Abstract
Prior field studies by our group have demonstrated a relationship between fluvial deposition of heavy metals and hyporheic-zone microbial community structure. Here, we determined the rates of change in hyporheic microbial communities in response to heavy-metal contamination and assessed group-level differences in resiliency in response to heavy metals. A controlled laboratory study was performed using 20 flowthrough river mesocosms and a repeated-measurement factorial design. A single hyporheic microbial community was exposed to five different levels of an environmentally relevant metal treatment (0, 4, 8, 16, and 30% sterilized contaminated sediments). Community-level responses were monitored at 1, 2, 4, 8, and 12 weeks via denaturing gradient gel electrophoresis and quantitative PCR using group-specific primer sets for indigenous populations most closely related to the α-, β-, and γ-proteobacteria. There was a consistent, strong curvilinear relationship between community composition and heavy-metal contamination (R2 = 0.83; P < 0.001), which was evident after only 7 days of metal exposure (i.e., short-term response). The abundance of each phylogenetic group was negatively affected by the heavy-metal treatments; however, each group recovered from the metal treatments to a different extent and at a unique rate during the course of the experiment. The structure of hyporheic microbial communities responded rapidly and at contamination levels an order of magnitude lower than those shown to elicit a response in aquatic macroinvertebrate assemblages. These studies indicate that hyporheic microbial communities are a sensitive and useful indicator of heavy-metal contamination in streams.
The hyporheic zone, the region of saturated sediments beneath a river, which is inundated with a mixture of surface and ground water, is an important component of lotic ecosystems (27, 57, 59). This zone supports a microbial community that performs essential functional roles for the lotic ecosystem (21, 29, 48, 50, 58). For example, hyporheic microbial communities are involved in the cycling of nutrients (58) and nutrient retention (48), constitute the majority of the biomass and activity in lotic ecosystems (21, 29, 56), and can account for 76 to 96% of ecosystem respiration (50). Thus, changes in microbial community structure within the hyporheic zone may alter the functioning of lotic ecosystems.
Contamination of aquatic environments as a result of large-scale mining activities is widespread (47). Acid mine drainage and the introduction of mining wastes into streams have been shown to alter the geochemistry of streambeds (47) and the hyporheic zone (6, 10, 74). Metals released from mining wastes reduce water quality and harm many eukaryotic organisms (7, 11, 12, 43, 47, 69). In similarity to prior work in terrestrial ecosystems demonstrating that heavy-metal contamination alters the activity and composition of microbial communities (3, 23, 63, 73), previous observational studies performed by our laboratory provided evidence that the structure of hyporheic microbial communities is affected by fluvially deposited heavy metals (24, 25). These studies demonstrated a relationship between microbial community structure and the level of heavy-metal contamination in the shallow hyporheic zone. However, questions remain regarding the rate of change in hyporheic microbial community structure in response to heavy-metal exposure and whether various members of the hyporheic microbial community differ in their levels of resiliency in response to fluvial heavy-metal deposition. Herein, we describe a controlled laboratory experiment designed to address these questions.
Heavy-metal effects on microbial communities have generally been investigated through the controlled application of individual or mixed metal salts (22, 31, 41, 68) or by comparing contaminated and uncontaminated environments (31, 53, 54, 61, 73). The former approach provides the greatest experimental control while sacrificing realism, whereas the latter offers greater realism but reduced control over potentially confounding environmental factors (53). The present experiment is structured to maintain experimental control while exposing microbial communities to environmentally relevant heavy-metal treatments. Results of this experiment establish rates at which a hyporheic microbial community responds to fluvial heavy-metal contamination and demonstrate that hyporheic microbial communities can be useful indicators of heavy-metal contamination in lotic ecosystems.
MATERIALS AND METHODS
Experimental design.
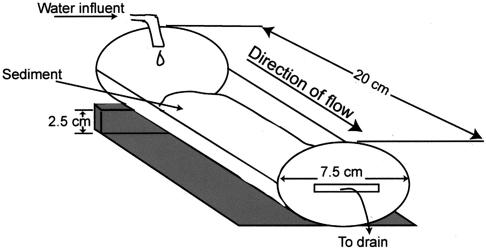
Using a repeated-measurement factorial design, the same sediment-associated hyporheic microbial community was exposed to five different levels of an environmentally relevant metal treatment (four replicates each of 0 [control], 4, 8, 16, and 30% heavy-metal-contaminated sediment). To accomplish this, 20 flowthrough river mesocosms (Fig. 1) were constructed, with identical inocula of homogenized hyporheic sediment with its indigenous microbial community (i.e., “live sediment”) combined with a fixed amount of appropriate mixtures of sterilized contaminated and uncontaminated sediments as described below and in Table 1. All mesocosms were subsequently incubated under identical conditions, and community response variables were compared between treatments and controls for each time point.
FIG. 1.
Schematic of the river mesocosms used to test for heavy-metal effects on hyporheic microbial communities.
TABLE 1.
Construction of experimental treatments
| Treatment level (%) | Live sediment (g) | Sterile contaminated sediment (g) | Sterile uncontaminated sediment (g) | Total sediment (g) |
|---|---|---|---|---|
| Control | 105 | 0 | 45 | 150 |
| 4 | 105 | 6 | 39 | 150 |
| 8 | 105 | 12 | 33 | 150 |
| 16 | 105 | 24 | 21 | 150 |
| 30 | 105 | 45 | 0 | 150 |
Live sediment was harvested from a third-order stream (Rattlesnake Creek, Missoula, Mont., 46°52′20"N, 113°59′00"W) with no known impact from mining by collecting bulk sediment (0- to 20-cm depth) via hand sieving with stacked 2.36- and 1.70-mm-pore-size stainless steel sieves. The recovered sediments were placed in a sterile, closed container and transported submerged in stream water to the laboratory. Upon arrival at the laboratory, the sediments were maintained at 4°C while the experimental treatments were compiled. A 900-g aliquot of this sediment was separated and sterilized by autoclaving at 121°C for 30 min on two successive days. This sterilization treatment was previously determined to be sufficient for killing all organisms associated with similar sediments (30). Heavy-metal-contaminated sediments of the same size range were gathered previously from the floodplain of Silverbow Creek near Butte, Mont. (third-order stream; 46°06′28"N, 112°48′17"W; elevation, 4,912 ft), sterilized as described above, and stored at −20°C until used. Treatment combinations (Table 1) were prepared in bulk to reduce within-treatment variability, and each combination was inoculated into four replicate mesocosms in 150-g aliquots. The mean concentrations of metals in the mesocosms ranged between 3.8 and 48.6 μg/g of sediment for As, 12.5 and 80.5 μg/g for Cu, 2.6 and 39.7 μg/g for Pb, and 26.7 and 134.8 μg/g for Zn; in each case, the lower value indicates the background concentration in the unamended mesocosms and the higher value indicates the concentration in the 30% amendment mesocosms. Since concentrations of these metals covary in the contaminated sediments, levels of metal content in the treatments are presented as contamination index (CI) values hereafter.
This experimental design allowed for different levels of a metal contamination treatment while maintaining identical hyporheic microbial community biomass and composition characteristics between treatments and replicates. In this way, all mesocosms contained identical microbial inocula and each treatment differed only in the ratio of contaminated to uncontaminated sterile sediments making up the remainder of the matrix. The use of a common municipal water source (filtered through an activated carbon filter to remove any residual chlorine) ensured that there would be no differences in the dissolved nutrients supplied to each mesocosm. All mesocosms were supplied water through a manifold at the same rate such that there was constant flow and the sediments were completely submerged. Mesocosms were incubated in the dark in a temperature-controlled chamber at the mean ambient annual temperature (14°C) for the stream.
Since grain size and slope determine flow rates through sediment and therefore nutrient availability for sediment-associated microbial communities, we used the same sediment size fraction for both the metal-contaminated and clean sediments and incubated all mesocosms at the same slope. To ensure that organic carbon levels were the same between treatments, each mesocosm was amended with 0.2% particulate organic matter. Organic-matter amendments consisted of a mixture of equal amounts of alder, cottonwood, and willow leaves that had been dehydrated, crushed, and sterilized and then thoroughly mixed with each bulk treatment combination prior to assembly of the mesocosms.
Sampling regimen.
Mesocosms were sampled at 1, 2, 4, 8, and 12 weeks. At each time point, subsamples were taken through the depth profile (0 to 5 cm) at five points along the length of each mesocosm by the use of flame-sterilized stainless steel scoops, thus controlling for position effects within the unidirectional flowthrough columns. Samples were taken over a time course to allow the rate at which microbial communities respond to heavy-metal contamination to be determined and to assess whether this response is consistent over time. Sediments from each mesocosm were placed in Whirl-Pak sampling bags (Fisher Scientific, Pittsburgh, Pa.), put on dry ice, and lyophilized overnight in a Freezemobile 24 lyophilizer (Virtis, Gardner, N.Y.). Once dried, sediments were stored at −70°C prior to subsequent analyses. Water pH was measured for 25-ml water samples gathered from each mesocosm effluent by use of a model 340 pH meter (Corning, Kennebuck, Minn.). Water samples were taken just prior to each sediment-sampling event.
DNA extraction.
Samples of lyophilized sediment (1 g) were extracted using a method based on that of Yu and Mohn (76), with modifications as described previously (26).
DGGE and gel pattern analysis.
Microbial community compositions of treatments and replicates were determined by denaturing gradient gel electrophoresis (DGGE) analysis. PCR amplification conditions, the primer pairs utilized, DGGE gel constituents, and electrophoresis conditions were all as described previously (26). Briefly, PCR was performed using the generally conserved 16S rRNA gene primers 536fC (5′CGC CCG CCG CGC CCC GCG CCC GGC CCG CCG CCC CCG CCCC CWT AAT GGC GCC GMC GAC 3′) and 907r (5′CCC CGT CAA TTC CTT TGA GTT T 3′) (26). PCR amplicons were separated via DGGE using a D GENE system (Bio-Rad Laboratories, Hercules, Calif.). Each DGGE gel contained two sets of internal standards to allow cross-gel comparisons: a Gibco 100-bp ladder (Invitrogen Corp., Carlsbad, Calif.) and a separate lane with 100 ng each of PCR product amplified from purified chromosomal DNA of Clostridium perfringens and Micrococcus luteus.
A linear gradient of denaturants ranging from 25 to 60% urea:formamide in a 6% acrylamide gel matrix was used. Gels were run at 60°C and 30 V for 30 min; the voltage was then increased to 130 V for 5 h. DGGE bands were visualized by staining with SYBR Green I (BioWhittaker Molecular Applications, Rockland, Maine), and the resulting patterns were captured digitally with a Gel Doc 1000 system and Molecular Analyst software (Bio-Rad Laboratories).
Analysis of gel patterns for between-treatment and between-time-point pattern similarities was performed using GelCompar version 4.0 software (Applied Maths, Kortrijk, Belgium) as described previously (26). A mean similarity matrix relating DGGE pattern similarities among all within-treatment replicates and between-treatment similarities for the entire experiment was generated. Similarity matrix data were transformed to relate community composition to CI as described below. Dissimilarity scores were calculated as 100 − similarity score. Mean dissimilarity scores were calculated by summing the dissimilarity scores for each combination of treatment comparisons for each time point and dividing by the number of replicate comparisons.
Real-time qPCR.
A suite of three group-specific primer sets corresponding to three predominant indigenous phylogenetic groups (groups I, II, and III; most closely related to the α, β, and γ-proteobacteria, respectively) were designed and used to monitor group-level abundance. These three phylogroups were previously shown to be the most abundant groups in the hyporheic zone (26). Primer construction, PCR conditions, and development of real-time quantitative PCR (qPCR) standards were performed as described previously (24). Briefly, qPCR reactions were performed using a Bio-Rad iCycler and an SYBR Green I detection method. Separate standards were designed for each targeted phylogenetic group. Standard curves were typically linear across 5 orders of magnitude (107 to 102 copies). Individual samples were diluted or concentrated to bring target copy numbers to within the linear range of detection. All qPCR values are expressed as log 16S rDNA copy number g−1 of sediment (dry weight).
Geochemical analyses.
Previous work by our group (30) has demonstrated that sediment-associated metal concentrations do not differ significantly over the time frame of the experiment described herein. Therefore, the total recoverable metal content of each treatment mixture was analyzed only at the end of the experiment (i.e., the 12-week time point). This also minimized sampling disturbance during the course of the experiment by reducing the amount of sediment removed at each time point. Total recoverable metal levels were determined by extracting sediments with hot acid and analyzing the extracts on an inductively coupled plasma analysis spectrometer (IRIS model; Thermoelemental, Franklin, Mass.) according to U.S. Environmental Protection Agency test method 200.7 as described previously (24). Concentrations of four metals of interest (As, Cu, Pb, and Zn) were used to create the CI. CI was used as a measure of contamination relative to the background metal content of controls determined by the following formula:
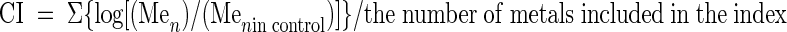 |
where n = As, Cu, Pb, and Zn.
Total carbon associated with the sediments making up each treatment combination was measured at the beginning of the experiment. For each carbon analysis, 10 g of sediment was dried overnight at 60°C and then crushed in a model 8000 mixer-mill (Spex Industries, Edison, N.J.) for 15 min. Ground samples (0.5 g) were measured for total carbon by the use of an EA 1110 elemental analyzer (CE Instruments, Lakewood, N.J.).
Statistical analysis of data.
Three separate approaches were used to analyze relationships between microbial community response variables and heavy-metal treatments: nonmetric dimensional scaling (NMDS), univariate analysis of variance (ANOVA) and multivariate ANOVA (MANOVA), and linear regression.
Relative differences in microbial community composition between metal levels and changes over time were determined by applying an NMDS analysis to the mean DGGE similarity matrix. ANOVA was used to determine whether there were significant changes in community composition and whether there were significant differences in the levels of abundance of three phylogenetic groups monitored with qPCR in response to metal treatments and across time. Tukey-Kramer multiple-comparison tests and direct contrasts between each treatment level and controls at each time point were used to determine which levels of metal amendment resulted in significant changes in group-level abundance at each time point. Linear regression modeled differences in microbial community composition in relation to CI, thus providing a predictive model of how the composition of microbial communities responds to fluvially deposited heavy metals. All statistical tests were performed using NCSS (Kaysville, Utah) 2001 software. The qPCR data was log transformed (log 16S rDNA copy number/g of dry weight) so that the assumptions of ANOVA and MANOVA were met. All other data met the assumptions of the multivariate statistics applied without transformation.
RESULTS
Metal treatments.
Metal levels between treatments are represented by an index of contamination (CI). The CI values exhibited a linear increase in the level of metal amendment from control to 30% treatment. Means and standard errors of CI values for each treatment were 0.007 ± 0.01, 0.31 ± 0.08, 0.44 ± 0.03, 0.75 ± 0.04, and 0.94 ± 0.10 for the control and the 4, 8, 16, and 30% metal amendments, respectively. The range of metal treatment levels utilized here was previously shown to induce linear decreases in bacterial community productivity in hyporheic sediments from the area, as determined by [14C]Leu incorporation levels (30). It was hypothesized that this same contaminant range would result in linear changes in microbial community structure. The pH of effluent water from each column was measured at the same time that sediment samples were taken to determine whether the metal treatments altered mesocosm pH. The pH values for all mesocosms were circumneutral for the entire experiment (range = 7.83 to 8.35), and there were no significant differences between treatments (FpH = 1.12; P = 0.38). Total organic carbon was also measured for each mesocosm (data not shown), and no significant differences among treatment levels were found (Forganic carbon = 1.17; P = 0.373; range = 0.04 to 0.09%).
Community composition.
The compositions of microbial communities within each mesocosm were compared by DGGE pattern analysis. Visual analysis of banding patterns indicated that there was little variation within treatments and that there were detectable differences between treatments (data not shown). In addition, visual gel analysis suggested that microbial community composition was highly variable during weeks 1, 2, and 4 and became less variable during weeks 8 and 12. However, visual comparison of banding patterns provides only a qualitative assessment of similarity. To overcome this limitation, the relationships between community composition and metal amendments were confirmed by applying multivariate statistics and linear regression to a mean DGGE similarity matrix generated as described previously (28).
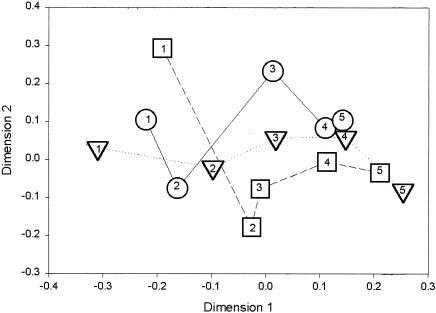
NMDS analysis of the mean DGGE similarity matrix was used to compare community composition within and between treatments during the experiment (Fig. 2). NMDS plots can be interpreted by evaluating observed distances between points on the graph or by finding patterns in the multidimensional space of the plot (9, 42). When applied to DGGE data, NMDS plots graphically represent relative differences in community composition between metal treatments at each time point and changes within treatments across time. Figure 2 indicates the results of NMDS analysis for control and 8 and 30% metal treatments. The 4 and 16% results were omitted for clarity of the figure and are therefore described here. Community composition with the 4% metal treatment followed the pattern exhibited by controls, with NMDS symbols for the 4% treatment lying between the control and 8% treatment results. Communities present in the 16% metal level treatment followed a pattern similar to that of the 30% treatment, with community structures at each time point being more similar to the 8% treatment than to the 30% treatment results. There were pronounced changes in community composition within each treatment detectable during weeks 1, 2, and 4, as indicated by the large relative separation of points plotted in Fig. 2. Less-dramatic changes were detected in the mesocosm communities after 8 and 12 weeks of incubation (Fig. 2).
FIG. 2.
Plot of the first and second dimensions of an NMDS analysis. Points represent mean similarities of DGGE patterns between metal treatments and time points. The mean similarity for each treatment was used to reduce the number of individual data points, and only three metal treatment levels are indicated for clarity, thus simplifying interpretation of the NMDS plot. ○, control; ▵, 8% metal treatment; □, 30% metal treatment. Numbers indicate the time points represented by the symbols: 1, 1 week; 2, 2 weeks; 3, 4 weeks; 4, 8 weeks; 5, 12 weeks. Lines connecting symbols are included to clarify changes in community composition for each treatment at each time point and are not intended to indicate the positions of communities at intermediate time points.
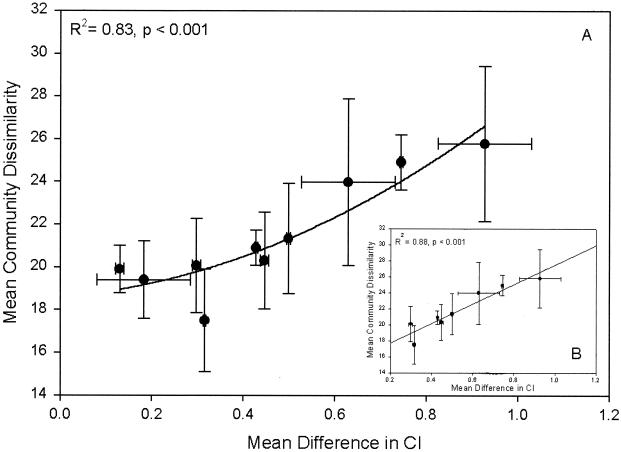
To determine whether differences in community composition between treatments were consistent over time, the mean percent dissimilarity in community composition between all treatments and across all time points was plotted against the difference in CI between treatments (Fig. 3A). This plot indicates that there is a significant curvilinear relationship between CI and the detectable community composition (R2 = 0.83; P < 0.001). Further examination of this relationship suggests that when the difference in CI between treatments is >0.2, there is a linear relationship (R2 = 0.88; P < 0.001) between community composition and heavy-metal contamination (Fig. 3B).
FIG. 3.
(A) Mean percentages of dissimilarity in community composition as determined by DGGE pattern analysis versus the difference in CI between treatments. (B) Mean percentages of dissimilarity in community composition versus the difference in CI for treatment levels that differ by more than 0.2 CI units.
Real-time qPCR.
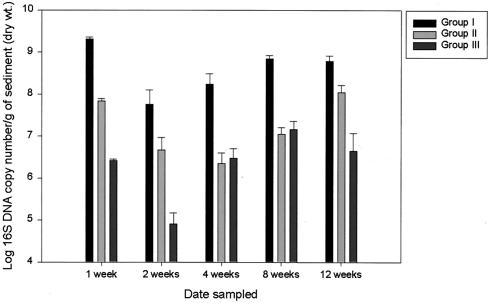
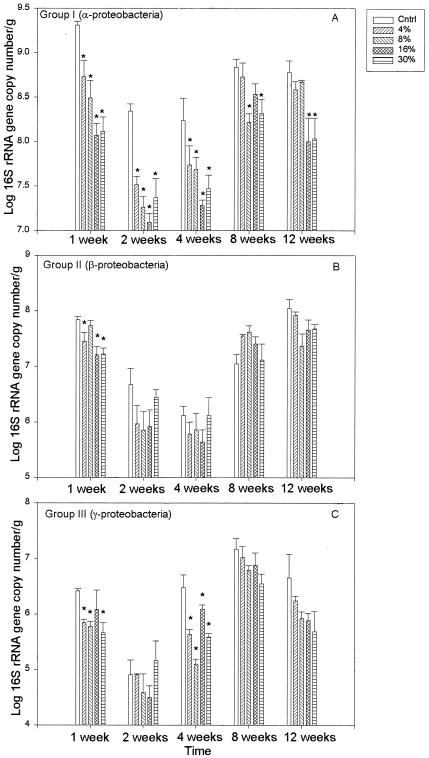
Group-level abundances of three separate phylogenetic groups were monitored with qPCR using primer sets developed from indigenous members of hyporheic microbial communities in western Montana (26). Groups I, II, and III are most closely affiliated with the α-, β-, and γ-proteobacteria, respectively (26). Similar patterns of change in group-level abundances were noted in the control treatments for all three phylogenetic groups (Fig. 4). The general pattern included a decrease in abundance between weeks 1 and 2 followed by a gradual increase back to week 1 levels by the end of the experiment (Fig. 4). The relative abundance of each monitored phylogenetic group was calculated by dividing the 16S rRNA gene copy number/group by the total 16S rRNA gene copy numbers detected for all three groups. While only an approximation of relative abundance within the total community, this allows us to directly compare the levels of abundance of the three groups being monitored within each sample. Group I was the most abundant phylogenetic group detected throughout the experiment, with relative abundances ranging from 83 to 97% of total 16S rDNA copy numbers g−l of sediment in control treatments. Group II tended to be the second most abundant group detected in the mesocosms, comprising 2 to 15% of total detected 16S rDNA copy numbers g−1 of sediment in controls. Group III was the least abundant group, with a percent abundance range of 0.1 to 2.5% of measured 16S rDNA copy numbers g−1 of controls.
FIG. 4.
Means and standard errors of group-level abundance measured in the control treatments over the course of the experiment.
Each phylogenetic group appeared to respond uniquely to the metal treatments. Therefore, the effect of heavy-metal amendments on the abundance of groups I, II, and III was ascertained by analyzing qPCR data with multivariate statistics. Significant differences in phylogenetic group abundance with respect to two factors, heavy metals (CI) and sampling date, were determined by analyzing qPCR data with MANOVA and ANOVA. MANOVA can simultaneously test for differences between means of two or more dependent variables (e.g., all three phylogenetic groups) with respect to multiple independent variables or factors (e.g., metal treatment and sampling date) (34). Univariate ANOVA can determine whether means of a single response variable (e.g., group I abundance) are different from one another with respect to two or more independent variables (e.g., metal treatment level and sampling date) (34).
MANOVA indicated that metal treatment, sampling date, and the interaction of metal treatment and sampling date significantly affected the abundance of the monitored phylogenetic groups (Table 2). Univariate ANOVA was then used to determine which phylogenetic groups and dates were driving the significant interactions detected by MANOVA. The abundance of all three monitored phylogenetic groups was significantly affected by metal treatments, sampling date, and the interaction of these two factors (Table 2). These multivariate analyses indicate that the abundances of groups I, II, and III are affected by the metal treatments and time.
TABLE 2.
Results from multivariate statistical analyisis of phylogenetic group abundances
| Factor | MANOVA results
|
ANOVA results
|
||||||
|---|---|---|---|---|---|---|---|---|
| Group I
|
Group II
|
Group III
|
||||||
| Wilks lambda | P | F | P | F | P | F | P | |
| Metal | 3.86 | <0.001 | 5.12 | 0.001 | 3.1 | 0.02 | 8.52 | <0.001 |
| Sampling date | 13.67 | <0.001 | 46.32 | <0.001 | 92.86 | <0.001 | 67.18 | <0.001 |
| Metal × sampling date | 2.7 | <0.001 | 2.79 | <0.001 | 2.1 | 0.02 | 4.21 | <0.001 |
Direct contrasts of group-level abundance in each metal-amended treatment level at each sampling date to group-level abundances in control treatments were utilized to determine whether groups I, II, and III differed in their levels of resiliency in response to the stress of the metal treatments (Fig. 5). Resiliency is defined here as a return of group-level abundance in a metal-amended treatment to the abundance of that group in control samples at the same time point. Group I abundance appeared to recover from metal stress only in the 4 and 8% metal amendments and only after 12 weeks of incubation (Fig. 5A). The abundance of this group was significantly lower in metal-amended mesocosms than in controls for the first 4 weeks of the experiment. However, by week 8, group I abundance in the 4% treatment was not different from that of the control. After 12 weeks of incubation, group I abundance with the 4 and 8% metal amendments was not different from that of the controls and was only significantly lower than that of the controls with the 16 and 30% treatments.
FIG. 5.
Means and standard errors of group-level abundance measured in each treatment level at 1-, 2-, 4-, 8-, and 12-week time points. (A) Means and standard errors of group I abundance. (B) Means and standard errors of group II abundance. (C) Means and standard errors of group III abundance. Stars indicate treatment levels at which group-level abundances are significantly (P < 0.05) lower than those seen with the control for each time point.
Group II exhibited a pattern strikingly different from that observed for group I (Fig. 5B). After 1 week of incubation, group II abundance was significantly lower than that of control samples in the majority of metal-amended mesocosms. For the remainder of the experiment, group II abundance was not significantly lower in metal-amended mesocosms than in control mesocosms.
The response of group III abundance to metal treatments was unlike that observed for group I or II (Fig. 5C). After 1 and 4 weeks of incubation, the abundance of group III was significantly lower for all metal treatment levels relative to that of the control (except for that of the 16% treatment at 1 week of incubation; however, this was most likely due to the high standard error of mean abundance in this treatment level). After 8 and 12 weeks of incubation, group III abundance was not statistically different from the control values.
Linear regression was employed to determine whether there were consistent predictable relationships between group-level abundance and CI. There was a strong significant negative correlation between CI and group I abundance throughout the experiment except at the 8-week time point; there, a negative trend was observed (Table 3). Group II abundance was negatively correlated with the CI at the 1-week time point only (Table 3). By contrast, the abundance of group III was significantly negatively correlated with CI at the 8- and 12-week time points only (Table 3).
TABLE 3.
Correlation coefficients (R2) and significance levels of Group I, II, and III abundance versus the CI for each time point and across the whole experiment
| Group |
R2 (P) for indicated time point or period
|
|||||
|---|---|---|---|---|---|---|
| 1 wk | 2 wk | 4 wk | 8 wk | 12 wk | Whole experiment | |
| I | −0.93 (0.01) | −0.83 (0.03) | −0.86 (0.02) | −0.72 (0.07) | −0.78 (0.05) | −0.89 (0.02) |
| II | −0.85 (0.03) | −0.04 (0.75) | −0.15 (0.51) | −0.00 (0.91) | −0.29 (0.35) | −0.13 (<0.001) |
| III | −0.44 (0.22) | −0.01 (0.93) | −0.21 (0.43) | −0.93 (< 0.01) | −0.90 (0.01) | −0.14 (<0.001) |
DISCUSSION
Metal treatments.
Environments contaminated with mining wastes tend to have elevated levels of multiple heavy metals that exist as complex chemical mixtures and exhibit various degrees of bioavailability (3). Therefore, controlled experiments that utilize individual metal salts as experimental treatments (22, 31, 41, 68) have limited external realism relevant to such systems. The metal amendments used here consist of sediments enriched in a variety of metals, including As, Cu, Pb, and Zn, through alluvial deposition in the environment following decades of mining activity in the region and thus have ecological relevance to the study of heavy-metal effects on hyporheic microbial communities. These surface-associated heavy metals covary with one another; therefore, it is difficult to determine the effect of the presence of any individual metal on microbial community structure (55). Instead we generated a CI on the basis of a suite of toxic metals present in the sediments to relate to the measured microbial response variables. The metals included in the CI were previously shown to be the most important in describing the relationship between fluvial heavy-metal deposition and hyporheic microbial community structure and function (24, 25, 30). Similar contamination indices have previously been used to evaluate levels of metal contamination and effects of heavy metals on community structure in a variety of systems (4, 17, 24, 51, 75).
Increased external relevance can be attained when environmental gradients are used to determine the degree to which microbial community structure is influenced by heavy-metal contamination (22, 24, 32, 53). However, environmental gradients can include factors that covary with the contaminant, thereby complicating analysis of results (52). Factors that influence aquatic microbial community structure include dissolved nutrient levels (8, 23, 35, 49), carbon quality and quantity (53), flow rates and sediment porosity (33, 77), pH (23), viral lysis (65, 71), and grazing (36, 40, 65, 70). These and other potential covariates were controlled for in the present experiment through the experimental design, in which microbial community inoculum, water chemistry, total carbon, temperature, and other environmental parameters were held constant while the degree of contamination with the metal mixture was adjusted. Thus, differences in community structure between controls and metal-amended mesocosms occurred in response to the applied heavy-metal amendments and not to some other unmeasured factor(s).
Community composition.
NMDS analysis of DGGE patterns indicated that microbial community compositions within each treatment differed during the experiment (Fig. 2). Changes in aquatic microbial community composition over time are not unusual (23, 36, 39, 45, 66). The pattern of variation observed here suggests that following 8 weeks of incubation, a steady-state community composition had been reached in the control mesocosms only (Fig. 2). That steady-state community composition changed little during the remainder of the experiment. These data suggest that it takes approximately 2 months for a hyporheic microbial community to reestablish a steady state following a major physical disturbance, such as the construction of our experimental treatments, in the absence of heavy-metal contamination. To the best of our knowledge, this is the first reported rate for hyporheic microbial community reestablishment following a physical disturbance. The continued changes observed in the metal-amended mesocosms suggest that the presence of heavy metals decreases the ability of the community to recover from physical disturbance (Fig. 2).
Although the composition of the microbial communities changed during the experiment, the relationship between community structure dissimilarity and the level of heavy-metal contamination (CI) was relatively constant (Fig. 3). This relationship was consistent and detectable after only 1 week of incubation. This suggests that the heavy-metal amendments imposed an immediate and continual selective pressure on the hyporheic microbial community and that changes in community structure in response to fluvial heavy-metal deposition are rapid. Short-term response to heavy metals in the form of rapid changes in aspects of microbial community structure such as bacterial cell densities and phospholipid fatty acid patterns have been noted in soils experimentally amended with heavy-metal salts (22, 32).
The curvilinear nature of the relationship between CI and hyporheic microbial community composition is different from the linear nature of the relationship described in previous field study reports (24, 25). This may be due to the smaller differences in CI tested here. The curved region of the relationship depicted in Fig. 3A suggests that very small differences in CI (<0.2), regardless of metal concentrations, will result in little to no detectable difference in hyporheic microbial community composition, potentially representing the threshold of resolution or simply reflecting the degree of experimental variability or sample-to-sample heterogeneity in the system. A similar dose-response relationship between microbial community response variables and heavy-metal contamination in metal-contaminated soils was previously noted (3). Detection of this curvilinear relationship suggests that for determining the level of contamination that is required to cause change in hyporheic microbial community composition, this controlled mesocosm experiment provided a resolution finer than was provided by the previous observational field studies of Feris et al. (24, 25). Shifts in community composition caused by metal treatments are likely due to selection for metal-tolerant populations (22, 54). This probable selection for metal-tolerant populations resulted in a 20 to 30% decrease in microbial community similarity between treatment levels. Previous work by our group indicated that a gradient of heavy-metal contamination across six different river sites accounted for a similar decrease in hyporheic microbial community similarity (24). Since environmental variables in the present experiment were controlled through experimental design, the change in community composition comprised the entire range of variation between samples. Similar degrees of change in community structure have been ascribed to heavy-metal contamination in terrestrial and aquatic environments. In soils contaminated with a mixture of Cu, Cd, Ni, and Zn, approximately 30% of the variation in microbial community structure, as measured by phospholipid fatty acid analysis, could be attributed to metals (54). A report of a study of 95 different streams, representing a range of metal contamination conditions, attributed 40% of variability in benthic invertebrate species richness to in-stream metal concentrations (17). The present report provides additional evidence that heavy-metal contamination in either terrestrial or aquatic environments doesn't result in whole-scale replacement of communities (17, 18). Rather, a shift in community structure occurs, with loss of some species and recruitment of new ones.
Shifts in hyporheic microbial community structure were detected at relatively low levels of contamination. Analysis of DGGE patterns with NMDS was suitable for distinguishing the community structure in controls from those seen with all other treatments. Since the 4 and 8% metal treatments were not significantly different from one another in terms of CI, the 8% metal amendment result was treated as the limit of detection for heavy-metal-induced change in hyporheic microbial community composition. The sediment metal concentrations represented by the 8% metal treatment (As = 9.0 μg/g of sediment, Cu = 15.7 μg/g, Pb = 10.9 μg/g, and Zn = 32.2 μg/g) are an order of magnitude lower than those that induce a response in benthic macroinvertebrates (2, 12-16, 19, 20, 44). A survey of the literature indicates that, on average, sediment metal values of As = 84 μg/g, Cu = 100 μg/g, Pb = 36 μg/g, and Zn = 346 μg/g are the limits at which a change in benthic macroinvertebrate communities can be detected.
Group-level abundance determined by qPCR.
DGGE pattern analysis revealed changes in community composition due to the metal treatments and how quickly these changes took place. However, that approach does not indicate how phylogenetic group abundance was affected by the presence of heavy metals.
Real-time qPCR can be used to monitor changes in the relative levels of abundance of individual species or groups of microorganisms in the laboratory and field (37, 67). Multivariate analyses and linear regression of qPCR data detected significant relationships between metal treatments and group-level abundance and indicated that these relationships changed during the course of the experiment (Table 2).
Group I abundance fluctuated over the course of the experiment. However, the effect of the metal amendments was consistently negative for each time point (Table 3). It appears that some members of this group are tolerant to low levels of heavy metals, but as metal levels increase, this tolerance is overcome and the abundance of group I declines. These results confirm the pattern detected in a prior field study, which indicated that the abundance of this group was negatively correlated with fluvially deposited heavy metals (24). This finding is, however, contrary to those of previous investigations of long-term effects of heavy-metal-contaminated sewage sludge amendments on soil bacterial communities. Sandaa et al. demonstrated an increase in α-proteobacterium levels in soils contaminated with a similar suite of metals (Cd, Cu, Ni, and Zn) compared to the results seen with control soils (61, 62). Thus, α-proteobacteria in the hyporheic zone appear to respond to heavy-metal contamination differently than those found in terrestrial environments. Considering the diversity of this phylogenetic group, some discrepancy between community responses in terrestrial and aquatic environments is perhaps not surprising.
Group II appeared to be the most resilient in response to the presence of heavy metals. Group II abundance was lower in the metal-amended mesocosms only at the beginning of the experiment. The high degree of resiliency in group II abundance suggests either that this phylogenetic group contains metal-tolerant members or possibly that it is more adept at expression of metal resistance than group I or III. Further, since a consistent relationship between the abundance of this phylogenetic group and the metal amendments is lacking (Table 3), we suggest that the abundance of β-proteobacteria associated with stream sediments would be a poor indicator for estimations of metal contamination in lotic environments.
The relationship between group III abundance and CI shows a pattern remarkably different from that observed for group I or II. Significant decreases in the abundance of this group could only be attributed to the metal treatments during weeks 1 and 4. After 8 and 12 weeks of incubation, the abundance of this group appeared to recover from heavy-metal stress at all levels of amendment, although a significant negative linear relationship with CI remained (Table 3). It is difficult to relate this response to previous studies, because there is a paucity of information regarding the response of microbial communities to heavy-metal contamination over time. However, reduced abundance of γ-proteobacteria in response to heavy-metal exposure is in agreement with previous measurements in soils (61, 62). We suggest that the linear relationship between the abundance of this group and CI could be useful for detecting and predicting longer-term (weeks to years) biotic effects of exposure to heavy metals in lotic environments.
In similarity to the observed effects on hyporheic microbial community composition, the level of contamination at which heavy-metal effects can be detected in group-level abundances is much lower than that seen where benthic macroinvertebrate populations demonstrate a response. Sediment metal levels of 9.0 μg of As/g of sediment, 15.7 μg of Cu/g, 10.9 μg of Pb/g, and 32.2 μg of Zn/g (represented by the 4% metal treatment) were sufficient to significantly lower the abundance of group I and group III (after only 1 week of exposure). These levels are an order of magnitude lower than those at which benthic macroinvertebrates have shown a response (2, 12-16, 19, 20, 44). That hyporheic microbial communities appear to be more sensitive to fluvial deposition of heavy metals than benthic macroinvertebrates is perhaps not surprising. Hyporheic microbes generally live in close association with sediment surfaces (5, 26, 28, 46, 56). Therefore, changes in sediment geochemistry due to deposition of heavy metals (6, 51, 72, 74) and toxic effects of the metals themselves (1, 38, 60, 64, 68) should present strong selective pressures on the local microbial community.
Conclusion.
This investigation provides additional evidence that hyporheic microbial community structure is affected by fluvial heavy-metal deposition. The response of these communities was rapid and could be detected at contamination levels an order of magnitude lower than those shown to elicit a response in aquatic macroinvertebrate communities. Since aquatic invertebrates actively graze on microbial communities, it is possible that changes in hyporheic microbial community structure due to low levels of metal contamination ultimately affect higher trophic levels. Therefore, using hyporheic microbial communities as indicators of short-term or low-level heavy-metal contamination in lotic environments may provide a useful tool for environmental quality analyses.
Acknowledgments
We thank Nathan Stevens for his assistance gathering the total recoverable metal and carbon data.
This research was funded by EPA EPSCoR grant R-82940001-0.
REFERENCES
- 1.Admiraal, W., H. Blanck, M. Buckert-de Jong, H. Guasch, N. Ivorra, V. Lehmann, B. A. H. Nystrom, M. Paulsson, and S. Sabater. 1999. Short-term toxicity of zinc to microbenthic algae and bacteria in a metal polluted stream. Water Res. 33:1989-1996. [Google Scholar]
- 2.Axtmann, E., D. Cain, and S. Luoma. 1997. Effect of tributary inflows on the distribution of trace metals in fine-grained bed sediments and benthic insects of the Clark Fork River, Montana. Environ. Sci. Technol. 31:750-758. [Google Scholar]
- 3.Bååth, E., M. Diaz-Ravina, A. Frostegard, and C. D. Campbell. 1998. Effect of metal-rich sludge amendments on the soil microbial community. Appl. Environ. Microbiol. 64:238-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backman, B., D. Bodis, P. Lahermo, S. Rapant, and T. Tarvainen. 1998. Cases and solutions: application of a groundwater contamination index in Finland and Slovakia. Environ. Geochem. 36:55-64. [Google Scholar]
- 5.Battin, T. J. 2000. Hydrodynamics is a major determinant of streambed biofilm activity: from the sediment to the reach scale. Limnol. Oceanogr. 45:1308-1319. [Google Scholar]
- 6.Benner, S., E. Smart, and J. Moore. 1995. Metal behavior during surface-groundwater interaction, Silver Bow Creek, Montana. Environ. Sci. Technol. 29:1789-1795. [DOI] [PubMed] [Google Scholar]
- 7.Bischof, C. 1996. Effects of heavy metal stress in free amino acids in the haemolymph and proteins in haemolymph and total body tissue of Lymantria dispar larvae parasitized by Glyptapanteles liparidis. Entomol. Exp. Appl. 79:61-68. [Google Scholar]
- 8.Böckelmann, D. B. U. 2001. Description and characterization of bacteria attached to lotic organic aggregates (river snow) in the Elbe River of Germany and the South Saskatchewan River of Canada. Dissertation. Technischen Universität Berlin, Berlin, Germany.
- 9.Borg, I., and J. Lingoes. 1987. Multidimensional similarity structure analysis. Springer Verlag, New York, N.Y.
- 10.Brick, C. M., and J. N. Moore. 1996. Diel variation of trace metals in the upper Clark Fork River, Montana. Environ. Sci. Technol. 30:1953-1960. [Google Scholar]
- 11.Burton, G. J., A. Drotar, J. Lazorchak, and L. Bahls. 1987. Relationship of microbial activity and Ceriodaphnia responses to mining impacts on the Clark Fork River, Montana. Arch. Environ. Contam. Toxicol. 16:523-530. [DOI] [PubMed] [Google Scholar]
- 12.Cain, D., J. Carter, S. Fend, S. Luoma, C. Alpers, and H. Taylor. 2000. Metal exposure in a benthic macroinvertebrate, Hydropsyche californica, related to mine drainage in the Sacramento River. Can. J. Fish. Aquat. Sci. 57:380-390. [Google Scholar]
- 13.Cain, D., and S. Luoma. 1998. Metal exposures to native populations of the caddisfly Hydropsyche (Trichoptera: Hydropsychidae) determined from cytosolic and whole body metal concentrations. Hydrobiologia 386:103-117. [Google Scholar]
- 14.Cain, D. J., S. N. Luoma, and E. V. Axtmann. 1995. Influence of gut content in immature aquatic insects on assessments of environmental metal contamination. Can. J. Fish. Aquat. Sci. 52:2736-2746. [Google Scholar]
- 15.Cain, D. J., S. N. Luoma, J. L. Carter, and S. Fend. 1992. Aquatic insects as bioindicators of trace element contamination in cobble-bottom rivers and streams. Can. J. Fish. Aquat. Sci. 49:2142-2154. [Google Scholar]
- 16.Clements, W., D. Carlisle, L. Courtney, and E. Harrahy. 2002. Integrating observational and experimental approaches to demonstrate causation in stream biomonitoring studies. Environ. Toxicol. Chem. 21:1138-1146. [PubMed] [Google Scholar]
- 17.Clements, W., D. Carlisle, J. Lazorchak, and P. Johnson. 2000. Heavy metals structure benthic communities in Colorado mountain streams. Ecol. Appl. 10:626-638. [Google Scholar]
- 18.Clements, W. H., D. S. Cherry, and J. J. Cairns. 1988. Impact of heavy metals on insect communities in streams: a comparison of observational and experimental results. Can. J. Fish. Aquat. Sci. 45:2017-2025. [Google Scholar]
- 19.Courtney, L., and W. Clements. 2002. Assessing the influence of water and substratum quality on benthic macroinvertebrate communities in a metal-polluted stream: an experimental approach. Freshwater Biol. 47:1766-1778. [Google Scholar]
- 20.Courtney, L., and W. Clements. 2000. Sensitivity to acidic pH in benthic invertebrate assemblages with different histories of exposure to metals. J. N. Am. Benthol. Soc. 19:112-127. [Google Scholar]
- 21.Craft, J., J. Stanford, and M. Pusch. 2002. Microbial respiration within a floodplain aquifer of a large gravel-bed river. Freshwater Biol. 47:251-261. [Google Scholar]
- 22.Diaz-Ravina, M., and E. Bååth. 1996. Development of metal tolerance in soil bacterial communities exposed to experimentally increased metal levels. Appl. Environ. Microbiol. 62:2970-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards, K. J., T. M. Gihring, and J. F. Banfield. 1999. Seasonal variations in microbial populations and environmental conditions in an extreme acid mine drainage environment. Appl. Environ. Microbiol. 65:3627-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feris, K., P. Ramsey, C. Frazar, J. N. Moore, J. E. Gannon, and W. E. Holben. 2003. Differences in hyporheic-zone microbial community structure along a heavy-metal contamination gradient. Appl. Environ. Microbiol. 69:5563-5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feris, K. P., P. W. Ramsey, C. F. Frazar, M. C. Rillig, J. E. Gannon, and W. E. Holben. 2003. Seasonal dynamics of shallow hyporheic zone microbial community structure along a heavy-metal contamination gradient. Appl. Environ. Microbiol. 70:2323-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feris, K. P., P. W. Ramsey, C. F. Frazar, M. C. Rillig, J. E. Gannon, and W. E. Holben. 2003. Structure and seasonal dynamics of hyporheic zone microbial communities in free-stone rivers of the western United States. Microb. Ecol. 46:200-215. [DOI] [PubMed] [Google Scholar]
- 27.Findlay, S. 1995. Importance of surface-subsurface exchange in stream ecosystems: the hyporheic zone. Limnol. Oceanogr. 40:159-164. [Google Scholar]
- 28.Findlay, S., D. Strayer, C. Goumbala, and K. Gould. 1993. Metabolism of streamwater dissolved organic carbon in the shallow hyporheic zone. Limnol. Oceanogr. 38:1493-1499. [Google Scholar]
- 29.Fischer, H., and M. Pusch. 2001. Comparison of bacterial production in sediments, epiphyton and the pelagic zone of a lowland river. Freshwater Biol. 46:1335-1348. [Google Scholar]
- 30.Frazar, C. F. 2002. Microbial community production and tolerance in heavy metals polluted sediment. Masters thesis. The University of Montana, Missoula.
- 31.Frostegard, A., A. Tunlid, and E. Bååth. 1996. Changes in microbial community structure during long-term incubation in two soils experimentally contaminated with metals. Soil Biol. Biochem. 28:55-63. [Google Scholar]
- 32.Frostegard, A., A. Tunlid, and E. Bååth. 1993. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 59:3605-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gasith, A., and V. H. Resh. 1999. Streams in Mediterranean climate regions: abiotic influences and biotic responses to predictable seasonal events. Annu. Rev. Ecol. Syst. 30:51-81. [Google Scholar]
- 34.Hair, J. F., R. E. Anderson, R. L. Tatham, and W. C. Black. 1992. Multivariate data analysis with readings, third ed. Macmillan Publishing Company, New York, N.Y.
- 35.Halda-Alija, L., S. P. Hendricks, and T. C. Johnston. 2001. Spatial and temporal variation of Enterobacter genotypes in sediments and the underlying hyporheic zone of an agricultural stream. Microb. Ecol. 42:286-294. [DOI] [PubMed] [Google Scholar]
- 36.Hofle, M. G., H. Haas, and K. Dominik. 1999. Seasonal dynamics of bacterioplankton community structure in a eutrophic lake as determined by 5S rRNA analysis. Appl. Environ. Microbiol. 65:3164-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hristova, K., B. Gebreyesus, D. Mackay, and K. M. Scow. 2003. Naturally occurring bacteria similar to the methyl tert-butyl ether (MTBE)-degrading strain PM1 are present in MTBE-contaminated groundwater. Appl. Environ. Microbiol. 69:2616-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivorra, N., C. Barranguet, M. Jonker, M. H. S. Kraak, and W. Admiraal. 2002. Metal-induced tolerance in the freshwater microbenthic diatom Gomphonema parvulum. Environ. Poll. 116:147-157. [DOI] [PubMed] [Google Scholar]
- 39.Jackson, C. R., P. f. Churchill, and E. E. Roden. 2001. Successional changes in bacterial assemblage structure during epilithic biofilm development. Ecology 82:555-566. [Google Scholar]
- 40.Kaufman, M. G., E. D. Walker, T. W. Smith, R. W. Merritt, and M. J. Klug. 1999. Effects of larval mosquitoes (Aedes triseriatus) and stemflow on microbial community dynamics in container habitats. Appl. Environ. Microbiol. 65:2661-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knight, B. P., S. P. McGrath, and A. M. Chaudri. 1997. Biomass carbon measurements and substrate utilization patterns of microbial populations from soils amended with cadmium, copper, or zinc. Appl. Environ. Microbiol. 63:39-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kruskal, J. B., and M. Wish. 1978. Multidimensional scaling. Sage Publications, Beverly Hills, Calif.
- 43.Lepowski, W. 1998. Arsenic crisis in Bangladesh. Chem. Eng. News 76:27-29. [Google Scholar]
- 44.Luoma, S. N. 1983. Bioavailability of trace metals to aquatic organisms. Sci. Total Environ. 28:1-22. [DOI] [PubMed] [Google Scholar]
- 45.Manz, W., K. Wendt-Potthoff, T. R. Neu, U. Szewzyk, and J. R. Lawrence. 1999. Phylogenetic composition, spatial structure, and dynamics of lotic bacterial biofilms investigated by fluorescent in situ hybridization and confocal laser scanning microscopy. Microb. Ecol. 37:225-237. [DOI] [PubMed] [Google Scholar]
- 46.Mermillod-Blondin, F., M. Creuze des Chatelliers, P. Marmonier, and M. J. Dole-Olivier. 2000. Distribution of solutes, microbes and invertebrates in river sediments along a riffle-pool-riffle sequence. Freshwater Biol. 44:255-269. [Google Scholar]
- 47.Moore, J. N., and S. N. Luoma. 1990. Hazardous wastes from large-scale metal extraction: a case study. Environ. Sci. Technol. 24:1278-1285. [Google Scholar]
- 48.Mulholland, P. J., E. R. Marzolf, J. R. Webster, D. R. Hart, and S. P. Hendricks. 1997. Evidence that hyporheic zones increase heterotrophic metabolism and phosphorous uptake in forest streams. Limnol. Oceanogr. 42:443-451. [Google Scholar]
- 49.Muylaert, K., K. Van der Gucht, N. Vloemans, L. D. Meester, M. Gillis, and W. Vyverman. 2002. Relationship between bacterial community composition and bottom-up versus top-down variables in four eutrophic shallow lakes. Appl. Environ. Microbiol. 68:4740-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naegeli, M. W., and U. Uehlinger. 1997. Contribution of the hyporheic zone to ecosystem metabolism in a prealpine gravel-bed river. J. N. Am. Benthol. Soc. 16:794-804. [Google Scholar]
- 51.Nimick, D. A., and J. N. Moore. 1991. Prediction of water-soluble metal concentrations in fluvially deposited tailings sediments, Upper Clark Fork Valley, Montana, USA. Appl. Geochem. 6:635-646. [Google Scholar]
- 52.Palmborg, C., L. Bringmark, E. Bringmark, and A. Nordgren. 1998. Multivariate analysis of microbial activity and soil organic matter at a forest site subjected to low-level heavy metal contamination. Ambio 27:53-58. [Google Scholar]
- 53.Palmborg, C., A. Nordgren, and E. Bååth. 1998. Multivariate modelling of soil microbial variables in forest soil contaminated by heavy metals using wet chemical analyses and pyrolysis GC/MS. Soil Biol. Biochem. 30:345-357. [Google Scholar]
- 54.Pennanen, T., A. Frostegard, H. Fritze, and E. Bååth. 1996. Phospholipid fatty acid composition and heavy-metal tolerance of soil microbial communities along two heavy metal-polluted gradients in coniferous forests. Appl. Environ. Microbiol. 62:420-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Philippi, T. E. 1993. Multiple regression: herbivory, p. 183-210. In S. M. Scheiner and J. Gurevitch (ed.), Design and analysis of ecological experiments. Chapman and Hall, New York, N.Y.
- 56.Pusch, M. 1997. Community respiration in the hyporheic zone of a riffle-pool sequence, p. 51-56. In J. Gibert, J. Mathieu, and F. Fournier (ed.), Groundwater/surface water ecotones: biological and hydrological interactions and management options. Cambridge University Press, Cambridge, United Kingdom.
- 57.Pusch, M. 1996. The metabolism of organic matter in the hyporheic zone of a mountain stream, and its spatial distribution. Hydrobiologia 323:107-118. [Google Scholar]
- 58.Pusch, M., D. Fiebig, I. Brettar, H. Eisenmann, B. K. Ellis, L. A. Kaplan, M. A. Lock, M. W. Naegeli, and W. Traunspurger. 1998. The role of micro-organisms in the ecological connectivity of running waters. Freshwater Biol. 40:453-495. [Google Scholar]
- 59.Pusch, M., and J. Schwoerbel. 1994. Community respiration in hyporheic sediments of a mountain stream (Steina, Black Forest). Arch. Hydrobiol. 130:35-52. [Google Scholar]
- 60.Roane, T. M., and I. L. Pepper. 1999. Microbial responses to environmentally toxic cadmium. Microb. Ecol. 38:358-364. [DOI] [PubMed] [Google Scholar]
- 61.Sandaa, R., V. Torsvik, O. Enger, F. Daae, T. Castberg, and D. Hahn. 1999. Analysis of bacterial communities in heavy-metal contaminated soils at different levels of resolution. FEMS Microbiol. Ecol. 30:237-251. [DOI] [PubMed] [Google Scholar]
- 62.Sandaa, R.-A., V. Torsvik, and O. Enger. 2001. Influence of long-term heavy-metal contamination on microbial communities in soil. Soil Biol. Biochem. 33:287-295. [Google Scholar]
- 63.Sandaa, R.-A., Ø. Enger, and V. L. Torsvik. 1999. Abundance and diversity of Archaea in heavy-metal-contaminated soils. Appl. Environ. Microbiol. 65:3293-3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silver, S., T. K. Misra, and R. A. Laddaga. 1989. Bacterial resistance to toxic heavy metals, p. 121-139. In R. K. Poole and G. M. Gadd (ed.), Metal-microbe interactions, vol. 26. IRL Press, New York, N.Y. [Google Scholar]
- 65.Simek, K., J. Pernthaler, M. G. Weinbauer, K. Hornak, J. R. Dolan, J. Nedoma, M. Masin, and R. Amann. 2001. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl. Environ. Microbiol. 67:2723-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smoot, J. C., and R. H. Findlay. 2001. Spatial and seasonal variation in a reservoir sedimentary microbial community as determined by phospholipid analysis. Microb. Ecol. 42:350-358. [DOI] [PubMed] [Google Scholar]
- 67.Soo-Youn, L., J. Bollinger, D. Bezdicek, and A. Ogram. 1996. Estimation of the abundance of an uncultured soil bacterial strain by a competitive quantitative PCR method. Appl. Environ. Microbiol. 62:3787-3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stephen, J. R., Y.-J. Chang, S. J. Macnaughton, G. A. Kowalchuk, K. T. Leung, C. A. Flemming, and D. C. White. 1999. Effect of toxic metals on indigenous soil β-subgroup proteobacterium ammonia oxidizer community structure and protection against toxicity by inoculated metal-resistant bacteria. Appl. Environ. Microbiol. 65:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Traina, S. J., and V. Laperche. 1999. Contaminant bioavailability in soils, sediments, and aquatic environments. Proc. Natl. Acad. Sci. USA 96:3365-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Hannen, E., W. Mooij, M. van Agterveld, H. Gons, and H. Laanbroek. 1999. Detritus-dependent development of the microbial community in an experimental system: qualitative analysis by denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 65:2478-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Hannen, E. J., G. Zwart, M. P. van Agterveld, H. J. Gons, J. Ebert, and H. J. Laanbroek. 1999. Changes in bacterial and eukaryotic community structure after mass lysis of filamentous cyanobacteria associated with viruses. Appl. Environ. Microbiol. 65:795-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walling, D. E., P. N. Owens, J. Carter, G. J. L. Leeks, S. Lewis, A. A. Meharg, and J. Wright. 2003. Storage of sediment-associated nutrients and contaminants in river channel and floodplain systems. Appl. Geochem. 18:195-220. [Google Scholar]
- 73.Wenderoth, D., and H. Reber. 1999. Correlation between structural diversity and catabolic versatility of metal affected prototrophic bacteria in soil. Soil Biol. Biochem. 31:345-352. [Google Scholar]
- 74.Wielinga, B., J. K. Lucy, J. N. Moore, O. F. Seastone, and J. E. Gannon. 1999. Microbiological and geochemical characterization of fluvially deposited sulfidic mine tailings. Appl. Environ. Microbiol. 65:1548-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams, D. D., N. E. Williams, and Y. Cao. 2000. Road salt contamination of groundwater in a major metropolitan area and development of a biological index to monitor its impact. Water Res. 34:127-138. [Google Scholar]
- 76.Yu, Z., and W. W. Mohn. 1999. Killing two birds with one stone: simultaneous extraction of DNA and RNA from activated sludge biomass. Can. J. Microbiol. 45:269-272. [Google Scholar]
- 77.Zhou, J., B. Xia, D. S. Treves, L.-Y. Wu, T. L. Marsh, R. V. O'Neill, A. V. Palumbo, and J. M. Tiedje. 2002. Spatial and resource factors influencing high microbial diversity in soil. Appl. Environ. Microbiol. 68:326-334. [DOI] [PMC free article] [PubMed] [Google Scholar]