Abstract
Processes that regulate quiescence, self-renewal, and senescence of hematopoietic stem cells (HSCs) are not well understood. Due in part to the ability of xenobiotic ligands to have persistent effects on the immune system in experimental animals, there has been much work to define a physiological role of the aryl hydrocarbon receptor (AhR) and relationships to human disease. Persistent AhR activation by dioxin, a potent agonist, results in altered numbers and function of HSCs in mice. HSCs from AhR null-allele (KO) mice are hyperproliferative and have altered cell cycle. Aging KO mice show characteristics consistent with premature bone marrow exhaustion. We propose that the increased proliferation of HSCs lacking AhR expression or activity is a result of loss of quiescence, and as such, AhR normally acts as a negative regulator to curb excessive or unnecessary proliferation. Similarly, prolonged and/or inappropriate stimulation of AhR activity may compromise the ability of HSCs to sense environmental signals that allow these cells to balance quiescence, proliferation, migration, and differentiation. These data, and others, support a hypothesis that deregulation of AhR function has an important role in HSC regulation and in the etiology and/or progression of certain hematopoietic diseases, many of which are associated with aging.
Keywords: Ah receptor, hematopoietic stem cells, quiescence
Introduction: Ah Receptor (AhR) Biology and Immunity
The AhR is a basic helix-loop-helix transcription factor originally identified as mediating the toxicity of and adaptive response to a large group of xenobiotics including the dioxins and planar polychlorinated biphenyls (PCBs).1 Since that time, a large and diverse number of AhR ligands have been identified, some of which are naturally occurring as well as therapeutic compounds.2 Due in part to the ability of many of these ligands, especially the xenobiotics, to have potent and persistent effects on the immune system in experimental animals models,3 there has been much work to define a normal function of this protein and possible relationships to human disease. Indeed, many investigations over the past 10 years have implicated a role of the AhR at several levels in the immune response.4-5 Furthermore, alteration of AhR activity has been associated with immune-related diseases as well as potential therapeutic activity.6-15 Nevertheless, the cellular and molecular mechanisms that are responsible for these diverse responses are not well understood. Despite, and perhaps because of, the multidimensional immunomodulation activity of the AhR, further understanding of the AhR-regulated signaling pathways that may control immunity has great potential to provide us with new opportunities for diagnosis, prevention, and therapeutic targets.
Hematopoietic Stem Cells and AhR in Hematopoietic Disease and Dysfunction
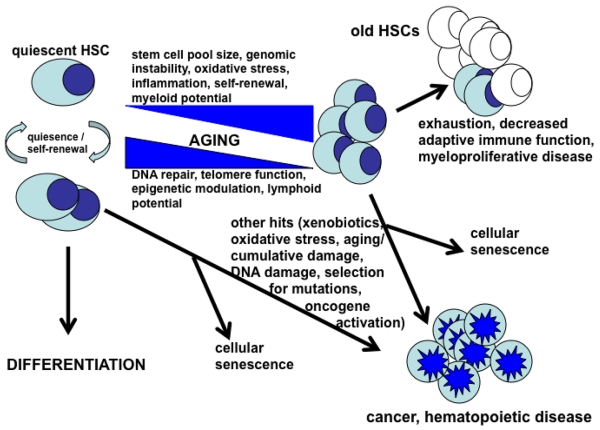
Hematopoietic stem cells (HSCs) are the source for the continuous replacement of ‘worn out’ differentiated cells in the immune system. Under normal homeostatic conditions, HSCs are mostly in a quiescent state so as to prevent premature exhaustion and their susceptibility to gene damage. Under conditions of stress, disease, tissue damage or pathogen exposure, HSCs can undergo extensive self-renewal, expansion and differentiation to mature lineage cells that fight infection and promote tissue repair. The appropriate balance between quiescence and proliferation of HSCs is an important aspect of their biology that allows for the preservation of the capability for long-term multi-lineage generation over the lifetime of an organism. Alterations in this balance may result in premature HSC exhaustion and subsequent bone marrow failure, or lead to senescence or malignant transformation (Figure 1).16-19 It is known that age-related changes in this balance take place, and, as such, it is not unexpected that immune-related disease processes occur at a greater frequency with age. Loss of immune function and increased incidence of certain leukemias and other myelodysplastic syndromes are, in fact, some of the most clinically significant consequences of aging.20,21 Age-related altered immune function also limits the success of therapies used to treat cancer and other disorders, and this further limits the life expectancy as well as quality of life in our aging population. As such, defining the regulatory networks and mechanisms that balance quiescence, self-renewal, differentiation and exhaustion and/or senescence of HSCs are crucially important in identifying biomarkers for early diagnosis as well as therapeutic targets to control and treat these diseases.
Figure 1.
A simple proposed model of relationships among stem cell quiescence, self-renewal and differentiation, exhaustion, senescence and development of disease. Modified from Jacob and Osato,19 and van Zant and Liang.20
In terms of hematopoietic disease, human exposure to the potent xenobiotic AhR agonists 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD; dioxin) or the planar PCBs has been reported to be associated with increased incidence of several hematopoietic disorders including lymphoma and leukemia.6,7,22 AhR-mediated pathways regulating miRNAs have been suggested to be involved in acute myeloid leukemia,10 and the Ahr gene promoter was found to be hypermethylated in human acute lymphoblastic leukemia (ALL) cells.9 The exposure of mice to TCDD and prolonged activation of the AhR results in several changes in HSCs including altered circadian rhythms and expression of clock genes,23 as well as altered numbers and phenotypic characteristics of HSC and progenitor populations,24-26 Although many studies have shown that altered phenotypic characteristics of HSCs do not always reflect altered function, TCDD exposure was also shown to inhibit the functional characteristics of HSCs as assessed by their ability to engraft into irradiated mice.26,27 Furthermore, studies using chimeric animals indicated that the alterations in both phenotypic and function were dependent on the presence of the AhR in hematopoietic cells but not in the stromal niche.27,28 Although providing evidence that is indirect, together these data are consistent with the hypothesis that the AhR has a role in the regulation of HSCs and that the alteration of this function may be either lead to and/or be associated with hematopoietic disease and dysfunction.
Cellular and Molecular Actions of TCDD in HSCs
Given the data suggesting the importance of the AhR in immunoregulation, and especially initial studies with TCDD indicated above, we engaged in studies to more critically define the mechanisms by which TCDD and AhR activation alter HSC activity. Further analysis of phenotypically-defined stem cell populations in bone marrow, showed that TCDD treatment to mice increased the relative percentages of cell subpopulations Lin−Sca-1+c-kit+(LSK)CD48−CD150− and LSKCD48−CD150+ that have been correlated with short-term HSCs and multipotent progenitors and long-term HSCs, respectively.29 To address the apparent paradox that increased percentages of phenotypically-defined HSCs occurred with an apparent decreased functional ability of these cells to generate progeny,26,27 we used a limiting dilution analysis to determine if TCDD affected the numbers of functional HSCs. Using this approach, it was determined that TCDD did not significantly affect the absolute proportions of functional long-term and short-term HSCs. Furthermore, the ability of LSK cells from TCDD-treated mice to undergo expansion and differentiation under ex vivo conditions was not different from cells taken from vehicle-treated animals.29 These data suggested that TCDD was affecting different steps in the engraftment process, e.g. migration to bone marrow, retention of these HSCs in vascular niches, and/or lineage differentiation. In order to address possible effects of TCDD on trafficking behavior of transplanted cells, we assessed the in vivo accumulation of cells in marrow after 24 h of transplantation, and, as a complementary study, measured the directional migration of TCDD-treated LSK cells through a semipermeable membrane to a chamber containing the chemokine CXCL12. Both studies demonstrated that TCDD-treatment decreases the migration and trafficking of phenotypically-defined HSCs. Thus, the observed decreased engraftment of cells from TCDD-treated mice is likely due to decreased migration rather than to decreased numbers of functional HSCs. A modified ability of HSCs and progenitors to move to and/or within the marrow niche may also contribute to the observed effects of TCDD on decreased thymic seeding and altered B cell numbers.24,30
Since the AhR is a transcription factor, we hypothesized that the phenotypic and cellular changes observed following TCDD exposure were consequences of AhR-mediated changes in gene expression. Microarray analyses of LSK cells from mice treated with TCDD were consistent with the phenotypic and functional changes observed. Significant gene changes occurred in pathways involved in antigen presentation, cell-to-cell signaling and interaction, cellular movement, hematological system development and function, and, in particular, immune cell trafficking.29 Among these, the most significant alterations occurred in genes expressing scinderin and metalloproteinase 8. Scinderin is a key regulator of chemotactic responses to CXCL12,31 and metalloproteinases are involved in the stability and function of cell surface proteins directing cell migration.32 Some of the other transcripts changed in the data sets (e.g. Fos, JunB, Ptgs2, and Egr1) encode transcriptional regulators, and it seems likely that some of the functional changes are secondary to initial AhR signaling. Thus, although these gene changes are consistent with the functional changes observed, they do suggest a complex crosstalk of the AhR with several pathways associated with the ability of HSCs to sense their microenvironment and control their trafficking behavior. Notably, an Ingenuity Pathway Analysis of these data sets also indicated a significant association with ‘cancer’ in the hematopoietic system,29 suggesting that disruptions in these pathways may promote hematological disease. This also implies a plausible (and testable) molecular basis for functional changes in HSCs that could lead to the observed increased incidence of lymphoma and leukemias in several populations exposed to xenobiotic AhR ligands.
Loss of AhR Results in HSCs with Abnormal Characteristics and Functions
To better understand the physiological role of the AhR, Ahr-null allele (AhR-KO) mice have been produced by several investigators.33,34 These animals have abnormal vascular development and develop other lesions in several tissues, some of which only become manifest with aging.35 The immune system also develops abnormally, with decreased mass and cellularity of liver, spleen and lymph nodes at one to two weeks of age.30,33-35 Although some of these alterations resolve by three weeks, these mice exhibit and earlier onset of spontaneous neoplasms including lymphomas.36 Nevertheless, up until just recently there has been only a limited characterization of the immune system, especially of HSCs, in these animals.
Further examination of the hematopoietic system in AhR-KO mice revealed several changes consistent with significant alterations in the HSC/progenitor cell populations. Young adult animals had enlarged spleens with increased B220+ and Mac-1+ populations. There was also a near doubling in the number of white cells in peripheral blood that was mainly due to increased numbers of lymphocytes, but a relative decrease in red blood cells. The total number of bone marrow cells, lineage cells, and numbers of phenotypically-defined HSCs/progenitor populations as well as functional HSCs, defined by competitive repopulation into irradiated mice, were also increased in KO mice. The most striking finding was that HSCs from young adult KO mice have inherently high rates of cell division as determined by BrdU incorporation, cell cycle analysis, and expansion under ex vivo conditions.37 That the splenomegaly in KO mice is transplantable by KO HSC/progenitor cells into wild-type mice,37 also indicates the dysfunctionality of these cells, and further suggests that the hematopoietic phenotype in these animals is a function of the increased cycling of HSCs with an increased throughput of lineage cells.
Together, these data support a contention that the AhR functions directly in HSCs to regulate their balance between quiescence and proliferation. In bone marrow, this could occur through a loss of a proliferation block and/or increased sensitivity to proliferative signals. The hypothesis is also consistent with a previous postulate, based on the finding that the Ahr promoter is silenced by hypermethylation in human acute lymphoblastic leukemia, that the AhR is a cell-specific negative regulator of proliferation.9 This role of the receptor is also supported by the finding that AhR antagonists promote the expansion of human HSCs,38 and that the Ahr gene is expressed during periods of quiescence but turned off during the proliferation and self-renewal of these cells.39
Loss of AhR Results in Premature Hematopoietic Stem Cell Exhaustion and Development of a Myeloproliferative Disorder in Aging Mice
Based on the above findings, we further hypothesized that that prolonged loss of AhR and a lifetime of excessive stem cell cycling (i.e. loss in quiescence), would result in sensitivity to stress conditions, premature exhaustion of HSCs, and hematopoietic disease. Indeed, the analyses of aging AhR-KO mice up to two years of age supported this hypothesis.40 Aging AhR-KO mice showed decreased survival, splenomegaly, increased circulating white cells, and hematopoietic cell accumulation in tissues. Although, like younger KO mice, aging mice showed an increased number of phenotypically-defined stem/progenitor cells in bone marrow, there was a significant decrease in self-renewal capacity as determined by competitive repopulation and serial transplantation. HSCs also showed increased levels of reactive oxygen species (ROS), increased staining for γ-H2A.X (an indicator of DNA damage), but decreased p16INK4a. The latter observation was of particular interest given that the expression of p16INK4a is increased in most cells with aging, and is believed to confer protection against mutations by inducing senescence.41 However, a recent report also indicated a decreased expression of p16 with age in acute myeloid leukemic cells, suggesting that this loss of protection may facilitate oncogenesis.42
These data in aging mice are further consistent with the hypothesis that AhR has some role in regulating the balance between quiescence and proliferation. A prolonged lack of AhR expression and/or activity allows HSCs/progenitors to escape quiescence, and a lifetime of cycling ultimately results in premature stem cell aging and exhaustion. All of these events increase the risk of DNA damage from ROS and other stressors that may also result in hematopoietic disease. Other studies have also shown that loss of quiescence can lead to HSC engraftment defects, HSC exhaustion, and eventually to myeloproliferative disease and leukemia.43 A previous report indicated an earlier onset of neoplasms, including lymphomas, in AhR-KO mice.36
Gene Changes in AhR-KO HSCs are Consistent with the Phenotype
To determine gene changes in AhR-KO HSCs that may lead to excessive cycling and premature exhaustion, we assessed the global gene expression profile in HSCs (LSKCD34−CD48−CD150+) cells from young adult KO and wild-type mice. The most significant gene changes (increased expression of Srpk2, Mir170, Creb1, Hes1, mTOR, Rad50, Pdp1, but decreased expression of Stra13) observed in KO mice have also been associated with HSC hyperproliferation, leukemia, and accelerated aging, and are consistent with the phenotype observed in these animals. Pathway analyses also indicated genes changes enriched for signaling pathways associated with oxidative stress, acute myelogenous leukemia, and aging. Notably, there was also an enrichment for genes possessing putative AhR response elements.40
Summary: A Role of the AhR in HSCs
Together, these studies and others support a critical role of the AhR in signaling pathways controlling the balance between HSC quiescence and proliferation. It seems likely that this occurs through both a direct regulation of gene expression and a complex cross talk of the AhR with other factors. Notably, a very recent study identified the AhR as one of several transcription factors, in addition to Egr1, Sox4, and Stat1, playing critical roles in HSC regulation and function.44 It is tempting to speculate further that the dysregulation of AhR function, and thus disruption of the quiescence-proliferation balance, has an important role in the etiology and progression of hematopoietic disease. While this has been suggested for humans exposed to particular xenobiotic AhR ligands,6,7 the potential that genetic and/or epigenetic changes could lead to this dysregulation has not been previously recognized.
Clearly more work is needed to define these relationships in terms of the signaling pathways directly regulated by the AhR that define HSC function or phenotype, the endogenous ligands which regulate AhR activity within the marrow compartment, and how expression of the AhR is itself regulated. It is of interest that kynurenine, which has immune regulatory activity, has been found to be a potent AhR ligand.2,45-47 It is also noteworthy, that expression of the AhR appears to be differentially expressed in quiescent versus proliferating HSCs,40 again suggesting a direct involvement of this transcription factor in regulating this balance. Finally, it is essential to consider and dissect the possible, indeed likely, role of stromal elements and the marrow niche in the regulation of the AhR either through the synthesis of endogenous AhR ligands and/or other factors that regulate AhR expression or its activity. The definition of the latter may be challenging especially if one of the roles of the AhR is to regulate how HSCs ‘sense’ the marrow microenvironment.
REFERENCES
- 1.Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu. Rev. Pharmacol. Toxicol. 1982;22:517–142. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- 2.Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 2011;124:1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerkvliet NI. AHR-mediated immunomodulation: the role of altered gene transcription. Biochem. Pharmacol. 2009;77:746–60. doi: 10.1016/j.bcp.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao N, Whitelaw ML. The emerging roles of AhR in physiology and immunity. Biochem. Pharmacol. 2013;86:561–70. doi: 10.1016/j.bcp.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Quintana FJ, Sherr DH. Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev. 2013;65:1148–61. doi: 10.1124/pr.113.007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertazzi PA, Consoni D, Bachetti S, Rubagotti M, Baccarelli A, Zocchetti C, Pesatori AC. Health effects of dioxin exposure: a 20-years mortality study. Am. J. Epidemiol. 2001;153:1031–1044. doi: 10.1093/aje/153.11.1031. [DOI] [PubMed] [Google Scholar]
- 7.Viel JF, Arveux P, Baverel J, Cahn JY. Soft-tissue sarcoma and non-Hodgkin’s lymphoma clusters around a municipal solid waste incinerator with high dioxin emission levels. Am. J. Epidemiol. 2000;152:13–19. doi: 10.1093/aje/152.1.13. [DOI] [PubMed] [Google Scholar]
- 8.Hayashibara T, Yamada Y, Mori N, Harasawa H, Sugahara K, Miyanishi T, Kamihara S, Tomonaga M. Possible involvement of aryl hydrocarbon receptor (AhR) in adult T-cell leukemia (ALT) leukemogenesis: constitutive activation of AHR in ALT. Biochem. Biophys. Res. Commun. 2003;300:128–134. doi: 10.1016/s0006-291x(02)02793-6. [DOI] [PubMed] [Google Scholar]
- 9.Mulero-Navarro S, Carvajal-Gonzalez JM, Herranz M, Ballestar E, Fraga MF, Rupero S, Esteller M, Fernandez-Salguero PM. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis. 2006;27:1099–1104. doi: 10.1093/carcin/bgi344. [DOI] [PubMed] [Google Scholar]
- 10.Rager JE, Fry RC. The aryl hydrocarbon receptor pathways: a key component of the microRNA-mediated AML signalisome. Int. J. Environ. Res. Public Health. 2012;9:1939–1953. doi: 10.3390/ijerph9051939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monteleone I, MacDonald TT, Pallone F, Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: linking the environment to disease pathogenesis. Curr. Opin. Gastroenterol. 2012;28:310–313. doi: 10.1097/MOG.0b013e328352ad69. [DOI] [PubMed] [Google Scholar]
- 12.Benson JM, Shepherd DM. Aryl hydrocarbon receptor activation by TCDD reduces inflammation associated with Crohn’s disease. Toxicol. Sci. 2011;120:68–78. doi: 10.1093/toxsci/kfq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang I, Ma J, Takeuchi M, Usui Y, Hattori T, Okunuki Y, et al. Suppression of experimental autoimmune uveoretinitis by inducing differentiation of regulatory T cells via activation of aryl hydrocarbon receptor. Invest. Ophthal. Vis. Sci. 2010;51:2109–2117. doi: 10.1167/iovs.09-3993. [DOI] [PubMed] [Google Scholar]
- 14.Baban B, Liu JY, Mozaffari MS. Aryl hydrocarbon receptor agonist, leflunomide, protects the ischemic-reperfused kidney: role of Tregs and stem cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012;303:R1136–R1146. doi: 10.1152/ajpregu.00315.2012. [DOI] [PubMed] [Google Scholar]
- 15.Hu W, Zhao J, Pei G. Activation of aryl hydrocarbon receptor (AhR) by Tranilast, an anti-allergy drug, promotes miR-302 expression and cell reprogramming. J. Biol. Chem. 2013;288:22972–22984. doi: 10.1074/jbc.M113.475624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rossi DJ, Jamieson CHM, Weissman IL. Stem cells and pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 17.Li J. Quiescence regulators for hematopoietic stem cells. Exp. Hematol. 2011;39:511–520. doi: 10.1016/j.exphem.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Waterstrat A, VanZant G. Effects of aging on hematopoietic stem and progenitor cells. Curr. Opin. Immunol. 2009;21:408–413. doi: 10.1016/j.coi.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Jacob B, Osato M. Stem cell exhaustion and leukemogenesis. J. Cell. Biochem. 2009;107:393–399. doi: 10.1002/jcb.22150. [DOI] [PubMed] [Google Scholar]
- 20.Van Zant G, Liang Y. Concise review: hematopoietic stem cell aging, life span, and transplantation. Stem Cell Trans. Med. 2012;1:651–657. doi: 10.5966/sctm.2012-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorshkind K, Swain S. Age-associated declines in immune system development and function: causes, consequences, and reversal. Curr. Opin. Immunol. 2009;21:404–407. doi: 10.1016/j.coi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer S, Hikel SM, Adams K, Hinds D, Moon K. Current status of the epidemiologic evidence linking polychlorinated biphenyls and Non-Hodgkin lymphoma, and the role of immune dysregulation. Env. Health Perspect. 2012;120:1067–1075. doi: 10.1289/ehp.1104652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garrett RW, Gasiewicz TA. The aryl hydrocarbon receptor agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin alters circadian rhythms, quiescence, and expression of clock genes in murine hematopoietic stem and progenitor cells. Mol. Pharmacol. 2006;69:2076–2083. doi: 10.1124/mol.105.021006. [DOI] [PubMed] [Google Scholar]
- 24.Fine JS, Silverstone AE, Gasiewicz TA. Impairment of prothymocyte activity by 2,3,7.8-tetrachlorodibenzo-p-dioxin. J. Immunol. 1990;144:1169–1176. [PubMed] [Google Scholar]
- 25.Murante FG, Gasiewicz TA. Hematopoietic progenitor cells are sensitive targets to 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6 mice. Toxicol. Sci. 2000;54:374–383. doi: 10.1093/toxsci/54.2.374. [DOI] [PubMed] [Google Scholar]
- 26.Singh KP, Wyman A, Casado FL, Garrett RW, Gasiewicz TA. Treatment of mice with the Ah receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis. 2009;30:11–19. doi: 10.1093/carcin/bgn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakai R, Kujiume T, Inoue H, Kanno R, Miyazaki M, Ninomiya Y, Kanno M. TCDD treatment eliminates the long-term reconstitution activity of hematopoietic stem cells. Toxicol. Sci. 2003;72:84–91. doi: 10.1093/toxsci/kfg002. [DOI] [PubMed] [Google Scholar]
- 28.Staples JE, Murante FG, Fiore NC, Gasiewicz TA, Silverstone AE. Thymic alterations induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin are strictly dependent on aryl hydrocarbon receptor activation in hematopoietic cells. J. Immunol. 1998;160:3844–3854. [PubMed] [Google Scholar]
- 29.Casado FL, Singh KP, Gasiewicz TA. Aryl hydrocarbon receptor activation in hematopoietic stem/progenitor cells alters cell function and pathway-specific gene modulation reflecting changes in cellular trafficking and migration. Mol. Pharmacol. 2011;80:673–682. doi: 10.1124/mol.111.071381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurmond TS, Staples JE, Silverstone AE, Gasiewicz TA. The aryl hydrocarbon receptor has a role in the in vivo maturation of murine bone marrow B lymphocytes and their response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 2000;165:227–236. doi: 10.1006/taap.2000.8942. [DOI] [PubMed] [Google Scholar]
- 31.Evans CA, Tonge R, Blinco D, Pierce A, Shaw J, Lu Y, Hamzah HG, Gray A, Downes CP, Gaskell SJ, et al. Comparative proteomics of primitive hematopoietic cell populations reveals differences in expression of proteins regulating motility. Blood. 2004;103:3751–3759. doi: 10.1182/blood-2003-09-3294. [DOI] [PubMed] [Google Scholar]
- 32.Shao HY, Miao ZY, Hui-Chen QFX, Chen XC, Tan S, Zhang HJ, Wang L, Gao YJ, Yang ZL, Zhang L. Nucleophosmin gene mutations promote NIH3T3 cell migration and invasion through CXCR4 and MMPs. Exp. Mol. Pathol. 2011;90:553–560. doi: 10.1016/j.yexmp.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc. Natl. Acad. Sci. USA. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet. Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 36.Hirabayashi Y, Inoue T. Aryl hydrocarbon receptor biology and xenobiotic responses in hematopoietic progenitor cells. Biochem. Pharmacol. 2009;77:521–535. doi: 10.1016/j.bcp.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 37.Singh KP, Garrett RW, Casado FL, Gasiewicz TA. Aryl hydrocarbon receptor-null allele mice have hematopoietic stem/progenitor cells with abnormal characteristics and functions. Stem Cells Develop. 2011;20:769–784. doi: 10.1089/scd.2010.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, Schultz PG, Cooke MP. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1348–1353. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venezia TA, Merchant AA, Ramos CA, Whitehouse NL, Young AS, Shaw CA, Goodell MA. Molecular signatures of proliferation and quiescence in hematopoietic stem cells. PLoS Biol. 2004;2:e301. doi: 10.1371/journal.pbio.0020301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh KP, Bennett JA, Casado FL, Walrath JL, Welle SL, Gasiewicz TA. Loss of aryl hydrocarbon receptor promotes gene changes associated with premature hematopoietic stem cell exhaustion and development of a myeloproliferative disorder in aging mice. Stem Cells Develop. 2013 Oct 19; doi: 10.1089/scd.2013.0346. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singer RAJ, Montecino-Rodrigues E, Witte ON, Dorshkind K. Aging and cancer resistance in lymphoid progenitors are linked processes conferred by p16INK4a and Arf. Gene Dev. 2008;22:3115–3120. doi: 10.1101/gad.1715808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jonge HJM, Woolthuis CM, de Bont ESJM, Huls G. Paradoxical down-regulation of p16INK4a mRNA with advancing age in acute myeloid leukemia. Aging. 2009;1:949–953. doi: 10.18632/aging.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jude CD, Gaudet JJ, Speck NA, Ernst P. Leukemia and hematopoietic stem cells: balancing proliferation and quiescence. Cell Cycle. 2008;7:586–591. doi: 10.4161/cc.7.5.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gazit R, Garrison BS, Rao TN, Shay T, Costello J, Ericson J, Kim F, Collins JJ, Regev A, Wagers AJ, Rossi DJ. Transcriptome analysis identifies regulators of hematopoietic stem and progenitor cells. Stem Cell Rep. 2013;1:266–280. doi: 10.1016/j.stemcr.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mandi Y, Vecsei L. The kynurenine system and immunomodulation. J. Neural. Transm. 2012;119:197–209. doi: 10.1007/s00702-011-0681-y. [DOI] [PubMed] [Google Scholar]
- 46.Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 2013;34:136–143. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlington WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]