Abstract
Overweight/obesity is a chronic disease that carries an increased risk of hypertension, diabetes mellitus, and premature death. Several epidemiological studies have demonstrated a clear relationship between salt intake and obesity, but the pathophysiologic mechanisms remain unknown. We hypothesized that ghrelin, which regulates appetite, food intake, and fat deposition, becomes elevated when one consumes a high-salt diet, contributing to the progression of obesity. We, therefore, investigated fasting ghrelin concentrations during a high-salt diet. Thirty-eight non-obese and normotensive subjects (aged 25 to 50 years) were selected from a rural community in Northern China. They were sequentially maintained on a normal diet for three days at baseline, a low-salt diet for seven days (3 g/day, NaCl), then a high-salt diet for seven days (18 g/day). The concentration of plasma ghrelin was measured using an immunoenzyme method (ELISA). High-salt intake significantly increased fasting ghrelin levels, which were higher during the high-salt diet (320.7 ± 30.6 pg/mL) than during the low-salt diet (172.9 ± 8.9 pg/mL). The comparison of ghrelin levels between the different salt diets was statistically-significantly different (p < 0.01). A positive correlation between 24-h urinary sodium excretion and fasting ghrelin levels was demonstrated. Our data indicate that a high-salt diet elevates fasting ghrelin in healthy human subjects, which may be a novel underlying mechanism of obesity.
Keywords: ghrelin, high salt, obesity, diet intervention
1. Introduction
Obesity has serious health consequences and is, therefore, a public health concern worldwide, according to the World Health Organization. By 2014 obesity was more than double what it had been in 1980. Of the 1.9 billion overweight adults in 2014, 600 million were classified as obese. Obesity at one time was a problem only in wealthy countries, but today it is common in low- and middle-income countries as well, particularly in the cities [1]. Health dangers caused by obesity include cardiovascular disease, the leading cause of death worldwide in 2012, diabetes mellitus, osteoarthritis, other degenerative joint diseases, and some forms of cancer, in particular endometrial, breast, and colon cancer. Increasing evidence shows that in both humans and experimental animals, obesity, like hypertension, is related to high salt consumption [2,3,4,5,6,7,8,9,10,11]. The belief that elevated sodium intake is a cause of obesity appears to have its origins in the idea that salt intake may increase the desire to eat certain types of foods and beverages, thus contributing to weight gain [2,3,8]. However, the pathophysiologic mechanisms remain unresolved.
Ghrelin is a 28-amino-acid peptide with an N-octanoyl alteration at serine-3, produced mainly in the stomach, which has been identified as the endogenous ligand of the growth hormone (GH) secretagogue receptor (GHS-R) [12]. Ghrelin has various physiological functions, such as stimulating the release of growth hormone and of appetite, as well as fat accumulation. Administration of exogenous ghrelin reportedly enhances appetite and increases food intake through the activation of hypothalamic neuropeptide Y/agouti-related peptide neurons, expressing GHS-R type 1a. In addition, ghrelin controls glucose homeostasis by regulating insulin secretion and sensitivity in pancreatic beta cells. Elimination of ghrelin in obese diabetic ob/ob mice increases basal insulin concentrations, enhances glucose-stimulated insulin secretion, and improves peripheral insulin sensitivity [12,13,14,15].
Data are still scarce in the literature with regard to the link between salt intake and ghrelin. We hypothesized that ghrelin contributes to the progression of obesity during high-salt loading. In the present study, which included non-obese and normotensive subjects, we investigated whether salt intake influences fasting ghrelin levels.
2. Materials and Methods
2.1. Subjects
From a total of 50 screened, normotensive subjects with similar dietary customs from a rural community in Northern China, 38 were enrolled in this study, and 12 were excluded because of hypertension or obesity. A brief medical questionnaire was administered. Subjects with a previous history of hypertension, obesity, liver, or renal disease or with diabetes mellitus were excluded. Hypertension was defined as mean systolic blood pressure ≥140 mmHg and/or mean diastolic blood pressure ≥90 mmHg. Obesity was defined as BMI ≥ 28 kg/m2. All subjects were non-smokers. The institutional ethics committee of Xi’an Jiaotong University Medical School (Code: XJTU1AF2013LSL-056) approved the study protocol, and each subject gave written informed consent. All of the procedures were performed in accordance with institutional guidelines.
2.2. Protocol
The protocol consisted of a series of investigations, including a three-day baseline period in which a clinical history and physical examination (height, weight, and blood pressure (BP)) were obtained and subjects consumed a normal-salt diet, followed by seven days of a low-salt diet (51.3 mmol or 3 g of NaCl per day), then 7 days of a high-salt diet (307.7 mmol or 18 g of NaCl per day) [16]. During the baseline investigational period, each subject was given detailed dietary instructions to avoid table salt, cooking salt, high-sodium foods, and food rich in nitrite/nitrate for the subsequent 14 days. All meals were prepared in research kitchens and consumed onsite. Dietary total energy intake was supplied according to their baseline energy intake assessed by a brief food frequency questionnaire. Food consumption of study participants was carefully recorded at each meal by study staff members.
2.3. Biochemical Analyses
Blood glucose was measured using the glucose oxidase method. Serum lipids (total cholesterol, triglycerides, HDL-C) were measured. Blood samples for measurement of fasting plasma ghrelin concentrations were drawn with EDTA-aprotinin tubes and immediately placed on ice. All tubes were centrifuged at 4 °C for collection of plasma and stored at −80 °C until the time of analysis. Ghrelin was determined using a validated sandwich ELISA using a ghrelin-specific antibody (Wuhan USCN Science and Technology Co., Ltd., Wuhan, China). Five plasma samples were used to evaluate intra- and interassay coefficients of variation, which, for ghrelin, ranged from 2.1%–4.3% (mean, 3.2) to 6.4%–9.2% (mean, 7.8), respectively.
2.4. 24-h Urinary Sodium and Potassium Determination
Twenty-four-hour urine samples were collected at baseline and on day seven of each intervention period. Twenty-four hour urine collection was obtained with the first voided urine upon waking on the day of collection being discarded and participants then collecting all voided urine up to, and including, the first void the following morning. Participants were instructed to keep collected samples inside cooler bags provided and stored in a cool, dark place until completion when a research assistant was contacted to collect the sample. The times at the beginning and the end of urine collection were recorded. The samples were kept frozen at −40 °C until analysis. Urinary concentrations of sodium were determined using ion-selective electrodes (Hitachi, Ltd., Tokyo, Japan). The 24-h urinary excretion of sodium and potassium was calculated by multiplying the concentration of sodium and potassium, respectively, by the 24-h total urine volume.
2.5. Statistical Analysis
The data are shown as mean ± SD. Differences between biochemical markers obtained at low- and high-salt intakes were calculated by analysis of variance with the repeated measures design. Age, sex, and body mass index (BMI) were adjusted using multivariable analysis. All calculations were performed with SPSS for Windows 16.0 software (SPSS, Inc., Chicago, IL, USA). Probability was assessed using a two-tailed p value of < 0.05 to describe statistical significance.
3. Result
3.1. Profiles of Study Subjects
All enrolled subjects completed this interventional study. Their average age was 50.9 ± 1.3 years and systolic BP and diastolic BP were 110.7 ± 2.2 and 72.6 ± 1.3 mmHg, respectively (Table 1). The 24-h urinary sodium excretion averaged 173.8 mmol/day (median: 131.5), which approximates an intake of 8 g of salt/day. Daily urinary potassium excretion averaged 48 ± 0.9 mmol/day (median: 44 mmol/day).
Table 1.
Baseline demographic and clinical characteristics.
| Parameter | Values |
|---|---|
| Mean age (year) | 50.6 ± 2.1 |
| Sex (male/female) | 21/17 |
| BMI (kg/m2) | 22.8 ± 0.4 |
| Systolic BP (mmHg) | 110.6 ± 5.8 |
| Diastolic BP (mmHg) | 72.1 ± 2.7 |
| Glucose, mmol/L | 3.91 ± 0.11 |
| Total cholesterol, mmol/L | 4.18 ± 0.14 |
| Triglycerides, mmol/L | 1.32 ± 0.11 |
| LDL-cholesterol, mmol/L | 2.35 ± 0.11 |
| HDL-cholesterol, mmol/L | 1.21 ± 0.04 |
3.2. Effects of Salt Intake on BP and 24-h Urinary Sodium Excretion
Table 2 shows BP responses to the low-salt and high-salt dietary interventions. BP significantly increased with the change from the low-salt to high-salt intervention (p < 0.05). The 24-h sodium excretions in the urine were calculated at the end of the intervention period to ensure compliance with the study protocol. As shown in Table 2, urinary sodium excretion significantly decreased with the change from baseline to the low-salt diet, but increased with the change from the low-salt to the high-salt diet (all p < 0.05). These results confirmed the subjects’ compliance with the dietary intervention protocol.
Table 2.
BP Levels (mmHg) and 24-h Urinary Sodium (mmol/day) at Baseline and During Dietary Interventions.
| SBP | DBP | 24 h Urinary Na+ (mmol/Day) | |
|---|---|---|---|
| Baseline | 110.6 ± 5.8 | 72.1 ± 2.7 | 175.8 ± 11.1 |
| Low-salt diet | 108.7 ± 2.8 | 73.5 ± 2.0 | 98.8 ± 9.3 |
| High-salt diet | 116.4 ± 5.8 * | 77.3 ± 4.2 * | 268 ± 10.2 * |
* p < 0.05 versus low-salt diet.
3.3. The Effect of High-Salt Intake on Fasting Ghrelin Levels
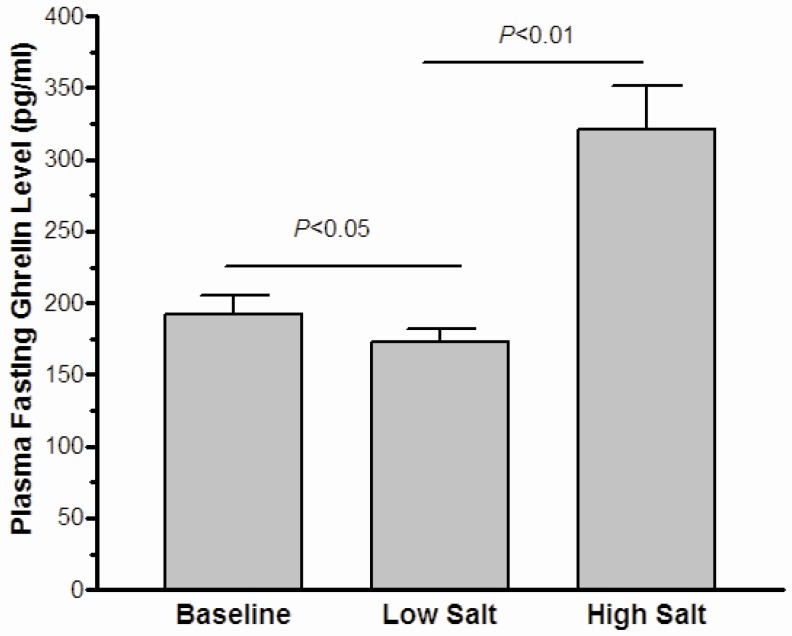
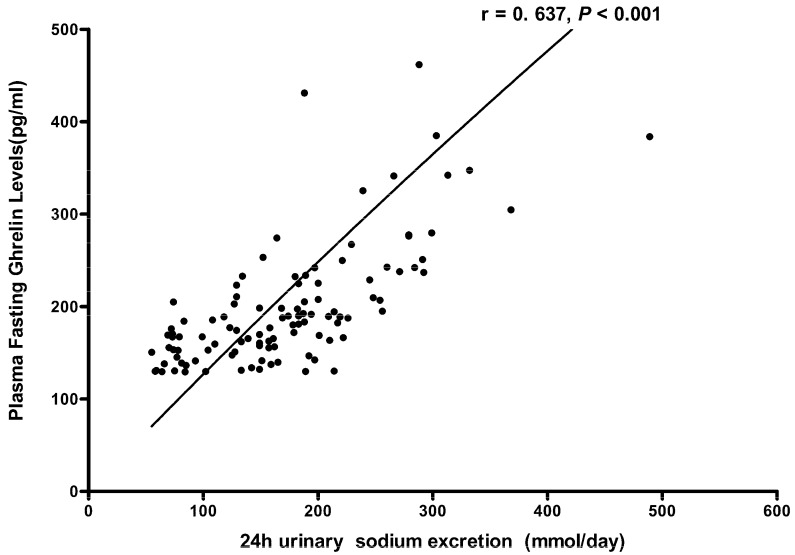
As shown in Figure 1, the statistical difference was slight in the fasting ghrelin levels between the baseline and the low-salt dietary intervention (192.4 ± 12.6 pg/mL vs. 172.9 ± 8.9 pg/mL, p = 0.037). Moreover, high-salt intake clearly enhanced plasma ghrelin levels (320.7 ± 30.6 pg/mL vs. 172.9 ± 8.9 pg/mL, p < 0.01). Further analyses (Figure 2) showed that the fasting ghrelin concentration correlated with the 24-h urinary sodium excretion during among baseline, low-salt, and high-salt dietary intervention periods (r = 0.637, p < 0.001).
Figure 1.
The effect of low-salt and high-salt intake on fasting ghrelin in all subjects.
Figure 2.
The correlation between plasma ghrelin levels and 24 h urinary sodium excretions in all subjects on baseline, a low-salt diet and on a high-salt diet.
4. Discussion
The initial finding of the present study is that high-salt intake is associated with fasting ghrelin elevation in non-obese and normotensive subjects. In addition, a positive correlation between 24-h urinary sodium excretion and fasting ghrelin levels was demonstrated in these Chinese subjects.
The well-established role of ghrelin as an important regulator of appetite and fat accumulation may be attributed to the pathogenesis of obesity. Chronic ghrelin administration increases body weight via diverse, concerted actions on food intake, energy expenditure, and fuel utilization [17,18,19,20,21,22,23]. On the contrary, congenital ablation of the ghrelin or ghrelin-receptor gene causes resistance to diet-induced obesity [24,25,26], and pharmacologic ghrelin blockade reduces food intake and body weight [21,27,28,29,30]. There may be a relationship between the genomic variation in the ghrelin gene and obesity [21,22,23,24,25,26,27,28,29,30,31,32,33]. In particular, the Leu72Met polymorphism in ghrelin was found to be associated with the early onset of obesity [31]. Ghrelin is produced primarily in the stomach, circulates in the blood, and serves as a peripheral signal, informing the arcuate nucleus of the central nervous system (via the vagus nerve) to stimulate hunger. Moreover, ghrelin also could be involved glucose metabolism through suppressing insulin secretion, which insulin plays a crucial role in obesity. It has been well documented that systemic administration of ghrelin or endogenous ghrelin could restrict glucose-induced insulin release and deteriorate glucose tolerance in vivo and in vivo [34,35,36], which leads to insulin resistance, indirectly take part in obesity. Therefore, ghrelin may possibly be a useful target against obesity.
Ghrelin may be the key to revealing the mechanisms of salt-induced obesity. Several epidemiological studies have demonstrated a clear relationship between salt intake and obesity [2,6,7], and interventional studies also show that a high-salt diet might induce subcutaneous and visceral adipose tissue [4,11]. A hypothesis has been proposed to explain the relationship between sodium intake and obesity: salty foods might be considered addictive substances that stimulate opioid receptors in the brain and the pleasure center, so that the consumption of salty foods every day produces an addiction to these foods, producing an increase in food consumption (tolerance to opiates), increased caloric intake, overweight, sedentary lifestyle, obesity, and related diseases [37,38]. In our study, we found that the high-salt diet elevated fasting ghrelin. Recently, Cai et al. reported that ghrelin is co-expressed with ENaC subunits in taste-receptor cells in fungiform papillae on the tongue and that Ghrelin/GHS-R/mice possess a significantly reduced salty taste sensitivity compared to wild-type mice [39]. Therefore, we conclude the salt-induced ghrelin increase may be a novel mechanism of obesity via informing the arcuate nucleus of the central nervous system to stimulate hunger.
Our study has several important strengths. First of all, the subjects were recruited from a rural community and were similar with respect to lifestyle and environmental risk factors, including diet and physical activity. Thus, confounding due to these exposures should be minimized. Moreover, participants with body mass index (BMI) more than 28 kg/m2 was excluded, and adjustment for BMI was conducted; therefore, confounding effects of BMI probably had no influence on salt intake and ghrelin. Participation in the dietary interventions was high, and compliance with the study interventions, as assessed by urinary excretion of sodium during each intervention period, was excellent.
The present study has some limitations that should be addressed. Because all of the subjects were recruited from the Chinese population, whether or not our observation could be generalized to other racial populations is unknown. Further studies are required to validate our findings in a larger and more diverse sample and to elucidate the mechanisms by which salt loading affects plasma ghrelin. Meanwhile, insulin resistance could be involved in salt-induced obesity, and further studies will be needed to reveal the relationship between ghrelin and insulin during high-salt diet.
5. Conclusions
In conclusion, our human intervention study found that salt loading could increase circulating ghrelin production in normotensive Asian subjects. Our findings indicate that the elevation of ghrelin might be the underlying mechanism of salt-induced obesity, which sheds some new light on prevention and a possible therapeutic target for obesity in the future.
Acknowledgments
This study was supported by grant 81200512 (to Liu) from the Natural Science Foundation of China.
Author Contributions
F.-Q.L. and J.-J.M. conceived and designed the experiments; J.-J.M. was responsible for subject recruitment; F.-Q.L., K.-Y.R., T.-S.G., Y.W., C.C., D.W., Y.Z. and F.-X.L. performed the experiments; Y.Z. J.-K.W., G.-C.G. and F.-X.L. analyzed the data; Y.Z. wrote the paper. All authors read, critically revised and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.World Health Organization Obesity and Overweight. Fact sheet N°311. [(accessed on 27 July 2015)]. Updated January 2015. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/
- 2.Navia B., Aparicio A., Perea J.M., Pérez-Farinós N., Villar-Villalba C., Labrado E., Ortega R.M. Sodium intake may promote weight gain: Results of the FANPE study in a representative sample of the adult Spanish population. Nutr. Hosp. 2014;29:1283–1289. doi: 10.3305/nh.2014.29.6.7361. [DOI] [PubMed] [Google Scholar]
- 3.Fowler S.P., Williams K., Resendez R.G., Hunt K.J., Hazuda H.P., Stern M.P. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity. 2008;16:1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- 4.Zhou X., Yuan F., Ji W.J., Guo Z.Z., Zhang L., Lu R.Y., Liu X., Liu H.M., Zhang W.C., Jiang T.M., et al. High-salt intake induced visceral adipose tissue hypoxia and its association with circulating monocyte subsets in humans. Obesity. 2014;22:1470–1476. doi: 10.1002/oby.20716. [DOI] [PubMed] [Google Scholar]
- 5.Baudrand R., Campino C., Carvajal C.A., Olivieri O., Guidi G., Faccini G., Vöhringer P.A., Cerda J., Owen G., Kalergis A.M., et al. High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin. Endocrinol. 2014;80:677–684. doi: 10.1111/cen.12225. [DOI] [PubMed] [Google Scholar]
- 6.Yoon Y.S., Oh S.W. Sodium density and obesity; The Korea National Health and Nutrition Examination Survey 2007–2010. Eur. J. Clin. Nutr. 2013;67:141–146. doi: 10.1038/ejcn.2012.204. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann I.S., Cubeddu L.X. Salt and the metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2009;19:123–128. doi: 10.1016/j.numecd.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Cocores J.A., Gold M.S. The Salted Food Addiction Hypothesis may explain overeating and the obesity epidemic. Med. Hypotheses. 2009;73:892–899. doi: 10.1016/j.mehy.2009.06.049. [DOI] [PubMed] [Google Scholar]
- 9.Yuan F., Guo Z.Z., Ji W.J., Ji W.J., Ma Y.Q., Zhang Z.L., Zhou X., Li Y.M. BOLD-MRI evaluation of subcutaneous and visceral adipose tissue oxygenation status: effect of dietary salt intake. Am. J. Transl. Res. 2015;7:598–606. [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Y., He F.J., MacGregor G.A. High salt intake: Independent risk factor for obesity? Hypertension. 2015;66:843–849. doi: 10.1161/HYPERTENSIONAHA.115.05948. [DOI] [PubMed] [Google Scholar]
- 11.Lee M., Kim M.K., Kim S.M., Park H., Park C.G., Park H.K. Gender-based differences on the association between salt-sensitive genes and obesity in Korean children aged between 8 and 9 years. PLoS ONE. 2015;10:323. doi: 10.1371/journal.pone.0120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima M., Hosoda H., Date Y., Nakazato M., Matsui H., Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 13.Sato T., Ida T., Nakamura Y., Shiimura Y., Kangawqa K., Kojima M. Physiological roles of ghrelin on obesity. Obes. Res. Clin. Pract. 2014;8:e405–e413. doi: 10.1016/j.orcp.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Sato T., Nakamura Y., Shiimura Y., Ohgusu H., Kangawa K., Kojima M. Structure, regulation and function of ghrelin. J. Biochem. 2012;151:119–128. doi: 10.1093/jb/mvr134. [DOI] [PubMed] [Google Scholar]
- 15.Wiedmer P., Nogueiras R., Broglio F., D’Alessio D., Tschöp M.H. Ghrelin, obesity and diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2007;3:705–712. doi: 10.1038/ncpendmet0625. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Liu F.Q., Wang D., Chu C., Mu J.J., Ren K.Y., Guo T.S., Chu C., Wang L., Geng L.K., et al. Effect of salt intake and potassium supplementation on serum renalase levels in Chinese adults: A randomized trial. Medicine. 2014;93:e44. doi: 10.1097/MD.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings D.E. Ghrelin and the short-and long-term regulation of appetite and body weight. Physiol. Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Qi Y., Inoue K., Fu M., Inui A., Herzog H. Chronic overproduction of ghrelin in the hypothalamus leads to temporal increase in food intake and body weight. Neuropeptides. 2015;50:23–28. doi: 10.1016/j.npep.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Nass R., Pezzoli S.S., Oliver M.C., Patrie J.T., Harrell F.E., Clasey J.L., Heymsfield S.B., Bach M.A., Vance M.L., Thorner M.O. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: A randomized trial. Ann. Intern. Med. 2008;149:601–611. doi: 10.7326/0003-4819-149-9-200811040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tschop M., Smiley D.L., Heiman M.L. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- 21.Nakazato M., Murakami N., Date Y., Kojima M., Matsuo H., Kangawa K., Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 22.Wren A.M., Small C.J., Abbott C.R., Dhillo W.S., Seal L.J., Cohen M.A., Batterham R.L., Taheri S., Stanley S.A., Ghatei M.A., et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- 23.Keen-Rhinehart E., Bartness T.J. Peripheral ghrelin injections stimulate food intake, foraging and food hoarding in Siberian hamsters. Am. J. Physiol. 2005;288:R716–R722. doi: 10.1152/ajpregu.00705.2004. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y., Wang P., Zheng H., Smith R.G. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc. Natl. Acad. Sci. USA. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wortley K.E., Anderson K.D., Garcia K., Murray J.D., Malinova L., Liu R., Moncrieffe M., Thabet K., Cox H.J., Yancopoulos G.D., et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc. Natl. Acad. Sci. USA. 2004;101:8227–8232. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y., Ahmed S., Smith R.G. Deletion of ghrelin impairs neither growth nor appetite. Mol. Cell. Biol. 2003;23:7973–7981. doi: 10.1128/MCB.23.22.7973-7981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asakawa A., Inui A., Kaga T., Katsuura G., Fujimiya M., Fujino M.A., Kasuga M. Antagonism of ghrelin receptor reduces food intake and body weight gain in mice. Gut. 2003;52:947–952. doi: 10.1136/gut.52.7.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shuto Y., Shibasaki T., Otagiri A., Kuriyama H., Ohata H., Tamura H., Kamegai J., Sugihara H., Oikawa S., Wakabayashi I. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J. Clin. Investig. 2002;109:1429–1436. doi: 10.1172/JCI0213300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagnasco M., Tulipano G., Melis M.R., Argiolas A., Cocchi D., Muller E.E. Endogenous ghrelin is an orexigenic peptide acting in the arcuate nucleus in response to fasting. Regul. Pept. 2003;111:161–167. doi: 10.1016/S0167-0115(02)00283-5. [DOI] [PubMed] [Google Scholar]
- 30.Murakami N., Hayashida T., Kuroiwa T., Nakahara K., Ida T., Mondal M.S., Nakazato M., Kojima M., Kangawa K. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J. Endocrinol. 2002;174:283–288. doi: 10.1677/joe.0.1740283. [DOI] [PubMed] [Google Scholar]
- 31.Del Giudice M., Santoro N., Cirillo G., Raimondo P., Grandone A., D’Aniello A., Di Nardo M., Perrone L. Molecular screening of the ghrelin gene in Italian obese children: The Leu72Met variant is associated with an earlier onset of obesity. Int. J. Obes. 2004;28:447–450. doi: 10.1038/sj.ijo.0802572. [DOI] [PubMed] [Google Scholar]
- 32.Pöykkö S., Ukkola O., Kauma H., Savolainen M.J., Kesäniemi Y.A. Ghrelin Arg51Gln mutation is a risk factor for Type 2 diabetes and hypertension in a random sample of middle-aged subjects. Diabetologia. 2003;46:455–458. doi: 10.1007/s00125-003-1058-z. [DOI] [PubMed] [Google Scholar]
- 33.Ukkola O., Ravussin E., Jacobson P., Pérusse L., Rankinen T., Tschöp M., Heiman M.L., Leon A.S., Rao D.C., Skinner J.S., et al. Role of ghrelin polymorphisms in obesity based on three different studies. Obes. Res. 2002;10:782–791. doi: 10.1038/oby.2002.106. [DOI] [PubMed] [Google Scholar]
- 34.Reimer M.K., Pacini G., Ahrén B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;44:916–921. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- 35.Dezaki K., Hosoda H., Kakei M., Hashiguchi S., Watanabe M., Kangawa K., Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: Implication in the glycemic control in rodents. Diabetes. 2004;53:3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- 36.Tong J., Prigeon R.L., Davis H.W., Bidlingmaier M., Kahn S.E., Cummings D.E., Tschöp M.H., D’Alessio D. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Na E.S., Morris M.J., Johnson A.K. Opioid mechanisms that mediate the palatability of and appetite for salt in sodium replete and deficient states. Physiol. Behav. 2012;106:164–170. doi: 10.1016/j.physbeh.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolhuis D.P., Costanzo A., Newman L.P., Keast R.S. Salt Promotes Passive Overconsumption of Dietary Fat in Humans. J. Nutr. 2016;146:838–845. doi: 10.3945/jn.115.226365. [DOI] [PubMed] [Google Scholar]
- 39.Cai H., Cong W.N., Daimon C.M., Wang R., Tschöp M.H., Sévigny J., Martin B., Maudsley S. Altered lipid and salt taste responsivity in ghrelin and GOAT null mice. PLoS ONE. 2013;8:323. doi: 10.1371/journal.pone.0076553. [DOI] [PMC free article] [PubMed] [Google Scholar]