Abstract
A nontoxic mutant diphtheria toxin fragment A (DTA) was genetically fused in single, double, or triple copy to the major surface protein antigen P1 (SpaP) and surface expressed in Streptococcus gordonii DL-1. The expression was verified by Western immunoblotting. Mouse antisera raised against the recombinant S. gordonii recognized the native diphtheria toxinm suggesting the recombinant DTA was immunogenic. When given intranasally to mice with cholera toxin subunit B as the adjuvant, the recombinant S. gordonii expressing double copies of DTA (SpaP-DTA2) induced a mucosal immunoglobulin A response and a weak systemic immunoglobulin G response. S. gordonii SpaP-DTA2 was able to orally colonize BALB/c mice for a 15-week period and elicited a mucosal response, but a serum immunoglobulin G response was not apparent. The antisera failed to neutralize diphtheria toxin cytotoxicity in a Vero cell assay.
Many childhood vaccines, such as the diphtheria-pertussis-tetanus vaccine, require multiple doses to achieve protection, which has led to reduced coverage of immunization. Live viral or bacterial vectors have been suggested as a solution to reduce the number of doses required for immunization. In addition, live vectors have the ability to induce a mucosal immune response following oral or nasal administration, which is typically difficult to obtain from conventional parenteral immunization. Several bacteria, such as Salmonella spp. (12), Mycobacterium bovis BCG (25), Streptococcus gordonii (17, 28), Lactobacillus plantarum (8), and Staphylococcus spp. (6, 11), have received attention as live vaccine delivery vehicles.
Among these bacteria, S. gordonii is a very attractive vector for childhood vaccines because it is one of the pioneer organisms in the human oral cavity. The organism can be detected in the oral cavity by 6 months of age and remains as a normal inhabitant of the oral mucosa and dental plaque in adults. Therefore, the organism has the potential of providing life-long protection following a single oral inoculation. In addition, this bacterium is relatively easy to manipulate genetically, and the expression of a number of heterologous antigens has been reported (18, 20, 22, 23, 24, 30). In the context of a live oral diphtheria-pertussis-tetanus vaccine, Medaglini et al. (24) showed that immunization with recombinant S. gordonii expressing tetanus toxin fragment C on its surface conferred protection from lethal tetanus toxin challenge in mice. In a previous work, we showed that the surface-expressed pertussis toxin S1 fragment (18, 19) and a secreted fusion protein consisting of pertussis toxin S1S3 fragments and filamentous hemagglutinin type I domain (20) produced by S. gordonii were immunogenic. These findings show that tetanus and pertussis antigens can be expressed by S. gordonii and that the antigens were immunogenic. However, the expression of diphtheria antigens in S. gordonii has not yet been reported.
Diphtheria toxin (DT) is a secreted 535-amino-acid protein which is proteolytically cleaved into two fragments, A and B (4, 9). The catalytic domain is located on fragment A (amino acids 1 to 193), and the receptor and translocation domains are on fragment B (amino acids 194 to 535). Fragment B is responsible for DT binding to specific cell surface receptors and translocation of fragment A into the cytosol. Fragment A catalyzes the ADP-ribosylation of elongation factor 2, resulting in inhibition of protein synthesis and cell death.
Expression of full-length DT and fragments of DT has been described in Escherichia coli (1, 2, 3), Salmonella enterica (7, 29), Staphylococcus carnosus (6), and Mycobacterium bovis (25). In E. coli and S. enterica, the recombinant protein was immunogenic but the antisera contained only weak neutralizing activity. In S. carnosus, the receptor-binding domain of DT was able to induce an immune response in mice following repeated immunization, and the antisera showed neutralizing activity against DT toxicity in Vero cells. A mutated nontoxic derivative of DT, CRM179, expressed by M. bovis was immunogenic, but the antibodies lacked neutralizing activity unless the recombinant antigen was coadministered with tetanus toxin fragment C.
In this study, we investigated the expression and immunogenicity of a nontoxic DT fragment A (DTA) in S. gordonii, with the long-term goal of developing a live oral diphtheria-pertussis-tetanus vaccine. DTA was surface expressed on S. gordonii as a fusion protein with the well-characterized major surface antigen P1 (SpaP or antigen I/II) from Streptococcus mutans (13, 14). We focused our work on DTA because it carries the catalytic domain and antibodies against this domain were reported to neutralize DT cytotoxicity (2, 7). The DTA fragment that we used in this study contained a single amino acid substitution (Glu148Ser), which rendered DT nontoxic (1).
MATERIALS AND METHODS
Bacteria and growth conditions.
S. gordonii was cultivated in Todd-Hewitt broth containing 0.5% yeast extract at 37°C aerobically without shaking. Kanamycin at 250 μg/ml was included in the medium to ensure plasmid maintenance. Recombinant E. coli was grown aerobically with vigorous shaking at 37°C in Luria-Bertani (LB) medium (1% tryptone, 0.5% yeast extract, and 1% NaCl [wt/vol]) containing either ampicillin (100 μg/ml) or kanamycin (50 μg/ml). All antibiotics were purchased from Sigma-Aldrich, Oakville, Canada.
Cloning of dta.
The DNA coding for DTA (amino acids 1 to 193) was amplified from pBRDT-S148 (1), which carried the nontoxic mutant DTE148S gene, with primers SL192 (5′-CTGATATCGGCGCTGATGATGTTG-3′; EcoRV site in italics) and SL200 (5′-TAGGTACCCCCCCGGGTCGCCTGACACGATTTC-3′; KpnI site in italics and SmaI site in bold). The 0.6-kb PCR product was digested with EcoRV and KpnI and ligated to the 5.3-kb EcoRV-KpnI fragment (a pUC18 derivative carrying the 5′ DNA of the spaP gene) from pSMI/II (14), creating pDTA-1. To provide the fusion gene with the 3′ end of spaP (containing the surface protein anchoring domain), the 3.6-kb EcoRV-KpnI fragment from pSMI/II was cloned into the EcoRV and KpnI sites of pDTA-1. The resulting plasmid isolated from E. coli XL1-Blue was designated pDTA-2. The cloning essentially inserted DTA into the N-terminal third of antigen P1 (Fig. 1). The 7.8-kb KpnI-ScaI fragment from pDTA-2 carrying the fusion gene was subcloned into the HincII and KpnI sites of pDL276, an E. coli-Streptococcus shuttle vector (5), creating the 14.8-kb pDTA.
FIG. 1.
Schematic representation of SpaP-DTA fusion proteins. The sizes of SpaP (open bar) and DTA (solid bar) are indicated by the number of amino acids (a.a.). Hatched bars indicate the C-terminal cell wall-anchoring domain of SpaP.
To facilitate the cloning of tandem copies of dta, the 4.2-kb KpnI-ScaI fragment from pDTA-1 was cloned into the KpnI and HincII sites of pDL276. The resulting plasmid was named pDTA-4. The plasmid carrying two copies of dta, pDTA2, was constructed by ligating the 4.2-kb EcoRV-KpnI fragment from pDTA-2 into the SmaI and KpnI sites of pDTA-4. The plasmid carrying three copies of dta, pDTA3, was constructed similarly by ligating the 4.8-kb EcoRV-KpnI fragment from pDTA2 into the SmaI and KpnI sites of pDTA-4. The expected fusion proteins encoded by pDTA2 and pDTA3 are depicted in Fig. 1.
pDTA, pDTA2, and pDTA3 were introduced into S. gordonii DL-1 via natural transformation by the method described previously (13). The transformants obtained were named S. gordonii DTA, DTA2, and DTA3, respectively.
Parenteral immunization.
The immunogenicity of the fusion proteins was investigated by immunizing cohorts of BALB/c mice (4 to 6 weeks old, female, n = 5) with S. gordonii DTA, S. gordonii DTA2, and S. gordonii DTA3. The mice were immunized intraperitoneally with 100 μl (109 CFU) of heat-killed whole-cell recombinant S. gordonii in equal volume of Freund's complete adjuvant (Sigma-Aldrich). The animals were given a booster dose with the same amount of cells in Freund's incomplete adjuvant at weeks 1 and 3 and half the amount of cells in Freund's incomplete adjuvant at weeks 5 and 9. Mice were euthanized at week 11. In a second experiment, one group of five mice was immunized intramuscularly with 109 live S. gordonii DTA2 cells in 50 μl of phosphate-buffered saline (PBS) containing 10 μg of immunostimulating CpG oligonucleotide 1018 ISS (TGACTGTGAACGTTCGAGATGA; Dynaxev Technologies, Berkeley, Calif.). A second group of mice was immunized similarly with 1018 ISS alone as a control. The animals received additional immunizations on days 22 and 36, and sera were obtained on day 45.
Intranasal immunization.
BALB/c mice (n = 5) were intranasally immunized with 109 live S. gordonii DTA2 cells in 25 μl of PBS containing 10 μg of cholera toxin subunit B (CTB; List Biological Laboratories, Inc., Campbell, Calif.) as the mucosal adjuvant. The method of immunization has been described elsewhere (20, 21). A second group of mice received 10 μg of CTB alone as a control. The animals received additional immunizations on days 21, 33, 47, and 54. The animals were euthanized on day 70, and serum samples and saliva and vaginal wash samples were obtained as described previously (10, 20).
Oral colonization.
In the initial experiment, 3-week-old BALB/c mice (female, n = 5) were colonized with S. gordonii DTA2 and S. gordonii SL3 (a DTA-negative and kanamycin-resistant derivative of parent strain DL-1) (19) using the method described previously (19). The results showed that the animals were colonized, but an immune response was not detected. Therefore, in a second experiment, the animals (5 weeks old) were primed with a single intraperitoneal injection of 100 μl of a commercial diphtheria, tetanus, and acellular pertussis vaccine (Quadracel; Aventis-Pasteur, Toronto, Canada). Three weeks later, the mice were fed 500 μg of kanamycin per ml of drinking water for 2 days to lower the oral microbial load and then inoculated orally with 100 μl (108 CFU) of recombinant S. gordonii (19). The mice continued to receive kanamycin (250 μg/ml) in their drinking water for another 24 h, at which time the mice were inoculated a second time.
Following the second inoculation, the animals were returned to normal drinking water for the remainder of the experiment except for 2 days at week 9, when they received a single dose of S. gordonii following a 2-day kanamycin-water treatment. Colonization was monitored on a weekly basis by oral swabbing as described previously (19). The colonies obtained were observed to be similar to S. gordonii DL-1 in colony morphology and were gram-positive cocci with short chains. Western immunoblot analysis confirmed the presence of the 229-kDa SpaP-DTA2 fusion protein from isolates recovered from S. gordonii DTA2-inoculated animals only. The number of isolates observed per swab varied between 1 and 50 for S. gordonii SL3 and 1 and >100 for recombinant S. gordonii DTA2.
Serum and saliva were collected at weeks 0 (just prior to priming), 3 (3 weeks postpriming), 6, 8, 11, 13, and 15 (at euthanasia). Vaginal fluids were collected from sedated animals by pipetting two 50-μl aliquots of sterile PBS into the vagina at weeks 0, 3, and 15 (20). Bronchoalveolar lavage (BAL) fluids were collected at euthanasia (10).
Generation of a mouse anti-DT antibody.
An anti-DT antibody was generated by immunizing five BALB/c mice (female, 8 weeks old) intraperitoneally with 5 μg of diphtheria toxoid (Massachusetts Public Health Biologic Laboratories, Jamaica Plain, Mass.) in Freund's complete adjuvant. The animals were boosted with the same antigen in Freund's incomplete adjuvant on days 28 and 55. Mice were euthanized on day 63, and the sera obtained were used as a positive control. The anti-DT titer of the serum was 3.3 × 106.
SDS-PAGE and Western immunoblotting.
Protein samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5% polyacrylamide gels (unless stated otherwise) with the buffer system of Laemmli (15). Proteins were transferred to nitrocellulose membranes by the method of Towbin et al. (31) and detected with either rabbit anti-P1 antibody (1:500) (16), mouse anti-DT antibody (1:2,000), or anti-recombinant S. gordonii serum (1:200) and the respective alkaline phosphatase conjugate, goat anti-rabbit immunoglobulin G (IgG) (1:30,000; Sigma-Aldrich) or goat anti-mouse IgG (1:20,000, Sigma-Aldrich).
Cellular distribution of SpaP-DTA fusion proteins.
An enzyme-linked immunosorbent assay (ELISA) was used to determine the presence of SpaP-DTA fusion proteins in culture supernatants and intact cells (i.e., cell surface) and was performed as described previously (13). In brief, mid-exponential-phase cultures grown in the chemically defined FMC medium (30a) were diluted to an optical density at 600 nm of 0.5 and centrifuged. Cells were washed in phosphate-buffered saline, diluted twofold in phosphate-buffered saline, and fixed on microtiter plates with 0.125% (wt/vol) glutaraldehyde. Culture supernatants were similarly diluted and used to coat microtiter plates. The fusion proteins on the cells and in the supernatants were detected with the mouse anti-DT antibody (1:2,000) followed by the goat anti-mouse IgG-alkaline phosphatase conjugate.
Immune responses.
Specific antibodies in samples were measured by ELISA in endpoint dilutions as described previously (20, 21). Briefly, twofold-diluted samples were assayed in triplicate in 96-well polystyrene microtiter plates that were coated with 200 ng of DT (List Biological Laboratories)/well. The starting dilutions of serum, saliva, vaginal wash, and BAL samples were 1:100, 1:10, 1:10, and 1:2, respectively. Specific IgG antibodies were detected with alkaline phosphatase-conjugated goat anti-mouse IgG. Specific IgA antibodies were detected with a biotinylated goat anti-mouse IgA (α-chain specific, 1:20,000; Sigma-Aldrich), followed by an avidin-alkaline phosphatase conjugate (1:20,000; Sigma-Aldrich). The titers of antibodies were expressed as the reciprocal of the dilution that produced an A405 reading 0.05 higher than that of the pooled preimmune samples or the control (1018 ISS alone) serum for the CpG experiment. The titers were analyzed by Student's t test, and P < 0.05 was considered statistically significant.
DT neutralization assay.
The DT neutralization assay was performed as described by Miyamura et al. (26) with modifications. Briefly, sera in triplicate were diluted in serial twofold dilutions (starting dilution at 1:2) with growth medium (minimal essential medium with 1% l-glutamine; Invitrogen Life Technologies, Burlington, Ontario, Canada) supplemented with 10% fetal bovine serum, 0.01% amphotericin B, 0.3% gentamicin, and 0.2% penicillin G. The diluted samples (12.5 μl) were then transferred to 96-well flat-bottomed Nunclon microplates (VWR International Ltd., Toronto, Canada), and an equal volume of native DT (0.8 ng/ml; List Biological Laboratories) was added. The mixtures were incubated at 37°C for 1.5 h, after which 50 μl of Vero cell suspension (1.2 × 105 cells/ml) and 150 μl of growth medium were added to each of the wells. The cultures were then incubated in a 5% CO2 incubator for 5 to 7 days at 37°C and scored with the aid of an inverted microscope for toxicity: +, confluent monolayer; ±, approximately 50% cell death; −, 100% cell death. Neutralization titer was defined as the reciprocal of the dilution that showed complete neutralization (+, confluent monolayer) of DT cytotoxicity.
RESULTS
Expression of DTA.
The correct fusions of SpaP-DTA, SpaP-DTA2, and SpaP-DTA3 carried on pDTA, pDTA2, and pDTA3 were verified by restriction analysis (data not shown). E. coli cells harboring these plasmids were shown to express the expected 207-, 229-, and 251-kDa fusion protein, respectively, that were recognized by the mouse anti-DT and rabbit anti-P1 antibodies (data not shown).
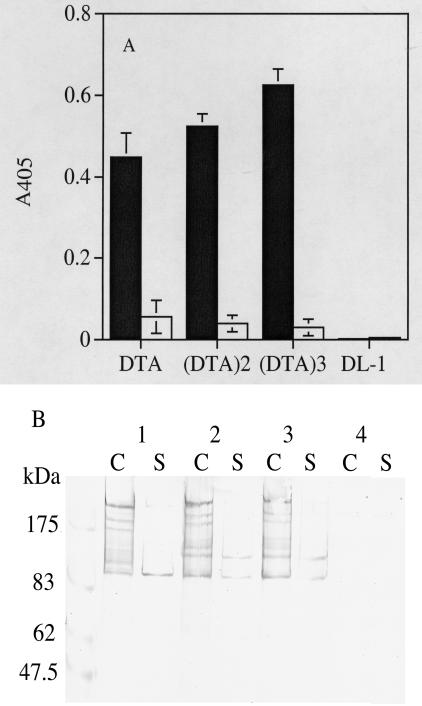
Expression of the SpaP-DTA fusion proteins by S. gordonii was demonstrated by ELISA and Western blotting. In ELISA, the recombinant DTA fusion proteins were detected mainly with intact cells of S. gordonii DTA, DTA2, and DTA3 (Fig. 2A). The culture supernatants contained only traces of the fusion proteins. Cells and supernatants from the control DL-1 cultures did not show any reaction with the anti-DT antibody. Western immunoblotting showed that S. gordonii DTA, DTA2, and DTA3 produced a high-molecular-weight protein band estimated to be 207, 229, and 251 kDa, respectively (Fig. 2B). In addition to these bands, four or five smaller proteins, presumably degraded products, were also detected by the same antibody. These bands were also detected by the rabbit anti-P1 antibody (data not shown). The high-molecular-weight immunoreactive bands were found in the cell fractions but not in the culture supernatants. These results suggest that DTA is produced in single, double, and triple copy by S. gordonii.
FIG. 2.
Expression of recombinant DTA by S. gordonii. (A) Recombinant DTA proteins on the cell surface (solid bars) and in culture supernatants (open bars) were detected by ELISA. A405 readings were determined from diluted samples equivalent to culture optical densities at 600 nm of 0.25. (B) Recombinant DTA proteins were detected in cell extracts and culture supernatants by Western immunoblotting. Lane 1, S. gordonii DTA; lane 2, S. gordonii DTA2; lane 3, S. gordonii DTA3; lane 4, cell extracts of S. gordonii DL-1 (negative control). (C) Proteins were extracted from cells by previously described methods (13). S, proteins from culture supernatant fluids. Prestained protein size markers are shown on the left. In both panels, the proteins were detected with a mouse anti-DT antibody.
Immunogenicity of the recombinant DTA.
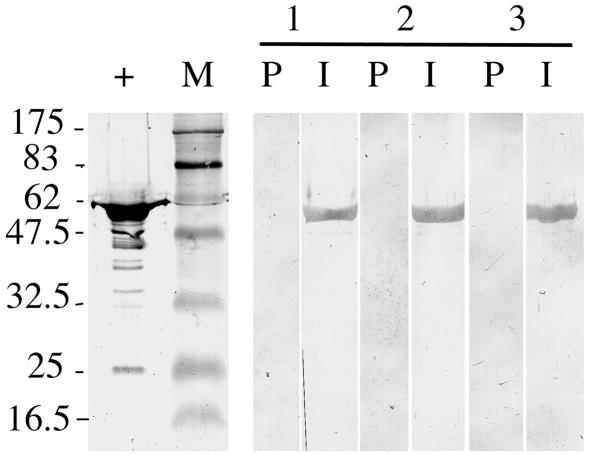
The immunogenicity of DTA was assessed by immunizing BALB/c mice intraperitoneally with five doses of heat-killed recombinant S. gordonii in Freund's adjuvant. Sera from animals immunized with S. gordonii DTA, DTA2, and DTA3 showed an anti-DT IgG titer (mean ± standard error) of 510 ± 282, 1,340 ± 567, and 360 ± 129, respectively. Western immunoblotting showed that all three antisera recognized the native DT (Fig. 3). These results indicated that the recombinant DTAs expressed by S. gordonii are capable of eliciting an immune response in mice but that the immune response was weak.
FIG. 3.
Western immunoblots showing recognition of native DT by antiserum obtained from mice immunized with heat-killed recombinant S. gordonii. DT (1 μg) was separated on an SDS-10% PAGE gel. Mouse antisera: lane 1, S. gordonii DTA; lane 2, S. gordonii DTA2; lane 3, S. gordonii DTA3. P, preimmune serum; I, immune serum; +, anti-DT antiserum; M, prestained protein size markers (sizes shown in kilodaltons).
A second immunogenicity experiment was conducted, in which the animals were given three doses of live S. gordonii DTA2 with an immunostimulatory CpG oligonucleotide, 1018 ISS, as the adjuvant. S. gordonii DTA2 was chosen for the second immunogenicity experiment and subsequent mucosal immunization experiments because it gave the highest immune response in the first experiment. After three doses, the mice responded with a serum IgG anti-DT titer of 83,306 ± 40,852.
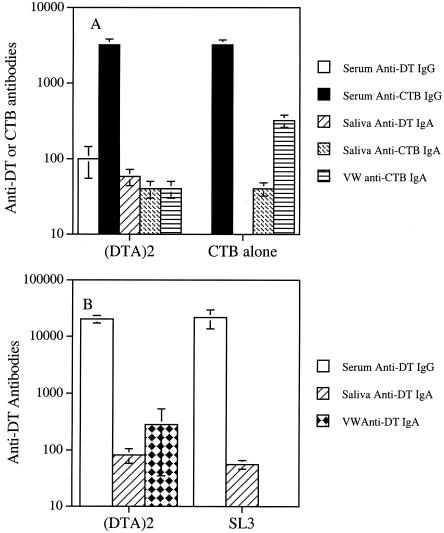
The ability of the recombinant S. gordonii DTA2 to induce an immune response was also assessed via intranasal immunization. As shown in Fig. 4A, animals immunized with S. gordonii DTA2 showed a weak serum IgG response. The serum anti-DT IgG titer was 100, and the anti-CTB IgG titer was 3,200. Saliva from the same group of animals gave an anti-DT IgA titer of 58. There was no anti-DT IgA in the vaginal wash sample. The anti-CTB IgA titers in saliva and vaginal wash samples of this group of animals were 40 and 40, respectively. In the CTB control group, there was no immune response to DT, but there was an immune response to CTB, as expected. The anti-CTB titers were 3,200, 40, and 320 in serum, saliva, and vaginal wash samples, respectively.
FIG. 4.
Immune responses to S. gordonii DTA2 in BALB/c mice. (A) Anti-DT and anti-CTB antibodies elicited by intranasal immunization with live S. gordonii DTA2 in the presence of CTB or with CTB alone. (B) Anti-DT antibodies induced by oral colonization with S. gordonii DTA2. The values shown are mean titers ± standard error for individual animal samples. In the CTB-alone and SL3 groups, the absence of a bar indicates that an immune response was not detected. VW, vaginal wash.
Oral colonization of mice with S. gordonii DTA2.
The ability of S. gordonii DTA2 to elicit an immune response following oral colonization was investigated. The results from an initial oral colonization experiment showed that the mice were colonized, but anti-DT IgG and IgA antibodies were not detected in serum or saliva. Thus, in a second experiment, the animals were primed parenterally with a single dose of a commercial vaccine, followed by oral colonization. Following the parenteral priming, the animals were given three oral inoculations of S. gordonii DTA2, and retention of recombinant S. gordonii in the oral cavity was monitored over a 15-week period. The recombinant S. gordonii DTA2 could be recovered from the oral cavity from two of the five animals throughout the experiment. In contrast, four of the five mice were colonized by the control S. gordonii SL3 up to week 5, and at week 15 only one mouse retained the organism.
The animals that had been primed with a single dose of the commercial vaccine followed by oral colonization with S. gordonii DTA2 showed a mucosal immune response (Fig. 4B). The salivary and vaginal wash samples obtained at euthanasia showed an anti-DT IgA titer (mean ± standard error) of 82 ± 24 and 284 ± 249, respectively. In comparison, the same samples from S. gordonii SL3-colonized mice showed an anti-DT IgA titer of 56 ± 10 (P = 0.12) and 0 (P = 0.159), respectively, but the difference was not statistically significant. No specific anti-DT IgA was detected in the BAL fluids from the two groups of animals. The serum obtained at euthanasia from the two groups of animals showed similar anti-DT IgG titers of 20,480 ± 3,135 and 21,760 ± 7,994, respectively. Serum obtained from the two groups at week 3 (i.e., 3 weeks after parenteral priming) showed the same anti-DT IgG titer of 12,800. Serum obtained at week 8 (i.e., 4 weeks after the first oral inoculation with S. gordonii) also showed similar anti-DT IgG titers of 51,200 (S. gordonii DTA2) and 102,400 (SL3).
DT neutralization.
Sera obtained after parenteral immunization with heat-killed and live cells and after colonization were assayed for the ability to neutralize DT toxicity. None of the sera displayed neutralization (titer < 2). In contrast, the mouse anti-DT antibodies and an antibody standard from the World Health Organization (at 1.6 IU/ml) showed neutralizing titers of 8,000 and 4,096, respectively.
DISCUSSION
In the present study, DTA was successfully expressed by S. gordonii. This is evident from Western blots showing that the fusion proteins were recognized by the monospecific anti-DT antibody. The fusion proteins are directed to the cell surface by the SpaP surface localization domain. The ELISA results showed that the fusion proteins were detected with the intact cells, indicating that the fusion proteins were indeed surface localized. This result is consistent with the surface localization of the SpaP-S1 fusion protein, constructed similarly and expressed in S. gordonii (18). Single, double, and triple copies of DTA were successfully expressed, indicating that the SpaP protein can accommodate a relatively large insert and suggesting that SpaP can be a good tool with which to express large multivalent fusion proteins.
Sera obtained after parenteral immunization recognized native DT in Western blots and ELISA, indicating that the fusion proteins were immunogenic. The level of immune response was relatively low when recombinant S. gordonii was given with Freund's adjuvant. In contrast, in a previous study, when mice were immunized similarly with a recombinant S. gordonii expressing SpaP/pertussis toxin S1 fusion on its surface, a high-titer anti-pertussis toxin serum was obtained (18). In an attempt to address the low immunogenicity of DTA, fusion proteins carrying two and three copies of DTA were constructed to increase the antigenic load. However, the levels of immune response generated by the three constructs were similar. In contrast, an exceptionally high-titer anti-DT serum was obtained with the immunostimulatory CpG sequence as the adjuvant, suggesting that the recombinant DTA expressed by S. gordonii is capable of eliciting an excellent immune response but requires an appropriate adjuvant.
The recombinant DTA expressed by S. gordonii was also immunogenic when administered mucosally. When given intranasally, S. gordonii DTA2 elicited salivary anti-DT IgA and serum anti-DT IgG antibodies. These specific antibodies were not detected in the control (CTB) group, indicating that the immune response is specific. Consistent with the results from parenteral immunization with Freund's adjuvant, the serum immune response was weak.
The ability of S. gordonii DTA2 to orally colonize mice was demonstrated. The animals retained the bacterium for 15 weeks. This is consistent with previous reports from our laboratory and others that long-term colonization of mice by recombinant S. gordonii is possible. Previously, we demonstrated that over 60% of BALB/c mice retained the SpaP/S1-expressing S. gordonii for up to 19 weeks (19). Medaglini and colleagues (22) also reported colonization of BALB/c mice by hornet venom allergen-expressing S. gordonii for up to 11 weeks, at which time only 10% of the mice still carried the bacterium. In this study, only two of the five animals were colonized by S. gordonii DTA2. The difference in the extent of colonization between this study and the SpaP/S1 study may be due to sample size. In the SpaP/S1 study, 24 animals were used, while only five were inoculated in this study.
The results from the initial colonization experiment failed to produce any anti-DT antibodies. Thus, in the second colonization experiment, the animals were given a single intraperitoneal injection of a commercial vaccine prior to colonization in an attempt to prime the immune system. The results showed that the S. gordonii DTA2-colonized mice had a high vaginal anti-DT IgA titer, while there was a lack of vaginal anti-DT IgA antibodies in the control group. Salivary anti-DT IgA in the S. gordonii DTA2 group was slightly higher than that in the control group. These results suggest that colonization by S. gordonii DTA2 yielded a mucosal immune response.
Colonization did not appear to stimulate a serum anti-DT IgG response, as the antibody titer was identical in S. gordonii DTA2- and S. gordonii SL3-infected mice. The anti-DT antibody detected in sera from both groups can be attributed to the single injection of the commercial vaccine. Failure to elicit a serum IgG response was observed in a previous colonization study with SpaP/S1-expressing S. gordonii (19). This is in contrast to results reported by Medaglini et al. (22) and Oggioni et al. (27) that antigen-specific serum IgG was detected following oral colonization. The reason for this discrepancy is not known but may be related to the different S. gordonii strains used by us and the Italian group or to low DTA expression in our system.
The DT neutralization assay results showed that sera raised against the recombinant DTA expressed by S. gordonii lacked neutralizing activity. Barbieri et al. (2) previously reported a weak neutralizing titer of 50 for a guinea pig serum raised against the pertussis toxin S1-DTA fusion protein. Orr et al. (29) did not find neutralizing activity in mouse sera against CRM179 expressed by Salmonella enterica serovar Typhi. Similarly, Miyaji et al. (25) found a lack of neutralizing activity in antibodies raised against CRM179 expressed by M. bovis BCG, but a weak neutralizing activity was detected when the animals were coimmunized with M. bovis expressing tetanus toxin fragment C. The antiserum against the receptor-binding domain of DT fragment B surface expressed by S. carnosus was reported to neutralize DT cytotoxicity (6); however, the actual neutralizing ability of the antibodies is hard to judge, as the serum was used at a single dilution (1:10). Therefore, our results are in general agreement with those reported by others, that neutralizing antibodies are difficult to generate from recombinant DT. In our study and those of others, the lack of neutralizing activity may be due to incorrect folding when DT is expressed in heterologous hosts.
In conclusion, a mutant DTA in single, double, and triple copy was surface expressed in S. gordonii as fusion proteins to the SpaP protein. The fusion proteins were immunogenic in mice, but the antibodies generated lacked neutralizing activity.
Acknowledgments
We thank Ann MacMillan and Yi-Jing Li for technical assistance with the oral colonization trial and Annette Morris for assistance with the DT neutralization assay. We also thank R. John Collier for the gift of pBRDT-S148 and Spencer Lee for providing the Vero cells. The immunostimulatory sequence 1018 ISS was a gift from Dynavex Technologies.
This study was supported by the Canadian Institutes of Health Research and in part by the Natural Sciences and Engineering Council of Canada. C.W.L. was a recipient of an IWK Health Centre Research Fellowship.
REFERENCES
- 1.Barbieri, J. T., and R. J. Collier. 1987. Expression of a mutant, full-length form of diphtheria toxin in Escherichia coli. Infect. Immun. 55:1647-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbieri, J. T., D. Armellini, J. Molkentin, and R. Rappuoli. 1992. Construction of a diphtheria toxin A fragment-C180 peptide fusion protein which elicits a neutralizing antibody response against diphtheria toxin and pertussis toxin. Infect. Immun. 60:5071-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bishai, W. R., R. Rappuoli, and J. R. Murphy. 1987. High-level expression of a proteolytic sensitive diphtheria toxin fragment in Escherichia coli. J. Bacteriol. 169:5140-5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collier, R. J. 1975. Diphtheria toxin: mode of action and structure. Bacteriol. Rev. 39:54-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunny, G. M., L. N. Lee, and D. J. LeBlanc. 1991. Improved electroporation and cloning vector system for gram-positive bacteria. Appl. Environ. Microbiol. 57:1194-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fromen-Romano, C., P. Drevet, A. Robert, A. Menez, and M. Leonetti. 1999. Recombinant Staphylococcus strains as live vectors for the induction of neutralizing anti-diphtheria toxin antisera. Infect. Immun. 67:5007-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Duarte, O. G., J. Galen, S. N. Chatfield, R. Rappuoli, L. Eidels, and M. M. Levine. 1995. Expression of fragment C of tetanus toxin fused to a carboxyl-terminal fragment of diphtheria toxin in Salmonella typhi CVD 908 vaccine strain. Vaccine 13:1596-1602. [DOI] [PubMed] [Google Scholar]
- 8.Grangette, C., H. Muller-Alouf, D. Goudercourt, M. C. Geoffroy, M. Turneer, and A. Mercenier. 2001. Mucosal immune responses and protection against tetanus toxin after intranasal immunization with recombinant Lactobacillus plantarum. Infect. Immun. 69:1547-1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenfield, L., M. J. Bjorn, G. Horn, D. Fong, G. A. Buck, R. J. Collier, and D. A. Kaplan. 1983. Nucleotide sequence of the structural gene for the diphtheria toxin carried by corynebacteriophage β. Proc. Natl. Acad. Sci. USA 80:6853-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halperin, S. A., T. B. Issekutz, and A. Kasina. 1991. Modulation of Bordetella pertussis infection with monoclonal antibodies to pertussis toxin. J. Infect. Dis. 163:355-361. [DOI] [PubMed] [Google Scholar]
- 11.Hansson, M., S. Stahl, T. N. Nguyen, T. Bachi, A. Robert, H. Binz, A. Sjolander, and M. Uhlen. 1992. Expression of recombinant proteins on the surface of the coagulase-negative bacterium Staphylococcus xylosus. J. Bacteriol. 174:4239-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are nonvirulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 13.Homonylo-McGavin, M. K., and S. F. Lee. 1996. Role of the C terminus in antigen P1 surface localization in Streptococcus mutans and two related cocci. J. Bacteriol. 178:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly, C., P. Evans, L. A. Bergmeier, S. F. Lee, A. Progulske-Fox, A. S. Bleiweis, and T. Lehner. 1989. Sequence analysis of the cloned streptococcal cell surface antigen I/II (P1). FEBS Lett. 258:127-132. [DOI] [PubMed] [Google Scholar]
- 15.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 16.Lee, S. F. 1992. Identification and characterization of a surface protein releasing enzymatic activity in Streptococcus mutans and other pathogenic streptococci. Infect. Immun. 60:4032-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee, S. F. 2003. Oral colonization and immune responses to Streptococcus gordonii: potential use as a vector to induce antibody against respiratory pathogens. Cur. Opin. Infect. Dis. 16:231-235. [DOI] [PubMed] [Google Scholar]
- 18.Lee, S. F., R. J. March, S. A. Halperin, G. Faulkner, and L. Gao. 1999. Surface expression of a protective recombinant pertussis toxin S1 subunit fragment in Streptococcus gordonii. Infect. Immun. 67:1511-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee, S. F., S. A. Halperin, H. Wang, and A. MacArthur. 2002. Oral colonization and immune responses to Streptococcus gordonii expressing a pertussis toxin S1 fragment in mice. FEMS Microbiol. Lett. 208:175-178. [DOI] [PubMed] [Google Scholar]
- 20.Lee, S. F., S. A. Halperin, J. B. Knight, and A. Tait. 2002. Purification and immunogenicity of a recombinant Bordetella pertussis S1S3FHA fusion protein expressed by Streptococcus gordonii. Appl. Environ. Microbiol. 68:4253-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, S. F., S. A. Halperin, D. F. Salloum, A. MacMillian, and A. Morris. 2003. Mucosal immunization with a genetically engineered pertussis toxin S1 fragment-cholera toxin subunit B chimera protein. Infect. Immun. 71:2272-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medaglini, D., G. Pozzi, T. P. King, and V. Fischetti. 1995. Mucosal and systemic immune responses to a recombinant protein expressed on the surface of the oral commensal bacterium Streptococcus gordonii after oral colonization. Proc. Natl. Acad. Sci. USA 92:6868-6872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medaglini, D., C. M. Rush, P. Sestini, and G. Pozzi. 1997. Commensal bacteria as vectors for mucosal vaccines against sexually transmitted diseases; vaginal colonization with recombinant streptococci induces local and systemic antibodies in mice. Vaccine 15:1330-1337. [DOI] [PubMed] [Google Scholar]
- 24.Medaglini, D., A. Ciabattini, M. R. Spinosa, T. Maggi, H. Marcotte, M. R. Oggioni, and G. Pozzi. 2001. Immunization with recombinant Streptococcus gordonii expressing tetanus toxin fragment C confers protection from lethal challenge in mice. Vaccine 19:1931-1939. [DOI] [PubMed] [Google Scholar]
- 25.Miyaji, E. N., R. P. Mazzatini, W. O. Dias, A. L. T. O. Nascimento, R. Marcovistz, D. S. Matos, I. Raw, N. Winter, B. Gicquel, R. Rappuoli, and L. C. C. Leite. 2001. Induction of neutralizing antibodies against diphtheria toxin by priming with recombinant Mycobacterium bovis BCG expressing CRM179, a mutant diphtheria toxin. Infect. Immun. 69:869-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyamura, K., S. Nishio, A. Ito, R. Murata, and R. Kono. 1974. Micro cell culture method for determination of diphtheria toxin and antitoxin titres using Vero cells. I. Studies on the factors affecting the toxin and antitoxin titration. J. Biol. Stand. 2:189-201. [DOI] [PubMed] [Google Scholar]
- 27.Oggioni, M. R., R. Manganelli, M. Controrni, M. Tommasino, and G. Pozzi. 1995. Immunization of mice by oral colonization with live recombinant commensal streptococci. Vaccine 13:775-779. [DOI] [PubMed] [Google Scholar]
- 28.Oggioni, M. R., D. Medaglini, T. Maggi, and G. Pozzi. 1999. Engineering the gram positive cell surface for construction of bacterial vaccine vectors. Methods 19:163-17338. [DOI] [PubMed] [Google Scholar]
- 29.Orr, N., J. E. Galen, and M. M. Levine. 1999. Expression and immunogenicity of a mutant diphtheria toxin molecule, CRM197, and its fragments in Salmonella typhi vaccine strain CVD 908-htrA. Infect. Immun. 67:4290-4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma, A., H. Nagata, N. Hamada, H. T. Sojar, D. E. Hruby, H. K. Kuramitsu, and R. J. Genco. 1996. Expression of functional Porphyromonas gingivalis fimbrillin polypeptide domains on the surface of Streptococcus gordonii. Infect. Immun. 62:3933-3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30a.Terleckyj, B., N. P. Willet, and G. D. Shickman. 1975. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect. Immun. 11:649-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]