Abstract
Model Cheddar cheeses were prepared from pasteurized milk artificially contaminated with high 104 to 105 CFU/ml) and low (101 to 102 CFU/ml) inocula of three different Mycobacterium paratuberculosis strains. A reference strain, NCTC 8578, and two strains (806PSS and 796PSS) previously isolated from pasteurized milk for retail sale were investigated in this study. The manufactured Cheddar cheeses were similar in pH, salt, moisture, and fat composition to commercial Cheddar. The survival of M. paratuberculosis cells was monitored over a 27-week ripening period by plating homogenized cheese samples onto HEYM agar medium supplemented with the antibiotics vancomycin, amphotericin B, and nalidixic acid without a decontamination step. A concentration effect was observed in M. paratuberculosis numbers between the inoculated milk and the 1-day old cheeses for each strain. For all manufactured cheeses, a slow gradual decrease in M. paratuberculosis CFU in cheese was observed over the ripening period. In all cases where high levels (>3.6 log10) of M. paratuberculosis were present in 1-day cheeses, the organism was culturable after the 27-week ripening period. The D values calculated for strains 806PSS, 796PSS, and NCTC 8578 were 107, 96, and 90 days, respectively. At low levels of contamination, M. paratuberculosis was only culturable from 27-week-old cheese spiked with strain 806PSS. M. paratuberculosis was recovered from the whey fraction in 10 of the 12 manufactured cheeses. Up to 4% of the initial M. paratuberculosis load was recovered in the culture-positive whey fractions at either the high or low initial inoculum.
Mycobacterium paratuberculosis is the etiologic agent of paratuberculosis (Johne's disease), a chronic granulomatous enteritis in ruminants and other animals (7, 8). The organism is excreted in the feces of animals with the pluribacillary form of the disease; colostrum and milk can also be infected systemically (40, 43) or contaminated with feces during the milking process. Crohn's disease, an intestinal inflammatory disorder that affects humans, shows many similarities to Johne's disease, both affecting the ileum and causing chronic diarrhea and weight loss. M. paratuberculosis has been isolated from humans suffering from Crohn's disease (4, 5, 6, 12, 44). However, when Johne's and Crohn's diseases are compared clinically and pathologically, several significant differences can be seen. These similarities and differences have been interpreted both in favor and in opposition to the view that M. paratuberculosis is a cause of Crohn's disease (13). In response to this, the United Kingdom government has advocated a precautionary approach and has supported actions to minimize exposure of the public to M. paratuberculosis.
Raw milk from an M. paratuberculosis-infected dairy herd is likely to be contaminated with M. paratuberculosis through direct excretion into the milk or via fecal contamination (32). Sweeney et al. (43) reported low levels (2 to 8 CFU/50 ml) of viable M. paratuberculosis cells present in milk from cows with clinical Johne's disease or asymptomatically infected cows in the latter stages of infection, whereas clinically infected animals can shed up to 1012 cells per day in feces (9). Therefore, raw cows' milk may act as a source of the bacterium for humans if control measures such as pasteurization are ineffective in killing this pathogen.
M. paratuberculosis is more resistant to adverse conditions such as low pH, high temperature, and salt than most other pathogenic bacteria (42). Several studies investigating the heat resistance of M. paratuberculosis have demonstrated the ineffectiveness of standard pasteurization regimens, including high-temperature, short-time pasteurization, to totally kill M. paratuberculosis in artificially infected raw milk (7, 15, 16, 23, 26, 30, 38, 41). M. paratuberculosis has also been cultured from naturally infected cows' milk after commercial-scale pasteurization (19). Furthermore, a more recent study has provided firm evidence of the presence of viable M. paratuberculosis in commercially pasteurized cows' milk manufactured for retail sale (18). Therefore, if M. paratuberculosis is present in raw milk and is not effectively inactivated by pasteurization, there is a possibility that the bacterium may exist in a viable form in other dairy products, such as cheese, manufactured from such milk.
Since the inefficacy of commercial high-temperature, short-time pasteurization to completely inactivate this potentially human-pathogenic mycobacterium has been demonstrated, efforts have concentrated on modifying the time and temperature parameters of pasteurization to improve its lethality for M. paratuberculosis in liquid milk (19, 22, 28). However, these modifications, while appropriate for liquid milk, may affect Cheddar cheese yield and textural properties (14), and therefore it is likely that standard pasteurization conditions (72°C for 15 s) or perhaps even those with a lower lethality will continue to be used for milk used in Cheddar cheese production.
In addition, M. paratuberculosis cells typically occur as clumps (20), which are dispersed to some extent during homogenization of retail milk prior to pasteurization (28). It has been suggested that declumped M. paratuberculosis was more susceptible to heat treatment (17). However, during Cheddar cheese making, liquid milk is not normally homogenized prior to pasteurization, which could increase the likelihood of clumped M. paratuberculosis cells being present in cheese milk. Also, during hard cheese manufacture, a major portion of the microbial load will be entrapped in the milk coagulum and during syneresis will be further concentrated in the cheese curd (35). Therefore, if viable M. paratuberculosis cells were to survive pasteurization, they would be concentrated in the cheese curd.
The inactivation of M. paratuberculosis in cheese milk will also depend on the environmental conditions of Cheddar manufacture and maturation vis à vis cheese cooking temperature, low moisture, low pH, salt content, prolonged low-temperature ripening, and the antagonistic effect of cheese starter and nonstarter microflora. The in vitro effect of these parameters on M. paratuberculosis survival cannot predict the persistence of M. paratuberculosis during the dynamic of Cheddar manufacture and ripening. To assess the cumulative effect of these conditions on the persistence of M. paratuberculosis, it is necessary to manufacture Cheddar cheese on a laboratory scale to simulate commercial production. There has been no published research on the survival of M. paratuberculosis in Cheddar cheese, the major hard cheese produced worldwide.
To our knowledge, only two studies have investigated M. paratuberculosis survival in cheese (35, 42). One prepared a soft Hispanic-style cheese under laboratory conditions (35), and the former used a pilot-scale process to manufacture model hard (Swiss Emmentaler) and semihard (Swiss Tisliter) cheese (42). Both of these cheeses have distinct characteristics that differ from Cheddar. The Hispanic-style soft cheese has higher water content, higher pH, and a lower maximum temperature during manufacture than Cheddar. The Swiss Emmentaler cheese, although a hard cheese, differs from Cheddar through lower salt content, higher cheese cooking temperature, and the inclusion of propionic acid bacteria in its manufacture. The calculated D value for the Hispanic-style soft cheese was 59.9 days compared to 27.8 days for Swiss hard cheese and 45.5 days for Swiss semihard cheese.
The aim of this work was to study the persistence of M. paratuberculosis during the manufacture and ripening of Cheddar cheese with a laboratory-scale Cheddar cheese production system, simulating commercial Cheddar production.
MATERIALS AND METHODS
M. paratuberculosis strains.
Three strains of M. paratuberculosis were tested: type strain NCTC 8578 (obtained from the National Collection of Type Cultures, London, United Kingdom) and strains 806PSS and 796PSS, which were originally isolated from pasteurized cows' milk (17) (kindly provided by I. Grant, Department of Food Science, Queens University of Belfast, Belfast, United Kingdom). The field isolates originated from different areas within the United Kingdom, and although they showed similar pulsed-field gel electrophoresis profiles, the strains were distinguishable by restriction fragment length polymorphism typing (I. Grant, personal communication).
Preparation of inoculum for cheese making.
Each strain was cultured in Middlebrook 7H9 broth medium containing 10% (vol/vol) Middlebrook OADC (oleic acid-albumin-dextrose-catalase; Becton Dickinson United Kingdom Ltd., Oxford, United Kingdom), 0.5% (vol/vol) Tween 80 (Sigma, Dorset, United Kingdom), and 0.0002% (wt/vol) mycobactin J (Synbiotics Europe SAS, Lyon, France) from 6-week-old (37°C) colonies grown on Herrold's egg yolk medium (HEYM) containing 2 μg of mycobactin J/ml (Synbiotics Europe SAS, Lyon, France). Culture broths (100 ml) were incubated at 37°C for 8 to 10 weeks. Cell suspensions were agitated with a Vortex Genie 2 (Scientific Industries Inc., Bohemia, N.Y.) for 3 min to disperse large clumps of M. paratuberculosis and decimally diluted in maximum recovery diluent (Oxoid, Basingstoke, United Kingdom) to achieve the desired inoculation level. Agitation of the cell suspension and its subsequent dilution were performed immediately before inoculation of the pasteurized cheese milk.
Origin of milk and testing.
A mixture of evening and morning raw bovine milk from a local dairy farm was used for cheese making. All milk was pasteurized (72°C for a minimum of 15 s) on-farm and poured into sealed pint glass bottles. Cheese making with the whole-milk samples was performed on the same day as pasteurization. Milk samples were maintained at or below 5°C between pasteurization and commencement of cheese making. All milk was subjected to phosphatase testing by the Aschaffenberg-Mullen method (1) to verify pasteurization and tested for the presence of inhibitory substances with the Delvotest SP (DSM Food Specialties, Delft, The Netherlands). The titratable acidity of milk and whey was used to monitor the manufacturing process (34). Milk samples were tested for the presence of M. paratuberculosis on the test media discussed below.
Cheddar cheese-making facility.
Cheddar cheeses were manufactured according to traditional procedures (34) with the cheese-making apparatus described by Banks and Muir (3). A rectangular stainless steel vat (305 by 200 by 200 mm, 10-liter capacity), with a centrally sloping floor, was fitted with a motorized paddle to stir the milk during cheese making. The vat was completely enclosed within a stainless steel jacket with a uniform 25-mm space all round through which water was circulated by an electronically controlled flow heater (Julabo F34; Jencons, Leighton Buzzard, United Kingdom). Cheese knives and a cheddaring box similar to those used by Banks and Muir (3) were used for the coagulum cutting and cheddaring processes, respectively. Strict safety precautions were taken to prevent any infection of personnel, contamination of the environment, and cross-contamination between trials. Cheddar manufacture and ripening were performed in a containment level 2 laboratory, and the cheese vat was located in a class 1 biological safety cabinet (200 by 70 by 60 cm; Rayair, Mach-Aire, Bolton, United Kingdom).
Manufacture and ripening of cheese.
A total of 12 M. paratuberculosis-spiked Cheddar cheeses were manufactured. For each of the three M. paratuberculosis strains, duplicate cheeses were prepared at high (104 to 105 CFU/ml) and low (101 to 102 CFU/ml) inoculation levels. Two control cheeses were prepared without artificial M. paratuberculosis contamination. Eight liters of pasteurized milk (4°C) was added to the cheese-making vat and inoculated with 8 ml of M. paratuberculosis culture. The milk was warmed to 32°C over a 45-min period, and the mesophilic/thermophilic homofermentative starter culture YY80 (Chr. Hansen, Berkshire, United Kingdom) was added to a final concentration of 0.009% (vol/vol). Starter inoculum was prepared by dispersing 10 g of frozen direct vat set culture in 90 ml of maximum recovery diluent. Rennet (Hannilase XL 205; Chr. Hansen) was added, after appropriate acid development, to induce coagulation. Curds were cut into cubes (approximately 4 by 4 by 8 mm), and the temperature was increased linearly to 40°C over 40 min. The temperature of the vat was maintained at 40°C for a further 60 min.
The cheese curd was allowed to settle (pitched), and the whey was drained. Cheddaring of the curd was performed first by manual folding of the curd mass, followed by progressive application of weights up to 16 kg over 60 min. The curd was milled by cutting into cubes (30 by 20 by 20 mm) and salted (2.5%, wt/wt). Cheeses were pressed in a 10-cm-diameter form in a Moorlands cheese press (type S2; Moorlands Cheesemakers, Somerset, United Kingdom) at a pressure of 50 kPa for 16 h at 10°C. The pressed curd was removed from the molds, sampled for microbiological analysis (see below), vacuum sealed in food-compatible polyamide-polyethylene pouches (Somerville Packaging, Lisburn, N. Ireland), vacuum packed (Mini Jumbo; Henkelman Vacuum Systems, Hertogenbosch, The Netherlands), and stored at 10°C for up to 27 weeks.
Sampling, culture, and enumeration.
A 50-ml portion of pasteurized cheese milk was taken before adding the M. paratuberculosis inoculum to check for natural contamination of the milk with M. paratuberculosis. The presence or absence of viable cells was confirmed by the decontamination and culture method, while immunomagnetic separation-PCR was used to confirm the presence or absence of live or dead cells in the pasteurized cheese milk (18).
During cheese manufacture, 10-ml aliquots of contaminated pasteurized milk and drained whey were used to determine the contamination level of the cheese milk and whey. Decimal dilutions of milk were prepared in maximum recovery diluent and inoculated onto the media described below.
Cheese samples were taken after 1 day and 3, 10, 18, and 27 weeks of ripening. The vacuum-sealed cheeses were unpacked, and two 2.5-g samples were taken with a cheese borer (1 by 10 cm). Cheeses were vacuum sealed again within 10 min. Five grams of cheese plug was aseptically transferred into a sterile stomacher bag containing 45 ml of cheese diluent. The cheese diluent comprised 0.5% (wt/vol) sodium chloride, 1% (wt/vol) Casitone (Difco, Detroit, Mich.), and 2.0% (wt/vol) sodium citrate. The mixture was homogenized in a Stomacher peristaltic Lab Blender 400 (Seward Medical, London, United Kingdom) for 2 min, heat sealed, and incubated for 1 h submerged in a water bath at 37°C. The resultant suspension was decimally diluted in maximum recovery diluent prior to microbiological analysis.
All liquid samples were inoculated (200 μl) onto two slopes of each of the following media: HEYM supplemented with an antibiotic cocktail containing vancomycin (8.4 μg/ml), amphotericin B (16.8 μg/ml), and nalidixic acid (25 μg/ml) (all from Sigma) (HEYM-VAN); 7H10-PANTA agar consisting of Middlebrook 7H10 agar base (Difco) supplemented with 10% (vol/vol) Middlebrook OADC enrichment, 2.0% (vol/vol) reconstituted PANTA Plus antibiotic supplement (polymyxin, amphotericin B, nalidixic acid, trimethoprim, and azlocillin; Becton Dickinson United Kingdom Ltd., Oxford, United Kingdom), and 0.5% glycerol. Two 0.5-ml aliquots of the cheese suspension were also injected into vials of Bactec 12B radiometric medium supplemented with 50 μl of reconstituted PANTA Plus antibiotic supplement (both from Becton Dickinson United Kingdom Ltd.). All culture media were supplemented with 2 μg mycobactin J ml−1. All media were incubated at 37°C for up to 12 weeks, and colonies typical of M. paratuberculosis on the agar medium were counted. A representative number of typical and atypical colonies were confirmed by Ziehl-Neelsen acid-fast staining and IS900 PCR (32). Bactec vials were read regularly on a Bactec 460TB instrument (Becton Dickinson United Kingdom), and growth index values were recorded.
Starter lactic acid bacteria were enumerated with MRS agar (Oxoid, Basingstoke, United Kingdom), and Rogosa agar (Oxoid) was used to monitor changes in lactobacilli throughout the ripening period. Both media were incubated at 30°C and 5% CO2 for a minimum of 48 h.
Cheese compositional analysis.
Standard procedures as described by Kirk and Sawyers (27) were used to analyze cheeses for pH, moisture, salt, and fat (Gerber method). Cheese plugs were decontaminated by irradiation prior to chemical analysis to ensure the safety of personnel undertaking the chemical analysis. Irradiation was performed to a dose of 25 kGy with a Gammabeam 650 facility (Nordion International Inc. Canada) available on site.
Statistical analysis.
Survival D values were calculated from the slope of the trend analysis line generated in Microsoft Excel (version 9.0) by plotting the log10 number of M. paratuberculosis per gram of cheese versus the ripening time. Analysis of variance was performed with Genstat Release 6.1 (for Windows 2000).
RESULTS
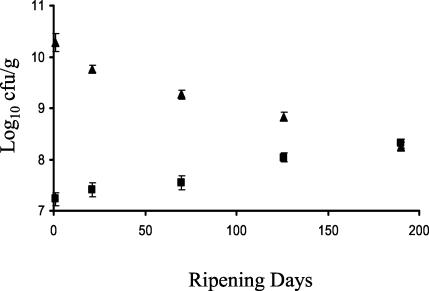
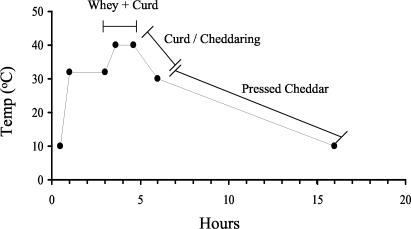
The manufactured Cheddar cheeses were similar in salt, moisture, and fat composition to commercial Cheddar (Table 1). The average pH value (5.21) was also similar to that of the commercial product. The design and health and safety measures in place for the laboratory-scale manufacturing process prevented cross-contamination between batches. The contamination of cheese milk with M. paratuberculosis did not appear to affect intrinsic Cheddar properties such as pH, fat, and moisture or the yield of cheese produced. All manufactured cheeses were within the specified parameters for first- or second-grade Cheddar. Starter lactic acid bacteria decreased slowly over the 27-week ripening period for all manufactured cheeses (Fig. 1). The titratable acidity values and cooking time and temperature parameters (Fig. 2), monitored throughout the manufacturing process were similar to those of commercial Cheddar manufacture.
TABLE 1.
Compositional analysis of model Cheddar cheese artificially contaminated with M. paratuberculosis
| Parametera | Value by M. paratuberculosis strain
|
Value by inoculum level
|
||||||
|---|---|---|---|---|---|---|---|---|
| 806PSS | 796PSS | NCTC 8578 | Control | SEMb | High | Low | SEM | |
| Yield (g) | 761 | 778 | 791 | 781 | 11.8 NS | 778 | 775 | 9.6 NS |
| pH | 5.20 | 5.20 | 5.21 | 5.21 | 0.017 NS | 5.21 | 5.20 | 0.014 NS |
| Moisture (%) | 37.5 | 37.4 | 37.5 | 38.0 | 0.24 NS | 37.1 | 37.8 | 0.20* |
| Fat (%) | 31.0 | 32.5 | 32.8 | 31.8 | 0.40 NS | 32.3 | 31.9 | 0.31 NS |
| Salt (%) | 1.53 | 1.51 | 1.61 | 1.52 | 0.03 NS | 1.55 | 1.55 | 0.02 NS |
| Nonfat (%) | 69.3 | 67.5 | 67.3 | 68.1 | 0.43* | 67.7 | 68.3 | 0.35 NS |
| Dry matter (%) | 62.3 | 63.1 | 63.0 | 62.0 | 0.42 NS | 62.9 | 62.7 | 0.34 NS |
| % Salt:moisture | 4.1 | 4.0 | 4.3 | 4.0 | 0.07 NS | 4.2 | 4.1 | 0.06 NS |
| % Moisture nonfat substance | 54.1 | 55.4 | 55.7 | 55.7 | 0.35* | 54.7 | 55.4 | 0.28 NS |
| % Fat:dry matter | 49.7 | 51.5 | 52.0 | 51.3 | 0.65 NS | 51.4 | 50.8 | 0.53 NS |
For % salt:moisture, the suggested range of percentages for first-grade cheeses was 52 to 54%; for second-grade cheeses, 50 to 56%. For % moisture nonfat substance, the suggested range of percentages for first-grade cheeses was 4.7 to 5.7%; for second-grade cheeses, 4.0 to 6.0%. For % fat:dry matter, the suggested range of percentages for first-grade cheeses was 52 to 56%; for second-grade cheeses, 50 to 57%.
P > 0.05 was considered not significant (NS). *, P < 0.05; **, P < 0.01.
FIG. 1.
Changes in lactic acid bacteria (triangles) and lactobacilli (squares) numbers during Cheddar cheese ripening. Error bars show standard deviation.
FIG. 2.
Temperature changes during the first 16 h of Cheddar cheese ripening.
Although all cheeses and cheese milk samples were inoculated onto HEYM/VAN and 7H10/PANTA agar media without a decontamination step, only the former was used to enumerate M. paratuberculosis. Counts of M. paratuberculosis could not be obtained from a number of 7H10/PANTA slopes due either to contamination within 6 weeks of incubation or to the absence of growth. In both cases, M. paratuberculosis could be enumerated on the corresponding HEYM/VAN slopes. Where growth occurred on both agar media, colony counts were similar. On HEYM/VAN slopes, CFU were countable at 6 to 8 weeks, and the numbers did not change with prolonged incubation.
The number of M. paratuberculosis in the 1-day-old cheeses (per gram) was greater than the levels detected in the inoculated milk for all strains tested (Table 2). This concentration occurred when both high (104 to 105 CFU/ml) and low (101 to 102 CFU/ml) levels were present in the pasteurized cheese milk. This increase in counts, due to the physical concentration of bacteria in the cheese curd, was significantly (P < 0.05) higher for cheese contaminated with strains 806PSS and 796PSS compared to NCTC 8578. The average concentration effect for strains 806PSS, 796PSS, and NCTC 8578 are 11.7-, 9.3-, and 4.2-fold, respectively.
TABLE 2.
Changes in organism numbersa
| Strain inoculated or inoculum level |
M. paratuberculosis count (log10 CFU/ml or g)
|
Concn effect (log10) | |
|---|---|---|---|
| Inoculated milk | Cheese (24 h) | ||
| Strain | |||
| 806PSS | 3.0 | 4.0 | 1.0 |
| 796PSS | 3.1 | 4.1 | 0.9 |
| NCTC 8578 | 2.6 | 3.1 | 0.6 |
| SEM | 0.70 NS | 0.12 NS | 0.11* |
| Inoculum | |||
| High | 4.0 | 4.7 | 0.8 |
| Low | 1.8 | 2.7 | 0.9 |
| SEM | 0.05 NS | 0.10 NS | 0.09 NS |
See Table 1, footnote b.
Figure 3 shows the survival of each of the three M. avium subsp. paratuberculosis strains at both high and low initial inocula over a 27-week ripening period. For all manufactured cheeses, a slow gradual decrease in M. paratuberculosis CFU/g of cheese was observed over the ripening period. In all cases where high levels (>3.6 log10) of M. paratuberculosis were present in 1-day cheeses, the organism was culturable after the 27-week ripening period. D values were calculated for cheeses spiked with high levels of M. paratuberculosis. The D values for strains 806PSS, 796PSS, and NCTC 8578 were 107, 96, and 90 days, respectively. However, the correlation of the trend analysis line for strain NCTC 8578 was poorer than that of the other strains at the high inocula. At these inocula, M. paratuberculosis was confirmed in the cheeses throughout the ripening period by the radiometric culture method.
FIG. 3.
Survival of M. paratuberculosis strains 806PSS (a), 796PSS (b), and NCTC 8578 (c) during Cheddar cheese ripening. Data are for each single model cheese at high and low initial inocula with trend analysis line applied. Open symbols, cheese 1; solid symbols, cheese 2. The dashed line indicates the detection limit.
At low levels of contamination, M. paratuberculosis was only culturable from 27-week-old cheese spiked with strain 806PSS. Despite the fact that cheeses containing low levels of strain 796PSS or strain 806PSS had similar levels of M. paratuberculosis at the start of the ripening period, counts of the former fell below the detection limit of 1.35 log10 CFU/ml after 27 weeks of ripening. The number in cheeses contaminated with low levels of the reference strain NCTC 8578 also fell below the detection limit after 27 weeks of ripening, although starting levels in 1-day-old cheeses were lower than in both the other strains. At 27 weeks of ripening, one of the cheeses containing low levels of NCTC 8578 produced a growth index reading on Bactec 12B radiometric culture medium, indicative of M. paratuberculosis, but a positive identification could not be confirmed by subculture and growth on HEYM. Negative results from the radiometric culture were consistent with the absence of M. paratuberculosis in parallel samples that were cultured. After 18 weeks of ripening, strain NCTC 8578 (at the low inoculum) was detectable at the minimum level of detection in the replicate cheeses. M. paratuberculosis was only recovered from one of the cheeses contaminated with strain 796PSS at low levels after 18 weeks of ripening.
M. paratuberculosis was recovered from the whey fraction in 10 of the 12 cheeses manufactured from spiked milk (Table 3). The organism was not culturable from whey released from either of the cheeses contaminated with low levels of the reference strain NCTC 8578 but was detectable in both cheeses by the radiometric culture method. For both cheeses contaminated with high levels of this strain, less than 1% of the initial M. paratuberculosis load in cheese milk was recovered in the whey fraction. In the remaining manufactured cheeses, 1 to 4% of the initial M. paratuberculosis load was recovered in the whey fraction.
TABLE 3.
Recovery of M. paratuberculosis from the whey fraction released during Cheddar cheese manufacture
| Strain (inoculum level) |
M. paratuberculosis in wheya
|
|
|---|---|---|
| HEYM/VAN, log10 CFU/ml (SEM) | Bactec 12B | |
| 806PSS (high) | 2.7 (0.07) | + |
| 806PSS (low) | 0.4 (0.00) | + |
| 796PSS (high) | 2.2 (0.11) | + |
| 796PSS (low) | 0.8 (0.11) | + |
| NCTC8578 (high) | 1.0 (0.29) | + |
| NCTC8578 (low) | ND | + |
+, gradual increase in growth index value observed. ND, not detected.
DISCUSSION
A laboratory-scale Cheddar cheese-making process was established which produced cheese with microbial and chemical composition similar to that of commercially produced Cheddar with recognized parameters. This process was used to monitor the persistence of M. paratuberculosis inoculated postpasteurization into the cheese milk throughout the manufacturing stage and during a 27-week ripening period (10°C). Raw milk for Cheddar cheese production is generally subject to the same pasteurization regimen as milk for retail sale, but occasionally a lesser heat treatment is used (24). Grant et al. (18) recovered low levels of viable M. paratuberculosis from pasteurized cows' milk for retail sale. Therefore, this study chose to artificially contaminate milk postpasteurization with low levels of M. paratuberculosis, given detection limitations, to reflect the possible numbers surviving pasteurization. Higher inocula were also used in order to determine the kinetics of inactivation throughout the manufacturing and ripening stages, considering the insensitivity of viable quantitation methods.
There was an approximately 10-fold concentration of M. paratuberculosis strains 806PSS and 796PSS, originally isolated from pasteurized milk, from cheese milk to cheese curd. This increase was expected, as our cheese yield (9.7%) suggests that each liter of cheese milk produces approximately 100 g of curd following entrapment and syneresis. Although many pathogenic bacteria, including Aeromonas hydrophila, Campylobacter jejuni, Escherichia coli, Salmonella enterica serovar Typhimurium, and Listeria monocytogenes, do not survive the manufacturing process of hard cheese (2), the survival of M. paratuberculosis is not surprising given the previous high recovery of this organism from the curd of Swiss hard cheese (35).
The reference strain NCTC 8578 showed a lesser concentration in numbers from milk to curd. This reduction in numbers of the reference strain could have been due, inter alia, to the loss of this strain in the whey fraction or the partial deactivation of the M. paratuberculosis load during the manufacturing process. Although M. paratuberculosis was recovered from the whey fraction of cheese artificially contaminated with this strain, the levels were lower than that recovered in cheeses containing strains 806PSS and 796PSS. This would suggest no excessive partitioning of this strain in the whey fraction but rather loss during the manufacturing process. The parameters affecting bacterial survival during the manufacturing process include starter culture composition, curd cooking temperature, pH and NaCl concentration, and, most likely, a combination of all of these factors. Given the fact that all manufactured cheeses used the same starter culture and had similar process control and subsequent compositional analysis, the loss of numbers for the reference strain between contaminated milk and cheese curd may be due to a difference in strain resistance to the manufacturing parameters. Differences in M. paratuberculosis strain thermal tolerance (41) and combined pH and NaCl concentrations (42) have been observed previously.
To our knowledge, this study provides the first data on the persistence of M. paratuberculosis during Cheddar cheese manufacture and ripening. Hamer and Babel (21) reported Cheddar cheese contaminated with Mycobacterium tuberculosis to be infective after 220 days, while Kastli and Binz (25) determined survival times of 22 and 305 days for Mycobacterium bovis in Swiss hard and semihard cheeses, respectively.
The D values estimated for each of the three M. paratuberculosis strains (107 days for strain 806PSS, 96 days for strain 796PSS, and 90 days for strain NCTC 8578) in our Cheddar cheeses were much higher than those reported previously, even compared to Swiss Emmentaler, a hard cheese having a moisture content similar to that of Cheddar (35). D values of 45.5 and 27.8 days were calculated for semihard (Tilsiter) and hard (Emmentaler) Swiss cheeses, respectively, made from spiked raw milk, indicating a faster rate of death of M. paratuberculosis in the progressively harder cheeses. A D value of 59.9 days has been determined for the survival of M. paratuberculosis in a Hispanic-style soft cheese made from spiked pasteurized milk (42). The Hispanic-style soft cheese was produced under laboratory conditions and contained 2% (wt/vol) NaCl, pH 6.15, with a maximum temperature of 37°C reached during manufacture.
In addition to moisture content, a number of other factors are likely to contribute to the inactivation of M. paratuberculosis in cheese, including temperature, pH, salt, and the antagonistic effect of lactic acid bacteria (starter and nonstarter microflora) with their concomitant lactic acid production (35). Sung and Collins (41) investigated the in vitro effect of pH and NaCl and reported a D value of 19 days in acetate buffer (pH 5.0), with D values increasing with increased pH. After 24 h, our model cheeses reached pH 5.1 to 5.3. This pH remained constant throughout the ripening period and was lower than those encountered during the later stages of Swiss Emmentaler ripening (120 days). A more bactericidal effect on M. paratuberculosis would have been expected with the lower pH encountered in Cheddar ripening.
Although high levels of salting can affect the survival rates of microorganisms in some cheeses (36), NaCl concentrations from 2 to 6% had little or no effect on the survival rate of M. paratuberculosis under in vitro conditions at pH 4 to 6.8 (42). Since the average salt content in our model cheeses (1.6%) was lower, we conclude that salt content contributed little to the death of M. paratuberculosis during Cheddar ripening. A similar negligible detrimental effect was reported for Swiss Emmentaler cheese containing 0.4% salt.
The cheese temperature and exposure time experienced during production vary depending on the variety manufactured. During our model Cheddar production, the maximum curd cooking temperature was 40°C (applied for 2 to 3 h), compared to 53°C applied for 45 min in Swiss hard cheese (Emmentaler) (35). The ripening temperature of Swiss hard cheese was comparatively high, 10 days at 12°C, then 60 days at 22°C, and 50 days at 12°C. In contrast, the model Cheddar cheeses in this study were ripened for 190 days at 10°C. Spahr and Schafroth (35) reported that curd cooking temperature had little effect on the behavior of M. paratuberculosis. However, it has been reported that pathogens surviving the manufacturing process show a more rapid rate of death at higher ripening and storage temperatures (10). In an earlier study, Keswani and Frank (26) observed minimal thermal inactivation of M. paratuberculosis at 55°C, albeit over a much shorter time of 12 min. Therefore, we conclude that the curd cooking temperature during Cheddar manufacture coupled with a low ripening temperature contributed little to the inactivation of M. paratuberculosis during the production process.
The survival times of M. paratuberculosis in Cheddar cheeses cannot be directly compared with their in vitro behavior against the parameters of temperature, pH, and salt (42). Furthermore, there are key differences, other than those alluded to above, between this study and the only study available on M. paratuberculosis survival in hard cheese (35). In Swiss Emmentaler manufacture, raw milk was spiked with M. paratuberculosis cells with no subsequent heat treatment, and thus raw milk microflora entered the manufacturing process, where they were supplemented with a starter culture (not named in the study) and propionic acid bacteria as ripening bacteria. In our study, M. paratuberculosis was added postpasteurization along with a mesophilic-thermophilic starter culture. The possible contribution of raw milk microflora, the active antimicrobial enzyme systems of fresh raw milk, antagonistic starter culture flora, and propionic acid-producing ripening bacteria on the reduction of M. paratuberculosis and perhaps unknown factors can only be speculated upon. However, Meyer et al. (29) suggested that rapidly growing propionic acid bacteria, characteristic of Swiss Emmentaler production, may be a factor responsible for the inactivation of mycobacteria. Also, this study used M. paratuberculosis strains previously isolated from commercially pasteurized milk. There is the possibility that such strains were more thermotolerant due to their passage through the pasteurization process.
This study has also shown the release of up to 4% of the M. paratuberculosis load from the cheese milk into the whey fraction during the manufacturing process. The previous studies investigating the persistence of M. paratuberculosis in Hispanic soft cheese (42) and Swiss semihard and hard cheeses (35) did not examine whey for the recovery of M. paratuberculosis. In this study, whey was sampled and analyzed immediately upon draining (pH 5.6 to 6.0) and therefore was not exposed to this pH for a considerable time, possibly preventing any inactivation of M. paratuberculosis that may occur in whey. The long-term survival of M. paratuberculosis in whey may be an important factor to consider when evaluating the use of whey as a cheese processing by-product or the effectiveness of wastewater treatment processes.
In conclusion, this study demonstrates that laboratory-grown M. paratuberculosis cells inoculated into milk postpasteurization were concentrated (approximately 10-fold) within the cheese curd and decreased slowly over a 27-week ripening period. M. paratuberculosis was also recovered from the whey fraction in the majority of the manufactured cheeses. Although the manufactured cheese closely resembled the commercial product in pH, moisture, fat, and salt content, the behavior of M. paratuberculosis may have been affected by the limitations of our experimental design, i.e., the inability to use naturally infected milk.
The number of M. paratuberculosis cells, if any, entering the cheese manufacturing process will depend on the frequency and degree of contamination of raw milk used and also the heat treatment applied to milk for cheese production. Studies have suggested infrequent and low levels of M. paratuberculosis in raw milk used for cheese making (15, 18, 28, 37, 39, 43), although rates of isolation may be influenced by the detection regimens used by different research groups (11). With regard the effectiveness of raw milk heat treatment, a number of studies with pilot-scale commercial simulation of high-temperature, short-time pasteurization have not recovered M. paratuberculosis when milk was inoculated with 102 to 106 CFU/ml of different strains (23, 37, 38). Other studies have reported more than a 4 log10 reduction in M. paratuberculosis numbers under similar conditions (28, 33). However, in one of these studies, very small numbers of M. paratuberculosis were recovered from milk heat treated at 72°C for 15 s (28).
M. paratuberculosis has been recovered after simulated high-temperature, short-time pasteurization of naturally infected milk (19) and has been cultured from pasteurized milk for retail sale (18). Therefore, the possibility exists that cheese milk for Cheddar production may contain viable M. paratuberculosis cells. The results of our study, with a reference strain and field isolates, suggest minimal inactivation during the manufacturing process and up to 10-fold concentration following microbial entrapment and syneresis of the curd. Subsequently, a minimum of 3 months of low-temperature ripening is required to achieve a 1 log10 reduction in M. paratuberculosis levels.
Cheddar cheese is sold for consumption as mild, medium, or mature depending on the length of ripening. In the United Kingdom, mild Cheddar is usually ripened for up to 4 months (16 weeks), while the mature variety is ripened up to and beyond 12 months. A larger margin of safety, in relation to M. paratuberculosis, may be provided by medium or mature Cheddar ripened longer than 16 weeks.
Acknowledgments
We thank Irene Grant, Department of Food Science, Queens University, Belfast, for providing M. paratuberculosis strains 806PSS and 796PSS and for advice on and critical review of the manuscript. Thanks are due to Trevor Oliver and Bill Graham, Food Chemistry Branch, Department of Agriculture and Rural Development, who performed fat analysis and irradiation on the cheese samples, respectively. We express our thanks to Alan Gordan, Biometrics Division, Department of Agriculture and Rural Development, for statistical advice and analysis.
REFERENCES
- 1.Aschaffenberg, R., and J. E. C. Mullen. 1949. A rapid and simple phosphatase test for milk. J. Dairy Res. 16:58-67. [Google Scholar]
- 2.Bachmann, H. P., and U. Spahr. 1995. The fate of potentially pathogenic bacteria in swiss hard and semihard cheeses made from raw milk. J. Dairy Sci. 78:476-483. [DOI] [PubMed] [Google Scholar]
- 3.Banks, J. M., and D. D. Muir. 1984. A laboratory-scale technique for controlled production of Cheddar cheese. J. Food Technol. 19:593-604. [Google Scholar]
- 4.Bull, T. J., E. J. Mc Minn, K. Sidi-Boumedine, A. Skull, D. Durkin, P. Neild, G. Rhodes, R. Pickup, and J. Hermon-Taylor. 2003. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn's disease. J. Clin. Microbiol. 40:2915-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamberlin, W., D. Y. Graham, K. Hulten, H. M. T. El-Zimaity, M. R. Schwartz, S. Naser, I. Shafran, and F. A. K. El-Zaatari. 2001. Review article: Mycobacterium paratuberculosis as one cause of Crohn's disease. Aliment. Pharmacol. Ther. 15:337-346. [DOI] [PubMed] [Google Scholar]
- 6.Chiodini, R. J. 1989. Crohn's disease and the mycobacterioses: a review and comparison of two disease entities. Clin. Microbiol. Rev. 2:90-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiodini, R. J., and J. Hermon-Taylor. 1993. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurisation. J. Vet. Diagn. Investig. 5:629-631. [DOI] [PubMed] [Google Scholar]
- 8.Chiodini, R. J., H. J. van Kruiningen, and R. S. Merkal. 1984. Ruminant paratuberculosis (Johne's disease): the current status and future prospects. Cornell Vet. 74:218-262. [PubMed] [Google Scholar]
- 9.Cocito, C., P. Gilot, M. Coene, M. Dekesel, P. Poupart, and P. Vannuffel. 1994. Paratuberculosis. Clin. Microbiol. Rev. 7:328-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Aoust, J. Y., D. W. Warburton, and A. M. Sewell. 1985. Salmonella typhimurium phage-type 10 from Cheddar cheese implicated in a major Canadian food-borne outbreak. J. Food Prot. 48:1062-1066. [DOI] [PubMed] [Google Scholar]
- 11.Dundee. L., I. R. Grant, H. J. Ball, and M. T. Rowe. 2001. Comparative evaluation of four decontamination protocols for the isolation of Mycobacterium avium subsp. paratuberculosis from milk. Lett. Appl. Microbiol. 33:173-177. [DOI] [PubMed] [Google Scholar]
- 12.El-Zaatari, F. A. K., M. S. Osato, and D. Y. Graham. 2001. Etiology of Crohn's disease: the role of Mycobacterium avium paratuberculosis. Trends Mol. Med. 7:247-252. [DOI] [PubMed] [Google Scholar]
- 13.European Commission. 2000. Possible links between Crohn's disease and paratuberculosis. Report of the Scientific Committee on Animal Health and Animal Welfare. European Commission, Geneva, Switzerland.
- 14.Fox, P. F., T. P. Guinee, T. M. Cogan, and P. L. H. McSweeney. 2000. Fundamentals of cheese science. Aspen Publishers Inc., Baltimore, Md.
- 15.Grant, I. R., H. J. Ball, S. D. Neill, and M. T. Rowe. 1996. Inactivation of Mycobacterium paratuberculosis in cow's milk at pasteurization temperatures. Appl. Environ. Microbiol. 62:631-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grant, I. R., H. J. Ball, and M. T. Rowe. 1998. Effect of high temperature, short time (HTST) pasteurisation on milk containing low numbers of Mycobacterium paratuberculosis. Lett. Appl. Microbiol. 26:166-170. [DOI] [PubMed] [Google Scholar]
- 17.Grant, I. R., H. J. Ball, and M. T. Rowe. 1999. Effect of higher pasteurisation temperatures, and longer holding times at 72oC, on the inactivation of Mycobacterium paratuberculosis in milk. Lett. Appl. Microbiol. 28:461-465. [DOI] [PubMed] [Google Scholar]
- 18.Grant, I. R., H. J. Ball, and M. T. Rowe. 2002. Incidence of Mycobacterium paratuberculosis in bulk raw and commercially pasteurized cows' milk from approved dairy processing establishments in the United Kingdom. Appl. Environ. Microbiol. 68:2428-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant, I. R., E. Hitchings, A. McCartney, F. Ferguson, and M. T. Rowe. 2002. Effect of commercial scale HTST pasteurization on the viability of Mycobacterium paratuberculosis in naturally infected cows' milk. Appl. Environ. Microbiol. 68:602-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammer, P. 2000. Mycobacterium paratuberculosis in cattle and milk hygiene. Bull. Int. Dairy Fed. 345:19-22. [Google Scholar]
- 21.Hammer, B. W., and F. J. Babel. 1957. Dairy bacteriology, 4th ed. John Wiley & Sons Inc., New York, N.Y.
- 22.Hammer, P., C. Kiesner, H. G. Walte, and P. Teufel. 2002. Heat inactivation of Mycobacterium avium subsp. paratuberculosis in milk, p. 320-322. In R. A. Juste, M. V. Geijo, and J. M. Garrido (ed.). Proceedings of the Seventh International Colloquium on Paratuberculosis, Bilbao, Spain. International Association for Paratuberculosis, Madison, Wis.
- 23.Hope, A. F., P. A. Tulk, and R. J. Condron. 1996. Pasteurisation of Mycobacterium paratuberculosis in whole milk, p. 377-382. In R. J. Chiodini, M. E. Hines, and M. T. Collins, (ed.) Proceedings of the Fifth International Colloquium on Paratuberculosis. Rehoboth, Mass. International Association for Paratuberculosis, Madison, Wis.
- 24.Johnson, E. A, J. H. Nelson, and M. Johnson. 1990. Microbiological safety of cheese made from heat treated milk. 11. Microbiology. J. Food Prot. 53:519-540. [DOI] [PubMed] [Google Scholar]
- 25.Kästli, P., and M. Binz. 1949. Die Lebensfahigkeit von Mycobacterium tuberculosis in verschiedenen Käsesorten. Milchwissenschaft 4:391-394. [Google Scholar]
- 26.Keswani, J., and J. F. Frank. 1998. Thermal inactivation of Mycobacterium paratuberculosis in milk. J. Food Prot. 61:974-978. [DOI] [PubMed] [Google Scholar]
- 27.Kirk, R., and R. Sawyers. 1991. Pearson's composition and analysis of foods, 9th ed. Longman Group UK Ltd, London, United Kingdom.
- 28.McDonald, W. L, K. J. O'Riley, C. J. Schroen, and R. J. Condron. 2002. Heat inactivation of Mycobacterium avium subsp. paratuberculosis in milk, p. 312-316. In R. A. Juste, M. V. Geijo, and J. M. Garrido (ed.), Proceedings of the Seventh International Colloquium on Paratuberculosis, Bilbao, Spain. International Association for Paratuberculosis, Madison, Wis.
- 29.Meyer, J., J. Touiller, and J. Malgras. 1952. Les facteurs bacteriolytiques antituberculeux des fromages. Lait 32:512-515. [Google Scholar]
- 30.Meylan, M., D. M. Rings, W. P. Shulaw, J. J. Kowalski, S. Bech-Nielsen, and G. F. Hoffsis. 1996. Survival of Mycobacterium paratuberculosis and preservation of immunoglobulin G in bovine colostrum under experimental conditions simulating pasteurisation. Am. J. Vet. Res. 57:1580-1585. [PubMed] [Google Scholar]
- 31.Moss, M. T., J. Sanderson, M. Tisard, J. Hermon-Taylor, F. El-Zaatari, D. Markesich, and D. Graham. 1992. PCR detection of Mycobacterium paratuberculosis in long-term cultures from Crohn's disease tissues. Gut. 33:1209-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nauta, M. J., and J. W. van der Gleeson. 1998. Human exposure to Mycobacterium paratuberculosis via pasteurised milk: a modelling approach. Vet. Rec. 143:293-296. [DOI] [PubMed] [Google Scholar]
- 33.Pearce, L. E, H. T. Truong, R. A. Crawford, G. F. Yates, S. Cavaignac, and G. W de Lisle. 2001. Effect of turbulent-flow pasteurization on survival of Mycobacterium avium subsp. paratuberculosis added to raw milk. Appl. Environ. Microbiol. 67:3964-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott, R. 1986. Cheesemaking practice. Elsevier Applied Science Publishers, London, England.
- 35.Spahr, U., and K. Schafroth. 2001. Fate of Mycobacterium avium subsp. paratuberculosis in Swiss hard and semihard cheese manufactured from raw milk. Appl. Environ. Microbiol. 67:4199-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spahr, U., and B. Uri. 1994. Behaviour of pathogenic bacteria in cheese—a synopsis of experimental data. Bull. Int. Dairy Fed. 298:2-16. [Google Scholar]
- 37.Stabel, J. R. 2000. Johne's disease and milk: Do consumers need to worry. J. Dairy Sci. 83:1659-1663. [DOI] [PubMed] [Google Scholar]
- 38.Stabel, J. R., E. M. Steadham, and C. A. Bolin. 1997. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Appl. Environ. Microbiol. 63:4975-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stabel, J. R., S. J. Wells, and B. A. Wagner. 2002. Relationships between fecal culture, ELISA and bulk tank milk test results for Johne's disease in US dairy herds. J. Dairy Sci. 85:525-534. [DOI] [PubMed] [Google Scholar]
- 40.Streeter, R. N., G. F. Hoffis, S. Bech-Nielson, W. P. Shulaw, and D. M. Rings. 1995. Isolation of Mycobacterium paratuberculosis from colostrums and milk of subclinically infected cows. Am. J. Vet. Res. 56:1322-1324. [PubMed] [Google Scholar]
- 41.Sung, N., and M. T. Collins. 1998. Thermal tolerance of Mycobacterium paratuberculosis. Appl. Environ. Microbiol. 64:999-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sung, N., and M. T. Collins. 2000. Effect of three factors in cheese production (pH, salt, and heat) on Mycobacterium avium subsp. paratuberculosis viability. Appl. Environ. Microbiol. 66:1334-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sweeney, R. W., R. H. Whitlock, and A. E. Rosenberger. 1992. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J. Clin. Microbiol. 30:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson, D. E. 1994. The role of mycobacteria in Crohn's disease. J. Med. Microbiol. 41:74-94. [DOI] [PubMed] [Google Scholar]