Abstract
The genus Carnobacterium is currently divided into the following eight species: Carnobacterium piscicola, C. divergens, C. gallinarum, C. mobile, C. funditum, C. alterfunditum, C. inhibens, and C. viridans. An identification tool for the rapid differentiation of these eight Carnobacterium species was developed, based on the 16S-23S ribosomal DNA (rDNA) intergenic spacer region (ISR). PCR-restriction fragment length polymorphism (PCR-RFLP) analysis of this 16S-23S rDNA ISR was performed in order to obtain restriction profiles for all of the species. Three PCR amplicons, which were designated small ISR (S-ISR), medium ISR (M-ISR), and large ISR (L-ISR), were obtained for all Carnobacterium species. The L-ISR sequence revealed the presence of two tRNA genes, tRNAAla and tRNAIle, which were separated by a spacer region that varied from 24 to 38 bp long. This region was variable among the species, allowing the design of species-specific primers. These primers were tested and proved to be species specific. The identification method based on the 16S-23S rDNA ISR, using PCR-RFLP and specific primers, is very suitable for the rapid low-cost identification and discrimination of all of the Carnobacterium species from other phylogenetically related lactic acid bacteria.
The genus Carnobacterium, proposed by Collins et al. (10), includes heterofermentative lactic acid bacteria (LAB) that are currently divided into eight different species. The five species isolated from foods, such as poultry, meat, sausage, cheese, and seafood, are Carnobacterium piscicola, C. divergens, C. mobile, C. gallinarum, and C. viridans (4, 13, 19, 20, 21, 31, 33, 34). C. funditum and C. alterfunditum were isolated from anoxic Antarctic lake waters (15), while C. inhibens was isolated from the intestine of an Atlantic salmon (23). A Lactobacillus species, Lactobacillus maltaromicus, was found to be very similar to C. piscicola, and recently these two species have been considered synonymous (35). Most research on the genus Carnobacterium has focused on the production of bacteriocins, the regulation of metabolic enzymes, and the role of these factors in spoilage and the inhibition of Listeria monocytogenes in cold-smoked salmon (6, 12, 14, 30, 32, 40, 41, 42, 45, 47).
The identification of Carnobacterium species by phenotypic methods is time-consuming, and certain species may be difficult to identify correctly. These are some of the reasons that the rapid identification of Carnobacterium species is essential. Some species, such as C. mobile, C. inhibens, C. funditum, and C. alterfunditum, are rare and difficult to isolate, and therefore a rapid and accurate identification method can facilitate their identification and perhaps lead to the discovery of new species. Indeed, some species, such as C. inhibens, C. funditum, and C. alterfunditum, are very closely related, so a rapid and accurate method is required to distinguish them. As mentioned above, members of the Carnobacterium genus occupy different ecological niches and are important microflora in fish products such as smoked salmon (30, 40), in shrimps (13), in meat and sausages (19, 21, 55), in cheese (33), and in the fish intestine (23). Hence, a rapid identification method will facilitate the study of microbial ecology, such as the roles of Carnobacterium in a complex bacterial population and also during the storage of certain products. In addition, it is interesting to explore the potential characteristics of Carnobacterium, such as bacteriocin production, that can be used for bioprotection (6, 41).
In the last few years, some new ways to differentiate Carnobacterium species have been developed. These studies have focused mainly on 16S ribosomal DNA (rDNA). In Eubacteria, ribosomal genes are found in the form of an operon (25). The classic order of the ribosomal operon is 5′-16S-23S-5S-3′. Since rRNA genes are structurally conserved during evolution, related bacteria exhibit more similarities in their rRNA than unrelated species do. This makes rRNA sequences a preferred target for studies of bacterial evolution and typing. However, 16S rRNA sequences only allow for comparisons from the species level up to the kingdom level. The molecular typing methods developed to identify Carnobacterium species are based on randomly amplified polymorphic DNA (27), sequencing of the 16S rrn gene (54), restriction fragment length polymorphism (RFLP) of amplified 16S rDNA or the 16S-23S intergenic spacer region (ISR) (24, 46), multiplex PCR (11), and the design of oligonucleotides for use as specific probes or PCR primers (2, 8, 38). The results obtained by the randomly amplified polymorphic DNA technique enabled only C. divergens to be distinguished from other Carnobacterium species (27). The identification of Carnobacterium species based on restriction analysis of the 16S rDNA required a fastidious double digestion with HaeIII and HinfI (46). The identification of carnobacteria at the genus level has been demonstrated by nucleic acid hybridization using 16S rRNA-targeted genus-specific probes (38). However, the number of polymorphic sites in the 16S rDNA of Carnobacterium species is rather low (54), and thus it is difficult to define specific 16S rrn sequences that can be used to differentiate closely related species.
In contrast to 16S rDNA, the ISR between the 16S and 23S loci of the rDNA operon is not subject to the same selective pressure. The rDNA structural genes have been shown to exhibit considerable variability in size and sequence between organisms (3, 17, 18, 22, 25, 28, 56). RFLP of the PCR-amplified 16S-23S rDNA ISR, which has proved to be a rapid method to characterize bacterial isolates and populations (22, 37), was applied to identify acetic acid bacteria to the species level (43, 52). Recently, closely related Carnobacterium species isolated from food, including C. piscicola, C. divergens, C. gallinarum, and C. mobile, were differentiated by a 16S-23S ISR PCR-based single digestion with the HindIII, HinfI, or TaqI enzyme (24).
Specific oligonucleotide probes and PCR amplification of the 16S-23S rRNA ISR have been used to identify a large number of LAB (9, 48, 49, 51). Species-specific primers designed from the 16S-23S ISR have been developed and their use has proved to be a rapid method for the identification of closely related species such as Lactobacillus spp. (5). Moreover, species-specific primers have been designed by the use of domains that exhibit low levels of homology in the 16S rDNA sequences of Carnobacterium species (2, 8). However, the PCR primers used in these studies were not specific enough to differentiate Carnobacterium spp. from other genera of LAB. Even so, there are no reports of species-specific primers for the differentiation of all of the Carnobacterium species. This kind of primer could be very useful for PCR-based rapid identification. Furthermore, the eight currently described Carnobacterium species have never been involved in a comparative genetic characterization.
For the present study, the 16S-23S rDNA ISR was used as a target for the development of a reliable RFLP diagnostic algorithm and for the design of species-specific primers that are useful for discriminating all of the species of Carnobacterium in a sensitive, easy-to-perform PCR protocol.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains used for this study are listed in Table 1. The strains were stored as 20% glycerol stock cultures at −80°C in DeMan Rogosa Sharp medium (Biokar, Beauvais, France). Cultures were grown at 30°C for 24 h in DeMan Rogosa Sharp medium.
TABLE 1.
Bacterial strains used for this study
| Species | Straina | Source (reference) |
|---|---|---|
| Carnobacterium alterfunditum | CIP 105796T | Anoxic lake waters (13) |
| Carnobacterium divergens | NCDO 2763T | Meat (30) |
| INRA 541 | Meat (30) | |
| INRA 695 | Meat (30) | |
| INRA 507 | Meat (30) | |
| INRA 548 | Meat (30) | |
| INRA 733 | Meat (30) | |
| INRA 508 | Meat (30) | |
| INRA 524 | Meat (30) | |
| INRA 586 | Meat (30) | |
| INRA 687 | Meat (30) | |
| INRA 515 | Meat (30) | |
| INRA 337 | Meat (30) | |
| ENITIAA V41 | Fish (37) | |
| Carnobacterium funditum | CIP 106503T | Anoxic lake waters (13) |
| Carnobacterium gallinarum | NCDO 2766T | Poultry (9) |
| INRA 680 | Poultry (9) | |
| Carnobacterium inhibens | DSM 13024 | Salmon intestine (20) |
| Carnobacterium mobile | DSM 4849 | Poultry (9) |
| CIP 103159 | Poultry (9) | |
| Carnobacterium piscicola | NCDO 2762T | Fish (9, 17) |
| IFREMER 644 | Smoked salmon (25) | |
| IFREMER 665 | Smoked salmon (25) | |
| IFREMER 666 | Smoked salmon (25) | |
| IFREMER 692 | Smoked salmon (25) | |
| IFREMER 694 | Smoked salmon (25) | |
| IFREMER 668 | Smoked salmon (25) | |
| INRA 545 | Meat (30) | |
| INRA 725 | Meat (30) | |
| INRA 722 | Meat (30) | |
| INRA 501 | Meat (30) | |
| INRA 527 | Meat (30) | |
| INRA 572 | Meat (30) | |
| INRA 543 | Meat (30) | |
| INRA 528 | Meat (30) | |
| ENITIAA VI | Trout (37) | |
| INRA 336 | Meat (30) | |
| INRA 338 | Meat (30) | |
| INRA 526 | Meat (30) | |
| ENSAIA 1 | Cheese (28) | |
| ENSAIA 7 | Cheese (28) | |
| ENSAIA 16 | Cheese (28) | |
| ENSAIA 29 | Cheese (28) | |
| ENSAIA 32 | Cheese (28) | |
| Carnobacterium viridans | DSM 14451T | Bologna sausage (18) |
| Desemzia incerta | DSM 20581 | |
| Enterococcus faecalis | CIP 103015 | |
| Lactobacillus sakei | ATCC 15521 | |
| Lactobacillus curvatus | DSM 20019 | |
| Lactobacillus maltaromicus | CIP 103135 | |
| Leuconostoc lactis | DSM 20192 | |
| Leuconostoc mesenteroides | DSM 20240 | |
| Leuconostoc pseudomesenteroides | DSM 20193 | |
| Lactococcus lactis | ATCC 11454 | |
| Streptococcus thermophilus | INRA CNRZ 216 | |
| Weissella confusa | DSM 20186 |
DSM, Deutsche Sammlung von Microorganismen und Zellkulturen GmbH, Braunschweig, Germany; NCDO, National Collection of Dairy Organisms, Reading, United Kingdom; CIP, Collection de l'Institut Pasteur, Paris, France; INRA, Institut National de Recherche Agronomique; Theix, France; ENSAIA, Ecole Nationale Supérieure d'Agronomie et des Industries Alimentaires, Nancy, France; IFREMER, Institut Français de Recherche pour l'Exploitation de la Mer, Nantes, France; INRA CNRZ, Institut National de Recherche Agronomique, Centre National de Recherches Zootechniques, Jouy-en-Josas, France.
The Escherichia coli INV αF′ strain (Invitrogen Life Technology, Cergy Pontoise, France), used for cloning procedures, was grown in Luria-Bertani broth (0.1% tryptone, 0.1% sodium chloride, 0.05% yeast extract, pH 7.0) for 16 h at 37°C. Transformed E. coli cells were grown on Luria-Bertani agar plates containing 100 μg of ampicillin/ml, 0.5 mM isopropyl-β-d-thiogalactopyranoside, and 80 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside/ml.
DNA extraction and PCR amplification.
Total DNAs were extracted as described by Tudor et al. (53). The oligonucleotide primers used for this study were obtained from Invitrogen and are listed in Table 2. The ISRs were amplified with primers 16S-2 and 23S-7. For species-specific amplification, the ISR primers Cpis, Cdiv, Cmob, Cgal, Cinh, Cfun, Calt, and Cvir were designed from the nucleotide sequence data obtained in this study, and each of them was paired with the primer 23S-7 for the 23S rRNA gene. For restriction enzyme analysis, the ISRs were amplified with the primer set tRNAAla and 23S-10. The tRNAAla primer was designed from a conserved sequence of the tRNAAla gene located in the 16S-23S ISR of Oenococcus oeni (29).
TABLE 2.
Sequences of oligonucleotide primers used for PCR amplification and sequencing
| Primer | Position | Oligonucleotide sequence (5′ → 3′) | Tm(°C) | Reference |
|---|---|---|---|---|
| 16S-2 | 16S rRNA gene, forward (positions 1390 to 1407) | CTTGTACACACCGCCCGTC | 56 | 14 |
| 23S-7 | 23S rRNA gene, reverse (positions 188 to 208) | GGTACTTAGATGTTTCAGTTC | 56 | 14 |
| 23S-10 | 23S rRNA gene, reverse (positions 456 to 474) | CCTTTCCCTCACGGTACTG | 58 | 14 |
| tAla | tRNAAla gene, forward | TAGCTCAGCTGGGAGAGC | 58 | 24 |
| Calt | C. alterfunditum 16S-23S rRNA ISR gene, forward (positions 198 to 216) | GTATAAGATATTAGCTCTT | 53 | This study |
| Cdiv | C. divergens 16S-23S rRNA ISR gene, forward (positions 180 to 201) | TTCTAAAAAAATAGTACTCTTG | 56 | This study |
| Cfun | C. funditum 16S-23S rRNA ISR gene, forward (positions 177 to 201) | TATTTTGCACAAAAGATGACG | 53 | This study |
| Cgal | C. gallinarum 16S-23S rRNA ISR gene, forward (positions 179 to 199) | TAGTTTCACTCTAAAAAAAAC | 56 | This study |
| Cinh | C. inhibens 16S-23S rRNA ISR gene, forward (positions 177 to 198) | TTTTATTTGCACAAATA | 50 | This study |
| Cmob | C. mobile 16S-23S rRNA ISR gene, forward (positions 178 to 200) | CTCATTTAATTGCACAAAAAGG | 58 | This study |
| Cpis | C. piscicola 16S-23S rRNA ISR gene, forward (positions 193 to 213) | TTTATTTTTAATTAAATACCC | 53 | This study |
| Cvir | C. viridans 16S-23S rRNA ISR gene, forward (positions 193 to 212) | ATAAGTATAAGATGTTTTTT | 53 | This study |
PCRs were performed in a total volume of 50 μl containing 1× PCR buffer without MgCl2, 2.5 mM MgCl2, 1 μg of DNA/ml, a 0.3 μM concentration of each primer, a 0.25 mM concentration of each deoxynucleoside triphosphate, and 1 U of Taq DNA polymerase (Appligene Oncor, Espoo, Finland). PCRs were carried out in a PTC-100 thermocycler (MJ Research Inc., Watertown, Mass.). The amplification of ISRs consisted of 35 cycles of a 1-min denaturation step at 94°C, a 1-min annealing step at 56°C, and a 1-min extension step at 72°C. The annealing temperatures of each species-specific primer are listed in Table 2. Species-specific amplification consisted of 30 cycles of 1 min at 94°C, 45 s of an annealing step, and 45 s at 72°C. The first cycle of the amplification program was preceded by incubation for 5 min at 94°C and followed by a final 5-min extension at 72°C. Negative controls containing no DNA template were included in parallel. Five-microliter samples of the PCR products were analyzed by electrophoresis in a 1.5% (wt/vol) agarose gel in Tris-acetate-EDTA buffer and were subsequently visualized by UV illumination after ethidium bromide staining.
Restriction enzyme analysis and computer-assisted analysis of rDNA restriction patterns.
The enzymes used for restriction enzyme analysis were HindIII, HinfI, and αTaqI. Digestion reactions were performed in a final volume of 25 μl at the optimal temperature according to the manufacturer's protocols (BioLabs, Hitchin, United Kingdom). The total digested products were separated by electrophoresis in 2% (wt/vol) agarose gels in Tris-borate-EDTA buffer. The gels were stained with 0.5 μg of ethidium bromide/ml and visualized with UV light. Gel images were digitized with a charge-coupled device video camera (Sony, Clichy, France) and saved as TIFF files. These were converted, normalized with the molecular size markers in the 100-bp DNA ladder (BioLabs), and analyzed with Bio Profile 1D++ software (Vilbert Lourmat, Marne La Vallée, France). For ISR-RFLP analysis, a band-matching algorithm was selected to calculate pairwise similarity matrices with the Dice coefficient. A band-matching tolerance of 5% was chosen.
16S-23S ISR DNA cloning and sequencing.
Clone libraries of the PCR-amplified rDNAs of Carnobacterium species 16S-23S ISRs were constructed with primers 16S-2 and 23S-7 by the use of a pCR2.1 TA cloning kit (Invitrogen Life Technology). From each clone library, 30 white colonies were picked randomly. The clones were screened by PCR for the presence of rDNA-ISR inserts and by restriction mapping for the sizes of inserts. For each strain, three independent clones corresponding to the three types of 16S-23S ISR identified for Carnobacterium species were selected and sequenced. Double-stranded DNAs from the recombinant plasmids of the positive clones from the above screening assays were purified with a QIAprep Spin Miniprep kit (Qiagen S.A., Courtaboeuf, France). Nucleotide sequences of the cloned 16S-23S ISRs were determined by the dideoxynucleotide chain termination method (44) with an ABI 370 automated sequencer and a Taq Dye-Deoxy TM terminator cycle sequencing kit (Perkin-Elmer, Boston, Mass.). The M13 primers flanking the multiple cloning site of pCR2.1 DNA were used to sequence both DNA strands.
DNA analysis.
Sequences were submitted to the National Center for Biotechnology Information (Bethesda, Md.) for similarity searches in GenBank. The computer program CLUSTAL W (50) was used for sequence alignment and the BLAST 2 program (1) was used for the representation of sequence similarities, using sequences which did not include 16S or 23S rDNA.
Nucleotide sequence accession numbers.
The sequences of the 16S-23S ISR DNAs of C. funditum, C. alterfunditum, C. inhibens, and C. viridans were deposited in the GenBank database. The accession numbers for these sequences are as follows: AY 170833 (16S-23S rDNA S-ISR of C. funditum), AY 170832 (16S-23S rDNA M-ISR of C. funditum), AY 170831 (16S-23S rDNA L-ISR of C. funditum), AY 170836 (16S-23S rDNA S-ISR of C. alterfunditum), AY 170835 (16S-23S rDNA M-ISR of C. alterfunditum), AY 170834 (16S-23S rDNA L-ISR of C. alterfunditum), AY 170839 (16S-23S rDNA S-ISR of C. inhibens), AY 170838 (16S-23S rDNA M-ISR of C. inhibens), AY 170837 (16S-23S rDNA of L-ISR C. inhibens), AY 229996 (16S-23S rDNA S-ISR of C. viridans), AY 229995 (16S-23S rDNA of M-ISR C. viridans), and AY 229994 (16S-23S rDNA L-ISR of C. viridans).
RESULTS
ISR length polymorphism in Carnobacterium genus.
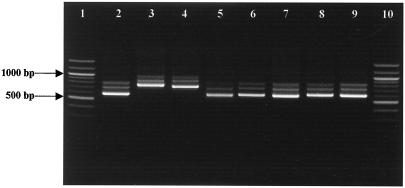
PCR amplification using primers 16S-2 and 23S-7, designed from the flanking terminal sequences of the 16S and 23S genes, was performed with chromosomal DNAs isolated from 45 strains of the eight Carnobacterium species. The PCR products from each Carnobacterium strain always revealed three major ISR size classes, designated small ISR (S-ISR), medium ISR (M-ISR), and large ISR (L-ISR). Their lengths allowed the differentiation of two groups of Carnobacterium species (Fig. 1). C. divergens NCDO 2763T, C. mobile DSM 4849T, C. funditum CIP 106503T, C. alterfunditum CIP 105796T, C. inhibens DSM 13024, and C. viridans DSM 14451T generated one major S-ISR band (ca. 600 bp) and two minor M-ISR and L-ISR bands (ca. 700 and ca. 750 bp, respectively). The ISRs amplified from C. gallinarum NCFB 2766T and C. piscicola NCDO 2762T were larger (S-ISR, ca. 700 bp; M-ISR, 800 bp; and L-ISR, 900 bp). These results showed that C. divergens, C. mobile, C. funditum, C. alterfunditum, C. inhibens, and C. viridans are included in group A, while C. gallinarum and C. piscicola appear to be the only members of group B, as defined by Kabadjova et al. (24).
FIG. 1.
Electrophoresis in a 1.5% agarose gel of PCR-amplified 16S-23S ISRs of carnobacteria. Lanes 1 and 10, molecular weight marker (100-bp DNA ladder); lane 2, C. divergens NCDO 2763T; lane 3, C. piscicola NCDO 2762T; lane 4, C. gallinarum NCDO 2766T; lane 5, C. mobile DSM 4849; lane 6, C. funditum CIP 106503T; lane 7, C. alterfunditum CIP 105796T; lane 8, C. inhibens DSM 13024; lane 9, C. viridans DSM 14451T.
PCR-based restriction analysis.
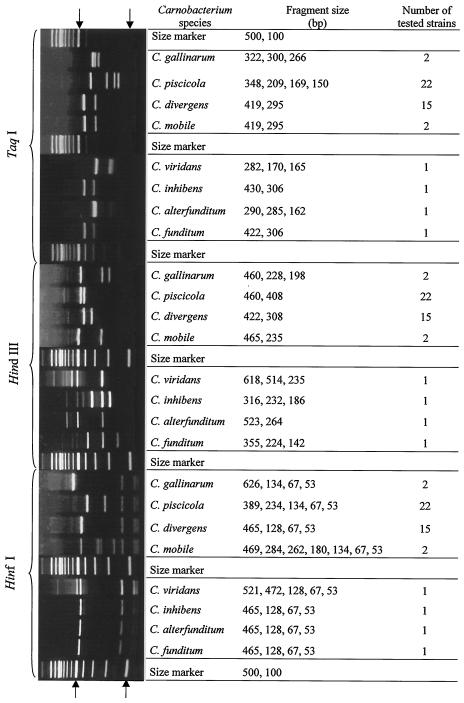
Since PCR amplification with primers 16S-2 and 23S-7 yielded three ISR amplicons of different lengths, it was difficult to obtain clear patterns in a PCR-RFLP analysis. The 16S-23S rDNA L-ISR and M-ISR of C. piscicola, C. divergens, C. mobile, and C. gallinarum contain a tRNAAla gene (24). The results showed that a tRNAAla gene is present in the 16S-23S rDNA ISR of C. funditum, C. alterfunditum, C. inhibens, and C. viridans, as already described for the other species. The PCR products amplified from each strain were digested separately with αTaqI, HinfI, and HindIII since they allow the differentiation of four closely related Carnobacterium species, i.e., C. piscicola, C. divergens, C. gallinarum, and C. mobile (24).
The observed individual TaqI, HinfI, and HindIII RFLP patterns of reference strains are shown in Fig. 2. The TaqI digestion revealed seven genotypes with well-resolved bands. The seven genotypes displayed the following patterns: four bands, at 150, 169, 209, and 348 bp, for C. piscicola; three bands, at 266, 300, and 322 bp, for C. gallinarum; two bands, at 295 and 419 bp, for C. divergens and C. mobile; one band at 282 bp and two bands at 165 to 170 bp for C. viridans; two bands, at 430 and 306 bp, for C. inhibens; two bands, at 422 and 306 bp, for C. funditum; and two bands at 285 to 290 bp and one band at 162 bp for C. alterfunditum. Hence, we determined that TaqI can only be used to distinguish four Carnobacterium species (C. viridans, C. alterfunditum, C. piscicola, and C. gallinarum) and to differentiate them from the group of the four other species (C. inhibens, C. funditum, C. divergens, and C. mobile). The HinfI digestion revealed five genotypes with four to seven bands. Four bands, at 53, 67, 128, and 465 bp, were distinguished for C. divergens, C. inhibens, C. alterfunditum, and C. funditum; four bands, at 53, 67, 134, and 626 bp, were seen for C. gallinarum; five bands, at 53, 67, 134, 234, and 389 bp, were seen for C. piscicola; seven bands, at 53, 67, 134, 180, 262, 284, and 469 bp, were seen for C. mobile; and five bands, at 521, 472, 128, 67, and 53 bp, were seen for C. viridans. We discovered that this enzyme could not distinguish between C. divergens, C. inhibens, C. alterfunditum, and C. funditum. However, HinfI permitted the differentiation of C. viridans, C. piscicola, C. gallinarum, and C. mobile from the group of C. divergens, C. inhibens, C. alterfunditum, and C. funditum. The HindIII restrictions resulted in two or three fragments, with sizes as follows: for C. mobile, 465 and 235 bp; for C. divergens, 422 and 308 bp; for C. gallinarum, 460, 228, and 198 bp; for C. piscicola, 460 and 408 bp; for C. viridans, 618, 514, and 235 bp; for C. inhibens, 316, 232, and 186 bp; and for C. funditum, 355, 224, and 142 bp. Meanwhile, C. alterfunditum had a two-fragment profile at 523 and 264 bp. A comparison between these profiles showed that HindIII allows for differentiation of the eight Carnobacterium species. The total lengths of the digested ISR DNA fragments ranged from 617 bp (C. viridans digested with TaqI) to 787 bp (C. alterfunditum digested with HindIII) and from 868 bp (C. piscicola digested with HindIII) to 888 bp (C. gallinarum digested with TaqI) for the strains belonging to groups A and B, respectively. However, the sum of the fragment sizes of the digested L-ISR was larger than the L-ISR size (750 bp) upon HinfI digestion for C. viridans (1,243 bp) and C. mobile (1,449 bp) and upon HindIII digestion for C. viridans (1,367 bp). This fact has been observed previously and was explained as a consequence of the differences in position of the HinfI and HindIII restriction sites located in the M-ISR and the L-ISR (24).
FIG. 2.
Gel electrophoresis of PCR-amplified 16S-23S ISR fragments of the Carnobacterium species digested with αTaqI, HinfI, and HindIII. Data for RFLP pattern species are presented by molecular size. The position of the 500-bp fragment of the 100-bp DNA ladder is indicated by arrows.
Nucleotide sequence analysis of 16S-23S rDNA ISR.
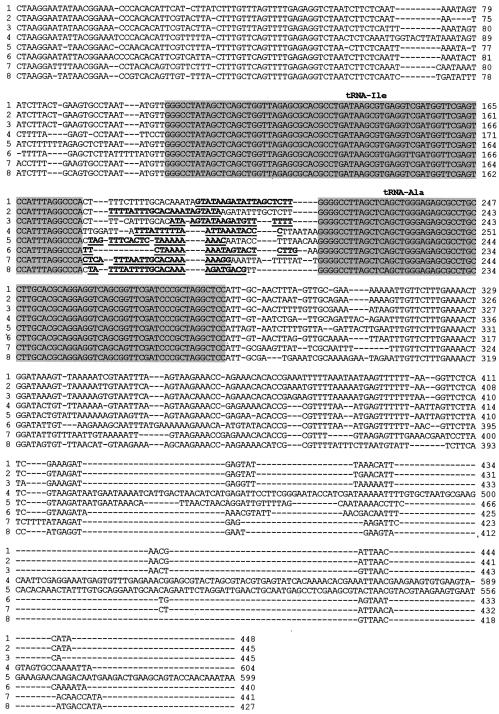
To compare structures between differently sized ISRs, we sequenced the S-ISRs, M-ISRs, and L-ISRs of C. funditum CIP 106503T, C. alterfunditum CIP 105796T, C. inhibens DSM 13024T, and C. viridans DSM 14451T. For each strain, the three ISR amplicons, cloned in plasmid pCR2.1, were screened by PCR amplification. Three independent clones containing the insert rDNAs corresponding to each of the three PCR ISR amplicons were identified and sequenced. The length of each ISR from each species is listed in Table 3, and a sequence alignment of L-ISRs is shown in Fig. 3.
TABLE 3.
Lengths of Carnobacterium ISRs
| Species | Length of ISR (bp)
|
||
|---|---|---|---|
| S-ISR | M-ISR | L-ISR | |
| C. piscicolaa | 391 | 473 | 604 |
| C. divergensa | 218 | 326 | 440 |
| C. mobilea | 204 | 312 | 441 |
| C. gallinaruma | 362 | 484 | 599 |
| C. funditum | 204 | 310 | 427 |
| C. alterfunditum | 225 | 337 | 448 |
| C. inhibens | 217 | 325 | 445 |
| C. viridans | 236 | 338 | 445 |
According to Kabadjova et al. (24).
FIG. 3.
Alignment of nucleotide sequences of Carnobacterium 16S-23S L-ISRs. Line 1, C. alterfunditum CIP 105796T; line 2, C. inhibens DSM 13024; line 3, C. viridans DSM 14451T; line 4, C. piscicola NCDO 2762T; line 5, C. gallinarum NCDO 2766T; line 6, C. divergens NCDO 2763T; line 7, C. mobile DSM 4849; line 8, C. funditum CIP 106503T. C. divergens, C. mobile, C. piscicola, and C. gallinarum were sequenced by Kabadjova et al. (24). GenBank accession numbers of the complete sequences are listed in Materials and Methods. The tRNAs are indicated by gray boxes. The specific primers are underlined.
The S-ISR sequences determined for C. funditum, C. alterfunditum, C. inhibens, and C. viridans revealed four different lengths, of 204, 225, 217, and 236 bp, respectively. The M-ISRs were 310 to 338 bp long and contained only the tRNAAla gene. For C. funditum, the tRNAAla gene begins at position 87 and ends at position 158. In the M-ISR of C. alterfunditum, the gene is located from positions 106 to 177, while in C. inhibens it begins at position 94 and ends at position 165. This gene is also found in the M-ISR of C. viridans, from positions 104 to 176. The central region was flanked by sequences that were identical or almost identical to those of the corresponding S-ISRs. The M-ISRs of these eight Carnobacterium species were similarly organized and comparable to the ISRs of Enterococcus, Lactococcus, Leuconostoc, and Streptococcus, which contain a unique tRNAAla gene (24, 28, 29, 36, 39, 57). The M-ISRs of the species belonging to group A (C. divergens, C. mobile, C. funditum, C. alterfunditum, C. inhibens, and C. viridans) exhibited 63.4% identity. The identity between the M-ISRs of all eight species was 44%. The L-ISRs of C. funditum, C. alterfunditum, C. inhibens, and C. viridans also revealed four different sequences. The L-ISR amplicons of C. funditum and C. alterfunditum consisted of 427 and 448 bp, respectively. Meanwhile, C. inhibens and C. viridans had the same L-ISR length of 445 bp. For C. funditum, C. alterfunditum, C. inhibens, and C. viridans, the tRNAIle gene (73 bp) is located at position 101, 102, 105, or 106 and the tRNAAla gene (71 bp) is located at position 204, 213, or 217, respectively. All carnobacterial L-ISRs were organized similarly to those of Lactobacillus species (5, 24). The most variable region in the L-ISR sequences was located between the genes for tRNAIle and tRNAAla (Fig. 3).
Phylogenetic relationships in Carnobacterium genus based on sequences of L-ISR.
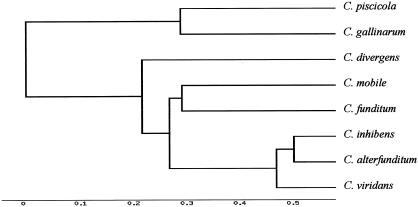
To evaluate the phylogenetic relationships between the species of the Carnobacterium genus on the basis of the L-ISRs, we created a phylogenetic tree including all of the sequences determined in this study (Fig. 4). The distance matrices were calculated by the Dayhoff matrix and the phylogenetic tree was constructed by cluster and topological algorithms with GeneBee software (7).
FIG. 4.
Phylogenetic relationship between strains of the species of the Carnobacterium genus determined by neighbor-joining analysis of the sequences of the 16S-23S rDNA L-ISRs. The distance matrices were calculated by the Dayhoff matrix and the phylogenetic tree was constructed by cluster and topological algorithms. Nucleotide sequence (L-ISR) accession numbers for C. funditum, C. alterfunditum, C. inhibens, and C. viridans are given in Materials and Methods. The nucleotide sequence accession numbers for L-ISRs of C. piscicola, C. divergens, C. gallinarum, and C. mobile are AF 374297, AF 374294, AF 374287, and AF 374291, respectively.
The phylogenetic relationships based on the L-ISRs resulted in the identification of two major groups of species. The first contains C. piscicola and C. gallinarum and the second contains C. divergens, C. mobile, C. funditum, C. alterfunditum, C. inhibens, and C. viridans. This analysis gathered the Carnobacterium species into groups A and B as distinguished by 16S-23S rDNA ISR size polymorphism.
In group A, two subgroups were formed. The first subgroup (A1) included C. divergens strains, while the second one (A2) included C. mobile, C. funditum, C. alterfunditum, C. inhibens, and C. viridans. C. divergens was separated in a branch from subgroup A2, indicating that C. divergens, even though it is related, represents an independent phylogenetic lineage that diverges from the members of subgroup A2. These data on ISR sequences confirmed the observation that C. inhibens and C. alterfunditum cannot be distinguished easily. The sequence similarity of L-ISRs containing tRNA genes supported the proposal that C. inhibens and C. alterfunditum are monophyletic and represent a single clade. The close relationship of C. mobile and C. funditum was confirmed by the sequences of their L-ISRs. Thereby, subgroup A2 can be divided into two subsubgroups, with the first one (A2-1) consisting of C. mobile and C. funditum and the second one (A2-2) consisting of C. viridans, C. inhibens, and C. alterfunditum (Fig. 4).
Design and specificity of species-specific primers.
In order to design primers that would specifically identify species, we screened the region showing the highest variability among Carnobacterium species. The highly variable spacer region between the two tRNA genes from the L-ISRs varied in length from 24 to 38 bp, and it turned out that this region met the requirement for the design of species-specific primers (Fig. 3). Eight specific primers for eight corresponding Carnobacterium species were designed. The primer sequences from this region are underlined in Fig. 3 and listed in Table 2. In order to validate the specificity of species-specific primers, we compared the oligonucleotide sequences to sequences in the GenBank database and to Ribosome Database Project sequences by using the BLAST program at the National Center for Biotechnology Information web site. The specific primers for C. divergens and C. gallinarum do not match significantly with any bacterial sequences available in the databases. No sequences identical to the other specific primer sequences were determined in the data banks. However, alignments showing 100% identity between genomic bacterial DNA sequences and 76 to 91% of the specific primer sequences were determined. The genomic DNA sequences showing partial identity with the Carnobacterium-specific primers belong to various genomes of gram-positive bacteria, such as Neisseria, Clostridium, Listeria, Bacillus, Mycoplasma, Lactococcus, and Staphylococcus. None of these genomic DNA sequences is located in a ribosomal operon. Consequently, to avoid the possibility of nonspecific amplification due to nonhomologous annealing of a specific primer to a bacterial genome, we chose a nested PCR approach. First, the ISR containing the species-specific sequence was amplified with primers 16S-2 and 23S-7. This amplicon was then used as a template for the species-specific PCR.
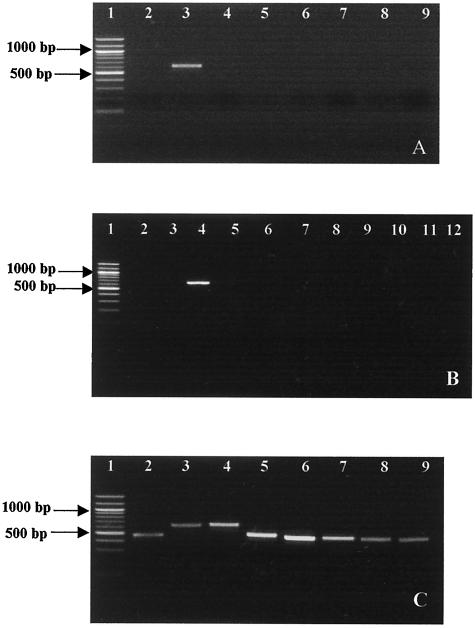
We used DNA samples from different strains in order to have a wide range for an assessment of primer specificity. The ISR target regions from C. piscicola NCDO 2762T, C. divergens NCDO 2763T, C. gallinarum NCDO 2766T, C. mobile DSM 4849T, C. funditum CIP 106503T, C. alterfunditum CIP 105796T, C. inhibens DSM 13024T, and C. viridans DSM 14451T were amplified by the nested PCR approach as described above or by one-step specific PCRs. The specificities of these primers were also tested on a large number of bacterial species, which are listed in Table 1. As an example, Fig. 5A and B show the specificity of the C. piscicola primer (Cpis). The Cpis and 23S-7 primers only allowed the synthesis of a fragment of 650 bp from C. piscicola DNA. Lactobacillus maltaromicus gave a positive response to the specific primer of C. piscicola (results not shown), which confirms that these two species are synonymous, as reported before (35). Each specific primer allowed amplification exclusively from the corresponding species by the nested or one-step specific PCR approach (results not shown). The positive responses for each species-specific primer are presented in Fig. 5C.
FIG. 5.
(A) PCR amplification of Carnobacterium species with specific primer for C. piscicola. (B) PCR amplification of different bacteria with the specific primer for C. piscicola. (C) PCR amplification of each Carnobacterium species with species-specific primers. Lanes for panels A and C: 1, molecular weight marker (100-bp DNA ladder); 2, C. divergens NCDO 2763T; 3, C. piscicola NCDO 2762T; 4, C. gallinarum NCDO 2766; 5, C. mobile DSM 4849; 6, C. funditum CIP 106503T; 7, C. alterfunditum CIP 105796T; 8, C. inhibens DSM 13024; 9, C. viridans DSM 14451T. Lanes for panel B: 1, molecular weight marker (100-bp DNA ladder); 2, Lactobacillus sakei ATCC 15521; 3, Lactobacillus curvatus DSM 20019; 4, C. piscicola NCDO 2762T; 5, Desemzia incerta DSM 20581; 6, Enterococcus faecalis CIP 103015; 7, Leuconostoc lactis DSM 20192; 8, Leuconostoc mesenteroides DSM 20240; 9, Leuconostoc pseudomesenteroides DSM 20193; 10, Lactococcus lactis ATCC 11454; 11, Streptococcus thermophilus INRA CNRZ 216; 12, Weissella confusa DSM 20186.
DISCUSSION
In this work, we compared the 16S-23S rDNA ISR organization of the eight Carnobacterium species. The three different 16S-23S ISR PCR products obtained for each strain indicated the presence of at least three types of rrn operon in Carnobacterium species. The variations in ISR length observed could be due in part to variations in the number and type of tRNA sequences found in this region.
The rRNA genes of Carnobacterium are separated by a spacer region, which varies in length from 204 to 391 bp for the S-ISR, 310 to 484 bp for the M-ISR, and 427 to 604 bp for the L-ISR. This spacer region includes the tRNA genes. The Carnobacterium species have three classes of spacer region and form three types of rrn operon (named rrn small [S], rrn medium [M], and rrn large [L]). rrn S and rrn L are similar to those identified for Lactobacillus species, in which rrn S contains no tRNA genes and rrn L has two tRNA genes (tRNAIle and tRNAAla), while Staphylococcus aureus (16) exhibits three types of rrn operon with structures identical to those of Carnobacterium species. The tRNA genes found in carnobacterial L-ISRs are homologous. This suggests that they originated from the same ancestral organism.
No tRNA genes were identified in the S-ISR of any species of the Carnobacterium genus. The species belonging to group A (C. divergens, C. mobile, C. funditum, C. alterfunditum, C. inhibens, and C. viridans) showed 56.8% identity among their S-ISRs, while a sequence alignment of all of the S-ISRs of the eight species resulted in 36.2% identity. Meanwhile, the species belonging to group A (C. divergens, C. mobile, C. funditum, C. alterfunditum, C. inhibens, and C. viridans) revealed 72.4% identity in their L-ISRs, while the identity among all of the L-ISRs of the Carnobacterium genus was 51.6%. L-ISRs are composed of the corresponding S-ISRs and M-ISRs interrupted by two large conserved blocks containing two tRNA genes coding for tRNAIle and tRNAAla.
The gene encoding tRNAAla includes an 18-nucleotide sequence that is conserved in all tRNAAla sequences compared, making this region a suitable target for PCR inside the ISR. Primer 23S-10 is the recommended primer for the detection of all copies of the spacer region (17). In all Carnobacterium species, the tRNAAla gene is located at the same distance from the 23S rRNA in both the M-ISR and the L-ISR. For this reason, PCRs using primers tRNAAla and 23S-10 allowed the amplification of two amplicons of the same length, which appeared as a single DNA fragment with a size of about 750 bp for group A (C. divergens, C. mobile, C. funditum, C. alterfunditum, C. inhibens, and C. viridans) and about 900 bp for group B (C. gallinarum and C. piscicola). These amplicons were appropriate for the RFLP analysis used in this study. PCR-RFLP of the 16S-23S rDNA ISR digested by three endonucleases, HinfI, HindIII, and αTaqI, was tested to cluster the Carnobacterium species. C. divergens, C. mobile, C. inhibens, and C. funditum could not be differentiated by αTaqI. The digestion of the ISR amplicons with HinfI only allowed the differentiation of C. piscicola, C. mobile, C. gallinarum, and C. viridans. Therefore, only HindIII allows the differentiation of the eight Carnobacterium species. The finding of two distinct Carnobacterium groups, group A (C. divergens, C. mobile, C. funditum, C. alterfunditum, C. inhibens, and C. viridans) and group B (C. gallinarum and C. piscicola), accurately defined by unique RFLP genotypes, correlates with a previous numerical phenetic study of the genus Carnobacterium (26). Furthermore, this fact is supported by the dendrogram presented in Fig. 4 showing the formation of groups A and B, consisting of C. gallinarum and C. piscicola and of C. divergens, C. mobile, C. funditum, C. alterfunditum, C. inhibens, and C. viridans, respectively.
The species-specific PCR primers, designed from 16S rDNA, were not specific enough to differentiate Carnobacterium spp. from other bacterial strains (8). The tRNA gene intergenic region in the L-ISRs has been shown to be highly variable and meets the requirement for the design of specific primers (5). Unlike a 16S rDNA-based PCR, in which the genus-specific primers are separated by a long stretch of target sequence, an ISR-based PCR would amplify a smaller PCR product, resulting in more efficient and sensitive target amplification. An alignment of the L-ISR sequences of the eight Carnobacterium species showed the presence of a variable region between tRNAIle and tRNAAla in the L-ISR. We designed species-specific primers from this region for each Carnobacterium species. Cross-examination was performed with the eight Carnobacterium species, and positive responses were only obtained for the species corresponding to the specific primers tested. These primers were also tested on various bacterial genera, such as Lactobacillus, Lactococcus, Leuconostoc, Weissella, Enterococcus, Streptococcus, and Desemzia, and these bacteria were not detectable by them, proving the specificity of the primers tested (Fig. 5B). An in silico analysis showed the low probability of a nonspecific amplification when the nested PCR approach is used. These results indicate that the PCR strategy proposed is highly specific.
Concerning the intraspecies stability of ISR-RFLP patterns, we can state that the spacer-based method was successfully evaluated with respect to expanded groups of strains within the carnobacterial species. The 16S-23S ISR HindIII RFLP profile and the designed primers clearly appear to be species specific. However, to our knowledge, there is only one isolated strain each described for C. funditum, C. alterfunditum, C. inhibens, and C. viridans. Consequently, the 16S-23S ISR HindIII RFLP profile and the specific primers for these species were only tested on the available reference strains. Hence, the identification strategy using the 16S-23S ISR HindIII RFLP profile confirmed by a nested PCR with specific primers, as described in this study, could be a useful and powerful tool to identify new strains of these species from different ecological niches.
In conclusion, the first molecular identification strategy for the eight closely related Carnobacterium species was proposed in this paper. PCR-RFLP analysis of 16S-23S rDNA ISRs digested by HindIII revealed different profiles for all Carnobacterium species. Sequence analysis of 16S-23S rDNA ISRs led to the construction of species-specific primers that are useful for PCR identification of the eight Carnobacterium species.
Acknowledgments
We especially thank Richard Holley (University of Manitoba, Winnipeg, Canada) for providing the C. viridans strain type.
We are grateful to the French Ministry of Research for financial support in the “Bioressources et Traçabilité pour le Post-Génome” project. This project was partially financed by European Union project “SEAFOOD plus.”
REFERENCES
- 1.Altschul, S., T. Madden, A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barakat, R. K., M. W. Griffiths, and L. J. Harris. 2000. Isolation and characterization of Carnobacterium, Lactococcus, and Enterococcus spp. from cooked, modified atmosphere packaged, refrigerated, poultry meat. Int. J. Food Microbiol. 62:83-94. [DOI] [PubMed] [Google Scholar]
- 3.Barry, T., C. M. Glennon, L. K. Dunican, and F. Gannon. 1991. The 16S/23S ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1:149. [DOI] [PubMed] [Google Scholar]
- 4.Baya, A. M., A. E. Toranzo, B. Lupiani, T. Li, B. S. Roberson, and F. M. Hetrick. 1991. Biochemical and serological characterization of Carnobacterium spp. isolated from farmed and natural populations of striped bass and catfish. Appl. Environ. Microbiol. 57:3114-3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berthier, F., and S. D. Ehrlich. 1998. Rapid species identification within two groups of closely related lactobacilli using PCR primers that target the 16S/23S rRNA spacer region. FEMS Microbiol. Lett. 161:97-106. [DOI] [PubMed] [Google Scholar]
- 6.Bhugaloo-Vial, P., X. Dousset, A. Metivier, O. Sorokine, P. Anglade, P. Boyaval, and D. Marion. 1996. Purification and amino acid sequences of piscicocins V1a and V1b, two class IIa bacteriocins secreted by Carnobacterium piscicola V1 that display significantly different levels of specific inhibitory activity. Appl. Environ. Microbiol. 62:4410-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodsky, L. I., A. L. Drachev, A. M. Leontovich, and S. I. Feranchuk. 1993. A novel method of multiple alignment of biopolymer sequences. Biosystems 30:65-79. [DOI] [PubMed] [Google Scholar]
- 8.Brooks, J. L., A. S. Moore, R. A. Patchett, M. D. Collins, and R. G. Kroll. 1992. Use of the polymerase chain reaction and oligonucleotide probes for the rapid detection and identification of Carnobacterium species from meat. J. Appl. Bacteriol. 72:294-301. [DOI] [PubMed] [Google Scholar]
- 9.Chagnaud, P., K. Machinis, L. A. Coutte, A. Marecat, and A. Mercenier. 2001. Rapid PCR-based procedure to identify lactic acid bacteria: application to six common Lactobacillus species. J. Microbiol. Methods 44:139-148. [DOI] [PubMed] [Google Scholar]
- 10.Collins, M. D., J. A. Farrow, B. Phillips, S. Ferusa, and D. Jones. 1987. Classification of Lactobacillus divergens, Lactobacillus piscicola and some catalase-negative, asporogenous, rod-shaped bacteria from poultry in a new genus, Carnobacterium. Int. J. Syst. Bacteriol. 37:310-316. [Google Scholar]
- 11.Connil, N., H. Prévost, and X. Dousset. 2002. Production of biogenic amines and divercin V41 in cold smoked salmon inoculated with Carnobacterium divergens V41, and specific detection of this strain by multiplex-PCR. J. Appl. Microbiol. 92:611-617. [DOI] [PubMed] [Google Scholar]
- 12.Coombs, J. M., and J. E. Brenchley. 1999. Biochemical and phylogenetic analyses of a cold-active beta-galactosidase from the lactic acid bacterium Carnobacterium piscicola BA. Appl. Environ. Microbiol. 65:5443-5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalgaard, P., M. Vancanneyt, N. Euras Vilalta, J. Swings, P. Fruekilde, and J. J. Leisner. 2003. Identification of lactic acid bacteria from spoilage associations of cooked and brined shrimps stored under modified atmosphere between 0°C and 25°C. J. Appl. Microbiol. 94:80-89. [DOI] [PubMed] [Google Scholar]
- 14.Duffes, F., F. Leroi, P. Boyaval, and X. Dousset. 1999. Inhibition of Listeria monocytogenes by Carnobacterium spp. strains in a simulated cold smoked fish system stored at 4°C. Int. J. Food Microbiol. 47:33-42. [DOI] [PubMed] [Google Scholar]
- 15.Franzmann, P. D., P. Hopfl, N. Weiss, and B. J. Tindall. 1991. Psychrotrophic, lactic acid-producing bacteria from anoxic waters in Ace Lake, Antarctica; Carnobacterium funditum sp. nov. and Carnobacterium alterfunditum sp. nov. Arch. Microbiol. 156:255-262. [DOI] [PubMed] [Google Scholar]
- 16.Gurtler, V., and H. D. Barrie. 1995. Typing of Staphylococcus aureus strains by PCR-amplification of variable-length 16S-23S rDNA spacer regions: characterization of spacer sequences. Microbiology 141:1255-1265. [DOI] [PubMed] [Google Scholar]
- 17.Gurtler, V., and V. A. Stanisich. 1996. New approaches to typing and identification of bacteria using the 16S-23S rDNA spacer region. Microbiology 142:3-16. [DOI] [PubMed] [Google Scholar]
- 18.Hain, T., N. Ward-Rainey, R. M. Kroppenstedt, E. Stackebrandt, and F. A. Rainey. 1997. Discrimination of Streptomyces albidoflavus strains based on the size and number of 16S-23S ribosomal DNA intergenic spacers. Int. J. Syst. Bacteriol. 47:202-206. [DOI] [PubMed] [Google Scholar]
- 19.Hitchener, L. J., A. F. Egan, and P. J. Rogers. 1982. Characteristics of lactic acid bacteria isolated from vacuum-packaged beef. J. Appl. Bacteriol. 52:31-37. [DOI] [PubMed] [Google Scholar]
- 20.Hiu, S. F., R. A. Holt, N. Sriranganathan, R. J. Seidler, and J. L. Fryer. 1984. Lactobacillus piscicola, a new species from salmonid fish. Int. J. Syst. Bacteriol. 34:393-400. [Google Scholar]
- 21.Holley, R. A., T. Y. Guan, M. Y. Peirson, and C. K. Yost. 2002. Carnobacterium viridans sp. nov., an alkaliphilic, facultative anaerobe isolated from refrigerated, vacuum-packed bologna sausage. Int. J. Syst. Evol. Microbiol. 52:1881-1885. [DOI] [PubMed] [Google Scholar]
- 22.Jensen, M. A., J. A. Webster, and N. Straus. 1993. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl. Environ. Microbiol. 59:945-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joborn, A., M. Dorsch, J. C. Olsson, A. Westerdahl, and S. Kjelleberg. 1999. Carnobacterium inhibens sp. nov., isolated from the intestine of Atlantic salmon (Salmo salar). Int. J. Syst. Bacteriol. 49:1891-1898. [DOI] [PubMed] [Google Scholar]
- 24.Kabadjova, P., X. Dousset, V. Le Cam, and H. Prevost. 2002. Differentiation of closely related Carnobacterium food isolates based on 16S-23S ribosomal DNA intergenic spacer region polymorphism. Appl. Environ. Microbiol. 68:5358-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krawiec, S., and M. Riley. 1990. Organization of the bacterial chromosome. Microbiol. Rev. 54:502-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai, S., and L. N. Manchester. 2000. Numerical phenetic study of the genus Carnobacterium. Antonie Leeuwenhoek 78:73-85. [DOI] [PubMed] [Google Scholar]
- 27.Lai, S., H. Shojaei, and L. N. Manchester. 2000. The differentiation of Carnobacterium divergens using the random amplification of polymorphic DNA polymerase chain reaction technique. Lett. Appl. Microbiol. 30:448-452. [DOI] [PubMed] [Google Scholar]
- 28.Leblond-Bourget, N., H. Philippe, I. Mangin, and B. Decaris. 1996. 16S rRNA and 16S to 23S internal transcribed spacer sequence analyses reveal inter- and intraspecific Bifidobacterium phylogeny. Int. J. Syst. Bacteriol. 46:102-111. [DOI] [PubMed] [Google Scholar]
- 29.Le Jeune, C., and A. Lonvaud-Funel. 1997. Sequence of DNA 16S/23S spacer region of Leuconostoc oenos (Oenococcus oeni): application to strain differentiation. Res. Microbiol. 148:79-86. [DOI] [PubMed] [Google Scholar]
- 30.Leroi, F., J. J. Joffraud, F. Chevalier, and M. Cardinal. 1998. Study of the microbial ecology of cold-smoked salmon during storage at 8°C. Int. J. Food Microbiol. 39:111-121. [DOI] [PubMed] [Google Scholar]
- 31.Mauguin, S., and G. Novel. 1994. Characterization of lactic acid bacteria isolated from seafood. J. Appl. Bacteriol. 76:616-625. [Google Scholar]
- 32.Metivier, A., M. F. Pilet, X. Dousset, O. Sorokine, P. Anglade, M. Zagorec, J. C. Piard, D. Marion, Y. Cenatiempo, and C. Fremaux. 1998. Divercin V41, a new bacteriocin with two disulphide bonds produced by Carnobacterium divergens V41: primary structure and genomic organization. Microbiology 144:2837-2844. [DOI] [PubMed] [Google Scholar]
- 33.Millière, J. B., M. Michael, F. Mathieu, and G. Lefebvre. 1994. Presence of Carnobacterium spp. in French surface mould-ripened soft-cheese. J. Appl. Bacteriol. 76:264-269. [Google Scholar]
- 34.Montel, M. C., R. Talon, J. Fournaud, and M. C. Champomier. 1991. A simplified key for identifying homofermentative Lactobacillus and Carnobacterium spp. from meat. J. Appl. Bacteriol. 70:469-472. [DOI] [PubMed] [Google Scholar]
- 35.Mora, D., M. Scarpellini, L. Franzetti, S. Colombo, and A. Galli. 2003. Reclassification of Lactobacillus maltaromicus (Miller et al. 1974) DSM 20342T and DSM 20344 and Carnobacterium piscicola (Collins et al. 1987) DSM 20730T and DSM 20722 as Carnobacterium maltaromaticum comb. nov. Int. J. Syst. Evol. Microbiol. 53:675-678. [DOI] [PubMed] [Google Scholar]
- 36.Naimi, A., G. Beck, and C. Branlant. 1997. Primary and secondary structures of rRNA spacer regions in enterococci. Microbiology 143:823-834. [DOI] [PubMed] [Google Scholar]
- 37.Navarro, E., P. Simonet, P. Normand, and R. Bardin. 1992. Characterization of natural populations of Nitrobacter spp. using PCR/RFLP analysis of the ribosomal intergenic spacer. Arch. Microbiol. 157:107-115. [DOI] [PubMed] [Google Scholar]
- 38.Nissen, H., A. Holck, and R. H. Dainty. 1994. Identification of Carnobacterium spp. and Leuconostoc spp. in meat by genus-specific 16S rRNA probes. Lett. Appl. Microbiol. 19:165-168. [DOI] [PubMed] [Google Scholar]
- 39.Nour, M., A. Naimi, G. Beck, and C. Branlant. 1995. 16S-23S and 23S-5S intergenic spacer regions of Streptococcus thermophilus and Streptococcus salivarius, primary and secondary structure. Curr. Microbiol. 31:270-278. [DOI] [PubMed] [Google Scholar]
- 40.Paludan-Muller, C., P. Dalgaard, H. H. Huss, and L. Gram. 1998. Evaluation of the role of Carnobacterium piscicola in spoilage of vacuum- and modified-atmosphere-packed cold-smoked salmon stored at 5°C. Int. J. Food Microbiol. 39:155-166. [DOI] [PubMed] [Google Scholar]
- 41.Pilet, M. F., X. Dousset, R. Barré, G. Novel, M. Desmazeaud, and J. C. Piard. 1995. Evidence for two bacteriocins produced by Carnobacterium piscicola and Carnobacterium divergens isolated from fish and active against Listeria monocytogenes. J. Food Prot. 58:256-262. [DOI] [PubMed] [Google Scholar]
- 42.Quadri, L. E., M. Sailer, K. L. Roy, J. C. Vederas, and M. E. Stiles. 1994. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J. Biol. Chem. 269:12204-12211. [PubMed] [Google Scholar]
- 43.Ruiz, A., M. Poblet, A. Mas, and J. M. Guillamon. 2000. Identification of acetic acid bacteria by RFLP of PCR-amplified 16S rDNA and 16S-23S rDNA intergenic spacer. Int. J. Syst. Evol. Microbiol. 50:1981-1987. [DOI] [PubMed] [Google Scholar]
- 44.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saucier, L., A. S. Paradkar, L. S. Frost, S. E. Jensen, and M. E. Stiles. 1997. Transcriptional analysis and regulation of carnobacteriocin production in Carnobacterium piscicola LV17. Gene 188:271-277. [DOI] [PubMed] [Google Scholar]
- 46.Scarpellini, M., D. Mora, S. Colombo, and L. Franzetti. 2002. Development of genus/species-specific PCR analysis for identification of Carnobacterium strains. Curr. Microbiol. 45:24-29. [DOI] [PubMed] [Google Scholar]
- 47.Schillinger, U., M. E. Stiles, and W. H. Holzapfel. 1993. Bacteriocin production by Carnobacterium piscicola LV 61. Int. J. Food Microbiol. 20:131-147. [DOI] [PubMed] [Google Scholar]
- 48.Song, Y., N. Kato, C. Liu, Y. Matsumiya, H. Kato, and K. Watanabe. 2000. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group- and species-specific primers derived from the 16S-23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol. Lett. 187:167-173. [DOI] [PubMed] [Google Scholar]
- 49.Tannock, G. W., A. Tilsala-Timisjarvi, S. Rodtong, J. Ng, K. Munro, and T. Alatossava. 1999. Identification of Lactobacillus isolates from the gastrointestinal tract, silage, and yoghurt by 16S-23S rRNA gene intergenic spacer region sequence comparisons. Appl. Environ. Microbiol. 65:4264-4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tilsala-Timisjarvi, A., and T. Alatossava. 1997. Development of oligonucleotide primers from the 16S-23S rRNA intergenic sequences for identifying different dairy and probiotic lactic acid bacteria by PCR. Int. J. Food Microbiol. 35:49-56. [DOI] [PubMed] [Google Scholar]
- 52.Trcek, J., and M. Teuber. 2002. Genetic and restriction analysis of the 16S-23S rDNA internal transcribed spacer regions of the acetic acid bacteria. FEMS Microbiol. Lett. 208:69-75. [DOI] [PubMed] [Google Scholar]
- 53.Tudor, J. J., L. Marri, P. J. Piggot, and L. Daneo-Moore. 1990. Size of the Streptococcus mutans GS-5 chromosome as determined by pulsed-field gel electrophoresis. Infect. Immun. 58:838-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wallbanks, S., A. J. Martinez-Murcia, J. L. Fryer, B. A. Phillips, and M. D. Collins. 1990. 16S rRNA sequence determination for members of the genus Carnobacterium and related lactic acid bacteria and description of Vagococcus salmoninarum sp. nov. Int. J. Syst. Bacteriol. 40:224-230. [DOI] [PubMed] [Google Scholar]
- 55.Yost, C. K., and F. M. Nattress. 2000. The use of multiplex PCR reactions to characterize populations of lactic acid bacteria associated with meat spoilage. Lett. Appl. Microbiol. 31:129-133. [DOI] [PubMed] [Google Scholar]
- 56.Young, R. A., R. Macklis, and J. A. Steitz. 1979. Sequence of the 16S-23S spacer region in two ribosomal RNA operons of Escherichia coli. J. Biol. Chem. 254:3264-3271. [PubMed] [Google Scholar]
- 57.Zavaleta, A. I., A. J. Martinez-Murcia, and F. Rodriguez-Valera. 1996. 16S-23S rDNA intergenic sequences indicate that Leuconostoc oenos is phylogenetically homogeneous. Microbiology 142:2105-2114. [DOI] [PubMed] [Google Scholar]