. [DOI] [PubMed] [Google Scholar]
- 47.Bertakis KD, Azari R. Obesity and the use of health care services. Obesity Res. 2005;13:372–379. doi: 10.1038/oby.2005.49. [DOI] [PubMed] [Google Scholar]
- 48.Kitahara CM, Neta G, Pfeiffer RM, et al. Common obesity-related genetic variants and papillary thyroid cancer risk. Cancer Epidemiol Biomarkers Prev. 2012;21:2268–2271. doi: 10.1158/1055-9965.EPI-12-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cléro É, Doyon F, Chungue V, et al. Dietary iodine and thyroid cancer risk in French Polynesia: a case-control study. Thyroid. 2012;22:422–429. doi: 10.1089/thy.2011.0173. [DOI] [PubMed] [Google Scholar]
- 50.Lawal O, Agbakwuru A, Olayinka OS, Adelusola K. Thyroid malignancy in endemic nodular goitres: prevalence, pattern and treatment. Eur J Surg Oncol. 2001;27:157–161. doi: 10.1053/ejso.2000.1085. [DOI] [PubMed] [Google Scholar]
- 51.Feldt-Rasmussen U. Iodine and cancer. Thyroid. 2001;11:483–486. doi: 10.1089/105072501300176435. [DOI] [PubMed] [Google Scholar]
- 52.Knobel M, Medeiros-Neto G. Relevance of iodine intake as a reputed predisposing factor for thyroid cancer. Arq Bras Endocrinol Metabol. 2007;51:701–712. doi: 10.1590/s0004-27302007000500007. [DOI] [PubMed] [Google Scholar]
- 53.Sehestedt T, Knudsen N, Perrild H, Johansen C. Iodine intake and incidence of thyroid cancer in Denmark. Clin Endocrinol (Oxf) 2006;65:229–233. doi: 10.1111/j.1365-2265.2006.02580.x. [DOI] [PubMed] [Google Scholar]
- 54.Burgess JR, Dwyer T, McArdle K, Tucker P, Shugg D. The changing incidence and spectrum of thyroid carcinoma in Tasmania (1978–1998) during a transition from iodine sufficiency to iodine deficiency. J Clin Endocrinol Metab. 2000;85:1513–1517. doi: 10.1210/jcem.85.4.6554. [DOI] [PubMed] [Google Scholar]
- 55.Rajoria S, Suriano R, Shanmugam A, et al. Metastatic phenotype is regulated by estrogen in thyroid cells. Thyroid. 2010;20:33–41. doi: 10.1089/thy.2009.0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajoria S, Suriano R, George A, et al. Estrogen induced metastatic modulators MMP-2 and MMP-9 are targets of 3,3’-diindolylmethane in thyroid cancer. PloS One. 2011;6:e15879. doi: 10.1371/journal.pone.0015879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kamat A, Rajoria S, George A, et al. Estrogen-mediated angiogenesis in thyroid tumor microenvironment is mediated through VEGF signaling pathways. Arch Otolaryngol Head Neck Surg. 2011;137:1146–1153. doi: 10.1001/archoto.2011.194. [DOI] [PubMed] [Google Scholar]
- 58.Zahid M, Goldner W, Beseler CL, Rogan EG, Cavalieri EL. Unbalanced estrogen metabolism in thyroid cancer. Int J Cancer. 2013;133:2642–2649. doi: 10.1002/ijc.28275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magri F, Capelli V, Rotondi M, et al. Expression of estrogen and androgen receptors in differentiated thyroid cancer: an additional criterion to assess the patient’s risk. Endocr Relat Cancer. 2012;19:463–471. doi: 10.1530/ERC-11-0389. [DOI] [PubMed] [Google Scholar]
- 60.Ward MH, Kilfoy BA, Weyer PJ, Anderson KE, Folsom AR, Cerhan JR. Nitrate intake and the risk of thyroid cancer and thyroid disease. Epidemiology. 2010;21:389–395. doi: 10.1097/EDE.0b013e3181d6201d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kilfoy BA, Zhang Y, Park Y, et al. Dietary nitrate and nitrite and the risk of thyroid cancer in the NIH-AARP Diet and Health Study. Int J Cancer. 2011;129:160–172. doi: 10.1002/ijc.25650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aschebrook-Kilfoy B, Shu XO, Gao YT, et al. Thyroid cancer risk and dietary nitrate and nitrite intake in the Shanghai women’s health study. Int J Cancer. 2013;132:897–904. doi: 10.1002/ijc.27659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tamimi DM. The association between chronic lymphocytic thyroiditis and thyroid tumors. Int J Surg Pathol. 2002;10:141–146. doi: 10.1177/106689690201000207. [DOI] [PubMed] [Google Scholar]
- 64.Jankovic B, Le KT, Hershman JM. Clinical review: Hashimoto’s thyroiditis and papillary thyroid carcinoma: is there a correlation? J Clin Endocrinol Metab. 2013;98:474–482. doi: 10.1210/jc.2012-2978. [DOI] [PubMed] [Google Scholar]