Abstract
Microbial spoilage of food causes losses of up to 40% of all food grown for human consumption worldwide. Yeast growth is a major factor in the spoilage of foods and beverages that are characterized by a high sugar content, low pH, and low water activity, and it is a significant economic problem. While growth of spoilage yeasts such as Zygosaccharomyces bailii and Saccharomyces cerevisiae can usually be retarded by weak organic acid preservatives, the inhibition often requires levels of preservative that are near or greater than the legal limits. We identified a novel synergistic effect of the chemical preservative benzoic acid and nitrogen starvation: while exposure of S. cerevisiae to either benzoic acid or nitrogen starvation is cytostatic under our conditions, the combination of the two treatments is cytocidal and can therefore be used beneficially in food preservation. In yeast, as in all eukaryotic organisms, survival under nitrogen starvation conditions requires a cellular response called macroautophagy. During macroautophagy, cytosolic material is sequestered by intracellular membranes. This material is then targeted for lysosomal degradation and recycled into molecular building blocks, such as amino acids and nucleotides. Macroautophagy is thought to allow cellular physiology to continue in the absence of external resources. Our analyses of the effects of benzoic acid on intracellular membrane trafficking revealed that there was specific inhibition of macroautophagy. The data suggest that the synergism between nitrogen starvation and benzoic acid is the result of inhibition of macroautophagy by benzoic acid and that a mechanistic understanding of this inhibition should be beneficial in the development of novel food preservation technologies.
Yeast spoilage of foods and beverages that are characterized by high sugar contents, low pHs, and low water activities is a major economic problem (42, 47). While growth of spoilage yeasts such as Zygosaccharomyces bailii and Saccharomyces cerevisiae can usually be retarded by weak organic acid preservatives, the inhibition often requires concentrations that are near the legal limits and is limited to a cytostatic effect (24). Thus, adaptation responses eventually allow growth and spoilage by these organisms even at these high levels of preservatives (9, 11).
The major weak organic acid food preservatives include benzoic, acetic, sorbic, and propionic acids. Together with sulfite, these compounds constitute the most widely used acid preservatives in industrial food and beverage production.
The adaptation response of S. cerevisiae and Z. bailii to weak organic acid stress has been the subject of a number of recent studies (reviewed in references 24 and 31). At low pH, acetic acid (pK 4.75), sorbic acid (pK 4.76), and benzoic acid (pK 4.19) exist substantially in the undissociated state. In this form, these molecules readily cross the cell membrane. However, once in the cytosol, where the pH is kept at 6 to 7, the acid dissociates, generating the conjugate base. This anion accumulates intracellularly to very high levels, since it cannot diffuse back across the membrane. The release of protons in the cytosol can potentially acidify the cytosol, and this mechanism was originally proposed to underlie the physiology of growth inhibition by this class of compounds (23). More recently, however, data indicate that this acidification does not correlate with growth inhibition and is therefore unlikely to represent the major mechanism of action for sorbic and benzoic acids (6).
Moreover, the same study showed that growth inhibition correlated with an increase in the ADP/ATP ratio, which in turn depends on the activation of a set of protective pumps that extrude acids from the cell (15, 16). On the other hand, disruption of these protective mechanisms results in an increase in weak acid sensitivity, while at the same time lowering the ADP/ATP ratio. Hence, the cell is willing to pay a high energetic price to avoid an even worse scenario, although the target of action in the absence of protective pumps, such as Pdr12, is not known. Intriguingly, weak acids have also been shown to cause oxidative stress, even at subinhibitory concentrations, suggesting a mode of action that involves disruption of membrane homeostasis and, possibly, disruption of mitochondrial physiology (33).
Several studies have supported the notion that weak organic acids function through disruption of membrane organization, as well as oxidative stress (14, 15, 32, 33, 39). Cells of S. cerevisiae that are exposed to weak organic acids tend to form petites by losing their mitochondria (33), and a concerted loss of mitochondria can occur though autophagy (43-45).
On the other hand, intracellular acidification may play a role in acids with short aliphatic chains, such as acetic acid, which require much higher concentrations (20 to 80 mM) for growth inhibition and appear to induce cell death phenomena at these concentrations (25, 26).
The previous studies also suggested that while all organic acids accumulate intracellularly due to the pH differential between the cytosol and the surrounding medium, their actual mechanisms of toxicity may be different once they are inside the cell. Indeed, 20 to 80 mM acetic acid induces a programmed cell death mechanism in S. cerevisiae (25, 26), while such phenomena have not been reported for benzoic or sorbic acid.
Disruption of membrane organization and homeostasis may take several forms. The most straightforward possibility is that the ability of the plasma membrane to act as a barrier is compromised (7). However, nonspecific disruption of the permeability barrier is expected to cause a cytocidal effect, while organic acids such as sorbic and benzoic acids are cytostatic under conditions used for food preservation (34, 38). A second possibility is that changes in membrane fluidity and changes in the organization of membrane microdomains occur, leading to disruption of membrane trafficking and dynamics.
The intracellular flux of lipids and proteins which constitutes vesicular trafficking in eukaryotic cells is a highly regulated process. Correct sorting of these components defines cellular compartmentalization and is thus vital for cellular function (35). Such fluxes may be monitored by analyzing the ability of the cell to deliver various proteins into specialized compartments, after synthesis on endoplasmic reticulum (ER)-bound or cytosolic ribosomes. The yeast vacuole is a compartment that allows relatively simple assays in this regard. Most of the resident vacuolar enzymes are synthesized as zymogens which are proteolytically digested, yielding active, mature proteins. A change in migration on denaturing gels can be used to assess the arrival of the protein in the vacuolar lumen (22).
All eukaryotic cells, when starved for nitrogen or challenged with specific hormonal stimuli, respond through a process in which bulk cytosolic material is targeted for lysosomal degradation. This catabolic response, called macroautophagy, involves the induction of membranes which envelop and sequester cytosolic material, including mitochondria, ribosomes, and other nonspecific soluble cargo, as well as some specific cargo (3, 18). As the sequestration process proceeds, the enveloping membrane fuses with itself to form a unique, double-bilayer vesicle called the autophagosome. In yeast, the autophagosome then fuses with the limiting membrane of the vacuole, releasing the inner vesicle containing the cytosolically derived material, termed the autophagic body, into the lumen of the vacuole (40). The autophagic body is subsequently degraded by various hydrolases, which allows the cell to replenish essential pools of biosynthetic building blocks. Consistent with this view, macroautophagy is essential for survival of yeast under nitrogen starvation conditions (46).
The autophagic trafficking pathway shares most of its molecular machinery with a related constitutive membrane trafficking pathway, the cytoplasm-to-vacuole targeting (Cvt) pathway. The Cvt pathway is active in yeast cells under normal growth conditions (19, 21). It is a biosynthetic conduit, carrying a yeast vacuolar protease, aminopeptidase I (Ape1), as well as other cargo, such as α-mannosidase, into the lumen of the vacuole. The soluble cytosolic precursor of Ape1, prApe1 (61 kDa), is synthesized on soluble ribosomes and is specifically sequestered in double-bilayer vesicles called Cvt vesicles (diameter, 140 to 160 nm), which are analogous to autophagosomes. The outer bilayer of a Cvt vesicle then fuses with the limiting membrane of the yeast vacuole, leading to the release of the inner vesicle (the Cvt body) into the lumen of the vacuole, where it is degraded, allowing the maturation of prApe1 to the active 50-kDa form through the action of proteinase B. While most of the gene products that are essential for macroautophagy are also essential for the Cvt pathway, a subset of the CVT genes is required only in the Cvt pathway, and a subset of the APG genes is autophagy specific. Specifically, Cvt pathway-specific genes are blocked at a point in the pathway that precedes vesicle completion, implying that the genetic requirements for forming Cvt vesicles are distinct from the requirements for forming autophagosomes (3). The various autophagic mutant classification systems have recently been consolidated into a single ATG nomenclature (20).
We have undertaken an analysis of the effects of benzoic acid, a food preservative that has been demonstrated to cause oxidative stress (33), on the behavior of discrete intracellular membrane trafficking pathways in yeast, including macroautophagy. We have found that growth-inhibitory concentrations of benzoic acid completely abrogate nitrogen starvation-induced macroautophagy, while they have no effect on the closely related Cvt pathway. The data suggest novel strategies for enhancing the effect of weak organic acids on food spoilage organisms.
MATERIALS AND METHODS
Strains, plasmids, and growth conditions.
Yeast was grown in YPD medium (containing 1% yeast extract, 2% peptone, and 2% glucose) or SD synthetic minimal medium (containing 0.67% yeast nitrogen base, 2% glucose, and auxotrophic amino acids and vitamins as required). The starvation medium was SD-N (0.17% yeast nitrogen base without ammonium sulfate or amino acids containing 2% glucose). For benzoate inhibition studies, media were supplemented with 0, 1, or 2 mM sodium benzoate plus 10 mM sodium citrate, and the pH was adjusted to 4.5. For acetic acid studies, media were supplemented with 2 mM acetic acid and with 20 mM acetic acid plus 10 mM sodium citrate at pH 4.5 and 3, respectively. Plasmids pRS305 and pRS306 were described previously by Sikorski and Hieter (36). Strains HAY 394 and HAY395 (Table 1) were described previously (2). Strain HAY75 (MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9) was a MATα segregant from strain HAY70 (1). Strain SLY2 was constructed by transforming strain HAY31 (MATa leu2-3,112 ura3-52) with an atg1Δ::URA3 construct generously provided by Y. Ohsumi and Y. Kamada. Strain SLY3 (MATa leu2-3,112::LEU2 ura3-52::URA3) was constructed by integrating NcoI-linearized pRS306 into the URA3 locus of HAY31, and both SLY2 and SLY3 were further modified by integration of BstEII-linearized pRS305 at the LEU2 locus, to obtain a prototrophic yeast strain. Yeast transformation was performed as described previously (12).
TABLE 1.
Yeast strains used in this study
Reagents.
A radioactive 35S-labeled cysteine-methionine mixture (Pro-mix) was obtained from Amersham Biosciences (Little Chalfont, United Kingdom). Enhanced chemiluminescence reagents were obtained from Pierce Biotechnology, Inc. (Rockford, Ill.). Other reagents were obtained from Sigma-Aldrich (Rehovot, Israel), unless otherwise specified.
Alkaline phosphatase assay.
Approximately 4 optical density at 600 nm (OD600) equivalents (1 OD600 unit was equivalent to the number of cells in 1 ml of a suspension at an OD600 of 1) of yeast cells was harvested, washed in ice-cold distilled water containing 2 mM phenylmethylsulfonyl fluoride, and resuspended in 100 μl of lysis buffer [20 mM piperazine-N,N′-bis(2-ethansulfonic acid) (PIPES) (pH 7.0), 0.5% Triton X-100, 50 mM KCl, 100 mM potassium acetate, 10 mM MgSO4, 10 μM ZnSO4, 1 mM phenylmethylsulfonyl fluoride]. An equal volume of acid-washed glass beads (diameter, 425 to 600 μm; Sigma) was added, the cells were broken by vortexing for 4 min, and the extract was diluted by adding 200 μl of lysis buffer. To start the assay, 20 μl of extract was added to 480 μl of reaction buffer (250 mM Tris-HCl [pH 8.5], 0.4% Triton X-100, 10 mM MgSO4, 1.25 mM nitrophenyl phosphate), and samples were incubated for 15 min at 37°C before the reaction was terminated by adding 500 μl of stop buffer (2 M glycine, pH 11). The evolution of nitrophenol was monitored by measuring the absorbance at 405 nM with a Spectronic Unicam UV-1 spectrophotometer, and the value for a time-zero blank was subtracted from the value for each sample. The nitrophenol concentration was calculated by using Beer's law with ɛ405 = 18,000 M−1 cm−1. The protein concentrations in the extracts were measured by the Pierce bicinchoninic acid assay (Pierce Chemical Co., Rockford, Ill.), and 1 U of activity was defined as production of 1 nmol of nitrophenol/min/mg of protein.
Preparation of whole-cell extracts for Western blot analysis.
Cells (10 OD600 units) were treated with trichloroacetic acid at a final concentration of 10% and washed twice with acetone. The dry cell pellet was then resuspended in 100 μl of cracking buffer (50 mM Tris [pH 6.8], 3.6 M urea, 1 mM EDTA, 1% sodium dodecyl sulfate [SDS]) and vortexed in a Disruptor Genie (Scientific Instruments, Bohemia, N.Y.) at the maximum speed with an equal volume of acid-washed glass beads (diameter, 425 to 600 μm) for 15 min. Unlysed cells were removed by centrifugation at 13,000 × g for 5 min, and an equal volume of 2× SDS loading buffer (100 mM Tris [pH 6.8], 20% glycerol, 2% SDS, 500 mM β-mercaptoethanol) was added to the supernatant. The samples (0.5 OD600 unit per lane) were incubated at 70°C for 10 min prior to loading onto gels.
In vivo labeling and immunoprecipitation.
Yeast cultures were grown to an OD600 of 0.5 in SD medium supplemented with the required amino acids. Cells (2 OD600 units per time point) were harvested by centrifugation at 1,800 × g and resuspended in 450 μl of SD medium with amino acids. A 10-min pulse with 100 μCi of 35S-labeled cysteine-methionine per time point was followed by addition of a chase solution (5 mM methionine and 1 mM cysteine [final concentrations]). Samples were taken at different times. The samples were precipitated with 10% trichloroacetic acid and then washed twice with cold acetone, dried, and resuspended in 100 μl of cracking buffer (50 mM Tris [pH 6.8], 1% SDS, 6 M urea, 1 mM EDTA) plus an equal volume of acid-washed glass beads. Following vortexing for 5 min, samples were diluted 10-fold in IP dilution buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 0.5% Tween 20, 5 mM EDTA, 100 μg of bovine serum albumin per ml) and centrifuged for 5 min at 13,000 × g. Each supernatant was precipitated overnight with anti-carboxypeptidase Y antibody (0.5 μl/2 OD600 units of cells), and this was followed by 2 h of incubation with protein A-conjugated Sepharose (final concentration, 2% [vol/vol]). Immune complexes were then washed and processed for SDS-polyacrylamide gel electrophoresis (PAGE). Dried gels were exposed to PhosphorImager plates and read with a Fuji BAS phosphorimager.
RESULTS
Benzoic acid inhibits nitrogen starvation-induced macroautophagy.
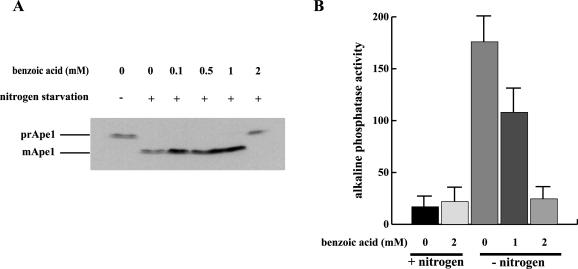
Work by other researchers has demonstrated that benzoic and sorbic acids act as membrane-perturbing agents (14, 15, 32, 33, 39). In addition, these acids induce loss of mitochondrial function, and one possibility that we entertained was that this could be the result of mitochondrial autophagy. We were therefore interested in determining whether exposure of yeast cells to benzoic acid affected their ability to induce macroautophagy in response to nitrogen starvation. Ape1, a resident vacuolar protease, is synthesized as a zymogen (prApe1, 61 kDa) and is carried into the yeast vacuole by one of two alternative routes. Under nitrogen-rich conditions, it is carried by the Cvt pathway, in which it is engulfed by intracellular membranes to form Cvt vesicles that fuse with the vacuole. When cells are starved, autophagosomes are induced that engulf and sequester prApe1 in a specific manner that is very similar to the transport of Ape1 via Cvt vesicles (18). In either case exposure to hydrolytic activities in the vacuole results in proteolytic maturation of prApe1 to the 50-kDa form (mApe1). To assay macroautophagy, we utilized a previously described procedure in which the maturation of prApe1 occurs exclusively through macroautophagy (3). This assay utilizes a characteristic of vac8Δ mutants, which are specifically blocked in the Cvt pathway but not in macroautophagy (2). The vac8Δ mutant cells accumulate prApe1 in nitrogen-rich medium, and induction of macroautophagy by nitrogen starvation or rapamycin treatment leads to maturation. As shown in Fig. 1A, maturation of prApe1 was observed in cells treated with 0, 0.1, 0.5, and 1 mM benzoic acid. However, in the presence of 2 mM benzoic acid, no maturation was observed, implying that this concentration of benzoic acid is inhibitory for macroautophagy. One possible explanation for this result is that Cvt pathway-independent functions of Vac8 are involved in these effects (30, 51). To rule out this possibility and also to corroborate the conclusion that macroautophagy is inhibited, we utilized a more general assay for macroautophagy which does not rely on a Cvt pathway mutant background. A truncated form of yeast alkaline phosphatase (Pho8) lacking the N-terminal 60 amino acids (Pho8Δ60) has been shown to accumulate as a cytosolic zymogen. Induction of macroautophagy then leads to engulfment of the zymogen with concomitant delivery to the vacuole, followed by activation; therefore, in such cells the level of alkaline phosphatase activity reflects the level of macroautophagy (29). When the Pho8Δ60 assay was conducted with nitrogen-starved cells, we found that 1 mM benzoic acid reduced the level of alkaline phosphatase activity by 50%, and 2 mM benzoic acid completely inhibited the response (Fig. 1B). These results indicate that the inhibition of Ape1 maturation in the vac8Δ cells was not specific for the vac8Δ mutation but reflected general inhibition of autophagy by benzoic acid.
FIG. 1.
Effects of benzoic acid on macroautophagy. (A) Benzoic acid inhibits autophagy-dependent maturation of of prApe1 in a vac8Δ mutant. Exponentially growing cells (HAY394) were harvested and resuspended in medium with (+) or without (−) nitrogen and various concentrations of benzoic acid. After 2 h of incubation, 0.5 OD600 unit of cells was harvested, and protein extracts were blotted and probed with anti-Ape1 antibody. The data are representative of the data from duplicate independent experiments in which identical results were obtained. (B) Benzoic acid inhibits autophagy-dependent maturation of Pho8Δ60. Cells expressing Pho8Δ60 (HAY369) were treated as described above for panel A, and alkaline phosphatase activity was determined in the extracts. One unit was defined as 1 nmol of nitrophenol evolved per mg of protein per min (4). The error bars indicate standard errors (n = 3).
Benzoic acid does not affect the Cvt pathway.
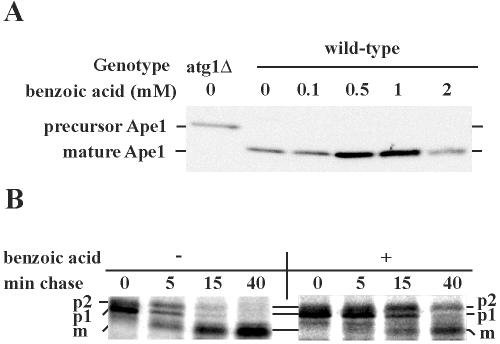
If the inhibition of macroautophagy shown in Fig. 1 simply reflects a general exhaustion of ATP stores or some other inhibition that has global effects on intracellular trafficking, one would expect all other trafficking pathways to similarly show complete inhibition. We first tested the effects of 2 mM benzoic acid on the closely related Cvt pathway, as this concentration completely blocked macroautophagy (Fig. 1) and also inhibits cell growth (31). As indicated above, the Cvt pathway is a biosynthetic analog of macroautophagy that is capable of carrying specific cargo proteins, such as prApe1 and α-mannosidase, to the vacuole through an engulfment process that is very similar to macroautophagy. Surprisingly, 4 h of incubation of wild-type cells in 2 mM benzoic acid did not result in any observable accumulation of prApe1(Fig. 2A). It therefore appears that the inhibition of macroautophagy shown in Fig. 1 is not due to a global metabolic block but is due to a disruption of some specific membrane processes.
FIG. 2.
Effects of benzoic acid on the trafficking of CPY and Ape1. (A) Benzoic acid at a concentration of 2 mM does not inhibit the Cvt pathway. Exponentially growing wild-type cells (HAY75) were harvested and resuspended in synthetic dextrose medium containing different concentrations of benzoic acid. Following 4 h of incubation, total protein was extracted, and 0.5 OD600 equivalent was separated by SDS-PAGE and probed with anti-Ape1 antibody. As a control to identify the relative mobility of prApe1, extracts from an atg1Δ strain, SLY2, were probed in parallel. (B) Benzoic acid at a concentration of 2 mM delays but does not block CPY maturation. Two OD600 units of wild-type cells (HAY75) per time point was pulsed for 5 min with 10 μCi of a radioactive cysteine-methionine mixture and chased with unlabeled amino acids plus 2 mM benzoic acid. Samples were taken at different times and separated into intracellular fractions and extracellular fractions. Total protein was extracted and immunoprecipitated with anti-CPY antibody and analyzed by SDS-PAGE and autoradiography.
Benzoic acid has an intermediate effect on CPY trafficking, suggesting that there is preferential inhibition of the ER-to-Golgi complex transport step.
Autophagy and the Cvt pathway are unusual intracellular trafficking pathways in the sense that the cellular compartments involved, apart from the destination (the vacuole), have not been identified in terms of known cellular compartmentalization schemes. We wanted to determine the effects of benzoic acid on a more thoroughly characterized trafficking pathway. Yeast carboxypeptidase Y (CPY) is a vacuolar protease that is synthesized in the lumen of the ER and undergoes cotranslational glycosylations and cleavage of the leader peptide to create the p1 form of the protein (67 kDa). Transport into the Golgi complex and addition of outer chain mannose yield the p2 form (69 kDa), and import into the vacuolar lumen gives rise to the mature form of the protein (61 kDa) through a proteolytic event (37). The different forms of the protein therefore report on the function of a number of cellular compartments and the trafficking pathways that link them. We found that 2 mM benzoic acid strongly inhibits the kinetics of appearance of the p2 form of the protein. However, once the p2 form accumulates, mature CPY appears, albeit with slower kinetics than those observed in the control (Fig. 2B). These data imply that benzoic acid partially inhibits CPY maturation, and the inhibition appears to target early phases of the pathway (i.e., the ER-to-Golgi complex step). Alternatively, some other aspect of Golgi complex organization may be compromised, leading to slower appearance of the p2 form.
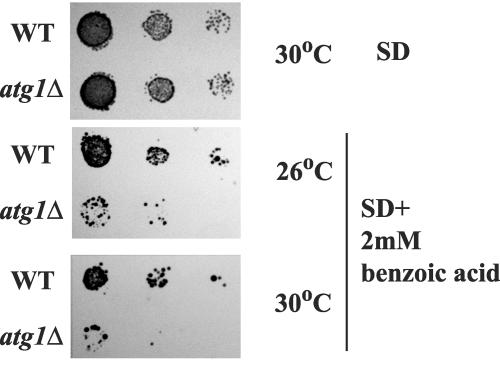
The atg1Δ autophagy mutant is defective in benzoic acid adaptation.
Given that autophagy is inhibited by benzoic acid, one may rationalize that if autophagy is in any way physiologically relevant to processes that occur following weak acid treatment, then mutants that are unable to induce autophagy may be defective in the adaptation response to benzoic acid. Atg1 is a protein kinase that is essential for both macroautophagy and the Cvt pathway (4, 17, 27). Indeed, atg1Δ cells showed a reduced ability to adapt to 2 mM benzoic acid, and this defect was exacerbated by increased temperature (Fig. 3), although the mutants did not show any growth defect at either 26 or 30°C in the absence of benzoic acid. This result suggests that Atg1 plays a functional role in adaptation to benzoic acid. Since our other data indicate that autophagy is inhibited by this concentration of benzoic acid, this finding may reflect a requirement for the function of Atg1 in an autophagic pathway distinct from nitrogen starvation-induced macroautophagy, such as the Cvt pathway or other organelle-specific autophagic pathways.
FIG. 3.
Mutants with ATG1 deleted are defective in adaptation to 2 mM benzoic acid. Wild-type (SLY3) and atg1Δ (SLY2) cells were grown to the log phase, and identical numbers of cells (1 × 106 cells) were serially diluted in a microtiter plate, plated on SD medium plates with 2 mM benzoic acid (pH 4.5), and grown at 26 and 30°C for 5 days. WT, wild type.
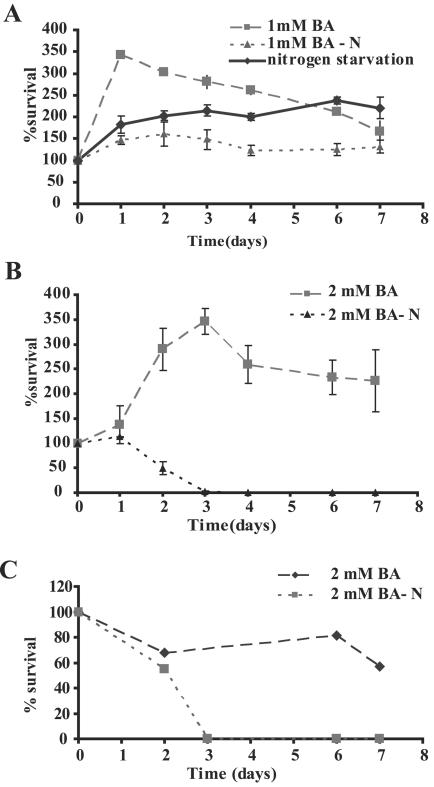
Benzoic acid is cytocidal under nitrogen starvation conditions.
Benzoic acid is a growth-retarding agent which does not normally cause cell death in yeast at the concentrations used in our experiments and in most food preservation applications (24). However, inhibition of macroautophagy under nitrogen starvation conditions is expected to lead to cell death, since macroautophagy is essential for survival under these conditions (46). We therefore tested this prediction by comparing the ability of wild-type cells to survive starvation in the presence and absence of benzoic acid. The results (Fig. 4) demonstrated that while cells incubated in the absence of a nitrogen source did not continue to multiply, they remained viable over the course of the experiment (Fig. 4A). Wild-type cells remain viable for 3 to 4 weeks under these conditions (Daniel J. Klionsky, personal communication). In contrast, cells incubated under nitrogen starvation conditions in the presence of 2 mM benzoic acid lost viability and had a half-life of approximately 2 to 3 days in cells without auxotrophic requirements, and amino acid auxotrophs lost viability even more rapidly (Fig. 4B and C). Hence, addition of benzoic acid abrogated the autophagic response of the cells, resulting in a loss of viability under nitrogen starvation conditions. It was recently shown that auxotrophic mutations strongly modify the behavior of yeast cells subjected to weak acid stress (for example, stress due to inhibition of tryptophan uptake in trp1 mutants) (5). We therefore repeated the experiments with prototrophic yeast cells (SLY3) and obtained identical results (Fig. 4C). Thus, while 2 mM benzoic acid alone or nitrogen starvation alone caused a cytostatic effect under our conditions, the combined treatment was cytocidal. This synergistic effect is consistent with our observation that benzoic acid inhibits macroautophagy, given that autophagy is essential for viability under nitrogen starvation conditions.
FIG. 4.
Nitrogen starvation enhances the toxicity of benzoic acid. Exponentially growing wild-type cells (HAY75 in panels A and B; SLY3 in panel C) were resuspended at a density of 1 OD600 in SD medium (pH 4.5) supplemented with 1 mM benzoic acid (A) or 2 mM benzoic acid (B and C) in the presence or absence of a nitrogen source, as well as with nitrogen starvation alone (A). The cell survival at different times is expressed as a percentage of the initial count. Values greater than 100% reflect continued cell growth. The error bars indicate standard deviations (n = 3). BA, benzoic acid; BA-N, benzoic acid with nitrogen starvation.
Chemical selectivity in the inhibition of macroautophagy by weak acids.
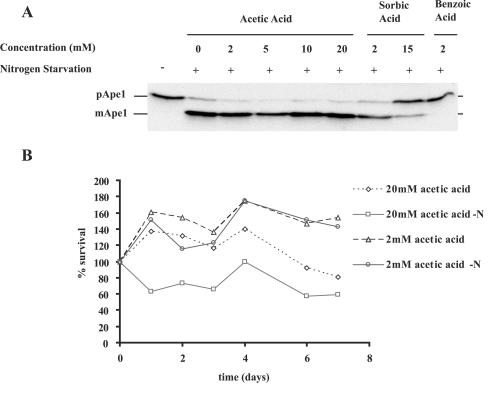
Acetic acid, like benzoic acid, is a weak organic acid that accumulates intracellularly under acidic conditions. In fact, the higher pK of acetic acid (pK 4.75) compared to the pK of benzoic acid (pK 4.19) dictated a greater influx of acetic acid into the cells under our working conditions (pH 4.5) due to the higher concentration of undissociated acid. A number of authors have pointed out, however, that the actions of benzoic and sorbic acids are distinct from the action of acetic acid, both in terms of the mechanism of action and in terms of the cellular stress responses that counteract these effects (6, 14, 31, 39). If the effects which we observed after benzoic acid addition were the result of intracellular acidification or ATP depletion, we expected that acetic acid would generate a similar, if not stronger, response. As shown in Fig. 5A, 2 mM acetic acid had no effect on nitrogen starvation-induced maturation of prApe1 in the vac8Δ mutant, in contrast to the complete inhibition observed with 2 mM benzoic acid (Fig. 1A). In fact, concentrations of acetic acid up to 20 mM had no effect on macroautophagy in our assays. Since 20 mM acetic acid does not inhibit macroautophagy, we did not expect a synergistic effect between nitrogen starvation and acetic acid. Again, at concentrations of acetic acid of up to 20 mM (Fig. 5B), we did not observe a synergistic cytocidal effect of the type seen with benzoic acid. Although some loss of viability was observed with 20 mM acetic acid (pH 3), this loss was probably related to the apoptotic effects reported by Ludovico et al. and did not result in a complete loss of viability at this concentration (26). In addition, it occurred to similar degrees (60 versus 80%) in starved and unstarved cells after a 7-day incubation.
FIG. 5.
Differential effects of sorbic, acetic, and benzoic acids. (A) Exponentially growing vac8Δ cells (HAY394) were resuspended at a density of 1 OD600 unit in SD medium (control) (pH 4.5), nitrogen starvation medium (SD-N) (pH 4.5), or nitrogen starvation medium supplemented with different concentrations of acetic acid (2 to 20 mM), benzoic acid (2 mM), and sorbic acid (2 and 15 mM) at pH 4.5. Following 2 h of incubation, cells were collected, and lysates were prepared and probed for prApe1 maturation by Western blotting as described in Materials and Methods. (B) Exponentially growing wild-type cells (HAY75) were resuspended at a density of 1 OD600 unit in citrate-buffered SD medium (pH 3) supplemented with 0, 2, 5, 10, or 20 mM acetic acid in the presence or absence of a nitrogen source. The cell survival at different times is expressed as a percentage of the initial count. Values greater than 100% reflect continued cell growth. The data are representative of the results of two independent experiments. acetic acid-N, acetic acid treatment with nitrogen starvation.
Unexpectedly, 2 mM sorbic acid also did not affect prApe1 maturation under these conditions. At higher concentrations of sorbic acid we observed inhibition, but even at 15 mM, close to the solubility limit for sorbate in aqueous solution, we were still able to observe Ape1 maturation, in contrast to the results obtained with benzoic acid, which completely inhibited Ape1 maturation at a concentration of 2 mM (Fig. 5A).
We concluded that the inhibition of macroautophagy by benzoic acid and the synergism between benzoic acid and nitrogen starvation are selective aspects of benzoic acid action on the cells and are not a simple consequence of cytosolic acidification or ATP depletion.
DISCUSSION
Food spoilage by yeasts belonging to the genera Saccharomyces and Zygosaccharomyces, as well as by related organisms, poses a significant economic problem (9). Weak organic acids, such as sorbic and benzoic acids, are commonly used to prevent yeast growth in foodstuffs. Due to the intrinsic ability of these organisms to adapt to the presence of weak monocarboxylic organic acids, sorbic acid and benzoic acid are added in millimolar amounts, concentrations that are near the tolerable toxicological limits. A comprehensive understanding of the adaptive response to weak organic acids and the physiological requirements for these adaptations is a prerequisite for designing ways to overcome the resistance of food spoilage organisms to growth-inhibiting compounds.
The suggestion that some organic acids may act by perturbing intracellular membrane dynamics under acidic conditions prompted us to survey the effects of benzoic acid on intracellular membrane trafficking. Previously published data have hinted at a connection between autophagic mechanisms and mitochondrial DNA escape. Intriguingly, Z. bailii YME2, a homolog of an S. cerevisiae gene which suppresses mitochondrial DNA escape (43), allows S. cerevisiae to catabolize sorbic and benzoic acids, hence increasing the levels of resistance to these compounds (28). This led us to study the effects of benzoic acid on membrane trafficking in S. cerevisiae, with the aim of determining the existence of any specific effects on macroautophagy.
Macroautophagy is a nutrient stress response that is essential for the survival of yeast under nitrogen starvation conditions (3, 18). This process allows cells to recycle available intracellular nitrogen in the presence of a suitable carbon source. Unlike other eukaryotes in which autophagy has been studied, S. cerevisiae possesses a biosynthetic analog of macroautophagy that is active under nitrogen-rich conditions. This parallel pathway, termed the Cvt pathway, is known to transport a resident vacuolar protease, Ape1, from its point of synthesis in the cytosol into the lumen of the vacuole (21). The Cvt pathway utilizes many of the molecular components required for macroautophagy, although Cvt pathway-specific genes have been identified, as have autophagy-specific genes (3).
Our data demonstrate that in the presence of 2 mM benzoic acid under conditions that allow growth of yeast on solid medium, nitrogen starvation-induced macroautophagy is completely inhibited. In contrast, we observed no effects of benzoic acid on the maturation of prApe1 under nitrogen-rich conditions, implying that the Cvt pathway is not inhibited.
In addition to the lack of inhibition of prApe1 maturation in rich medium, we found that benzoic acid causes only a delay in the maturation of CPY, a vacuolar protease that utilizes the classical transport route through the early secretory pathway into the endosomal system. Thus, while there is a clear effect on the kinetics of maturation, the pathway is not blocked, in contrast to macroautophagy. Interestingly, the main delay in maturation of CPY in the presence of benzoic acid is in the progression from the p1 form (ER) to the p2 form (Golgi complex). It was recently reported that specific genes that function in ER-to-Golgi complex transport are also required for macroautophagy (but not in the Cvt pathway) (13). Hence, the delay in the appearance of the p2 form of CPY may reflect inhibition of a function that is common to both ER-to-Golgi complex transport and macroautophagy. In addition, the accumulation of p2 CPY does not appear to reflect mistargeting via the secretory pathway, since spheroplasting and separation of intracellular and secreted fractions do not reveal significant amounts of extracellular p2 CPY (Hazan and Abeliovich, unpublished data).
If inhibition of macroautophagy is an important aspect of the action of benzoic acid on yeast, one would expect that benzoic acid would be cytotoxic under nitrogen starvation conditions. This central prediction was indeed validated by our experiments. While 2 mM benzoic acid is not toxic to yeast growing in standard synthetic medium in the presence of glucose as a carbon source, it is toxic under nitrogen starvation conditions, as shown in Fig. 4, both in cells that carry auxotrophic mutations and in prototrophic yeast cells. It is also interesting that the kinetics of the loss of viability closely resemble those of autophagy-deficient mutants under nitrogen stress conditions (2). This result has several implications. First, it implies that the efficiency of benzoic acid in inhibiting yeast-mediated food spoilage is dependent on the amount and quality of the nitrogen source available, in addition to the central importance of pH. This information may allow workers to predict the types of foodstuffs in which benzoic acid is an effective growth inhibitor by assessing nitrogen availability. In this way the use of nearly toxic levels of this compound would be avoided in products that do not benefit from its addition. Our data also show that the inhibition of macroautophagy and the associated synergism with nitrogen starvation do not occur in acetic acid-treated yeast at acetic acid concentrations up to 20 mM (Fig. 5), implying that these effects are not simply due to exhaustion of ATP stores or changes in the intracellular pH, as these phenomena would be predicted to occur equally strongly with acetic acid. Surprisingly, 2 mM sorbic acid did not affect macroautophagy in our assay. While both sorbic acid and benzoic acid have been suggested to act on intracellular membranes, some differences between the effects of these compounds have been reported previously (48), although not in specific assays for cellular mechanisms, as shown here. One possible explanation is that these acids are targeted to different intracellular compartments or that their effects on membrane organization are different.
Why does benzoic acid exert specific physiological effects on yeast cells? Benzoic acid is not an arbitrary organic acid. It is a product of plant secondary metabolism that accumulates at high levels in berries, as well as in other fruit (49, 50). One possibility is that this accumulation is specifically directed against microbial growth and that evolutionary pressures have dictated specific responses in eukaryotic microorganisms, such as yeasts, which colonize these plant tissues.
Intriguingly, we found that 2 mM benzoic acid causes an increased adaptation lag phase in autophagy-deficient atg1Δ cells compared with wild-type cells (Fig. 3). Since our data indicate that there is inhibition of starvation-induced macroautophagy by benzoic acid, one would not necessarily predict that adaptation to benzoic acid would be affected in the atg1Δ strain. Atg1 is a protein kinase involved in a number of autophagic pathways besides nitrogen starvation-induced macroautophagy. These pathways include the constitutive Cvt pathway, as well as pexophagy (3, 18). Other specific forms of autophagy, such as mitophagy (10), have also been postulated but have not been observed as regulated events in yeast. One possible explanation for our results is that benzoic acid adaptation requires the induction of an autophagy-like pathway that also utilizes Atg1, excluding this protein from functioning in nitrogen starvation-induced macroautophagy. Such a switch in the function of Atg1 in the presence of benzoic acid could then explain the inhibition of starvation-induced macroautophagy.
Identification of such an autophagy-related, benzoic acid-induced pathway is the target of future research. It is important to stress that it is unlikely that inhibition of starvation-induced macroautophagy is the molecular basis of cell growth inhibition since macroautophagy is not essential for cell growth. However, it is possible that a variant form of autophagy, which also requires the Atg1 protein kinase, is part of a stress response that allows cells to adapt to benzoic acid. Alternatively, the inhibition of the early secretory pathway (Fig. 2) could explain growth inhibition, since ER-to-Golgi complex transport is essential for cell growth and proliferation.
An important goal in food science is identification of stress elements that synergize to create optimal preservation strategies (7, 8). It has already been pointed out that several classical stress conditions enhance the effects of preservatives on yeast and bacterial cells (41) and that such findings can be used to optimize food preservation processes. Our results extend these findings to benzoic acid and nitrogen starvation.
This information may be used directly. For instance, in a process in which nitrogen-poor ingredients are added to nitrogen-rich ingredients, it may be advantageous to add the preservative to the nitrogen-poor component before the nitrogen-rich component is added in order to prevent inoculation of the nitrogen-rich material with vegetative spores and cells that survive the nitrogen-poor conditions and begin to grow upon addition of the nitrogen-rich component.
A less immediate implication of our findings is that a compound which activates the fungal nitrogen starvation response may be able to enhance the action of benzoic acid at levels that are nontoxic to humans. One possible lead compound for such studies may be rapamycin, a small macrolide antibiotic. Rapamycin inhibits the TOR signal transduction pathway in eukaryotic cells, leading to a nitrogen starvation response. Since rapamycin also affects mammalian cells by acting as an immunosuppressant, it is obviously not a candidate for a food additive. Nonetheless, rapamycin analogs that are yeast or fungus specific may be produced in the future, and such compounds will clearly have applications in food preservation and in the treatment of fungal infections.
The elucidation of the molecular mechanism by which benzoic acid inhibits macroautophagy and extension of these studies to other organic acids and spoilage organisms are likely to facilitate these goals.
Acknowledgments
We thank Oded Yarden and Peter Piper for helpful discussions, Dan Klionsky for anti-Ape1 and anti-CPY antibodies, and Yoshinori Ohsumi for the ATG1 deletion construct. We also thank Mina Herschmann for excellent technical assistance and Jeffrey E. Gerst for critical reading of the manuscript.
This work was funded by Vigevani Fund Hebrew University career development award 3014218 and by Charles H. Revson grant 496/03 from the Israel Academy of Science.
REFERENCES
- 1.Abeliovich, H., T. Darsow, and S. D. Emr. 1999. A t-SNARE/Sec1p complex composed of Tlg2p and Vps45p is required for cytoplasm to vacuole targeting of aminopeptidase I. EMBO J. 18:6005-6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abeliovich, H., W. A. J. Dunn, J. Kim, and D. J. Klionsky. 2000. Dissection of autophagosome biogenesis into distinct nucleation and expansion steps. J. Cell Biol. 151:1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abeliovich, H., and D. J. Klionsky. 2001. Autophagy in yeast: mechanistic insight and physiological function. Microbiol. Mol. Biol. Rev. 65:463-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abeliovich, H., C. Zheng, W. A. Dunn, K. M. Shokat, and D. J. Klionsky. 2003. Chemical genetic analysis of Apg1 reveals a non-kinase role in the induction of autophagy. Mol. Biol. Cell 14:477-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer, B. E., D. Rossington, M. Mollapour, Y. Mamnun, K. Kuchler, and P. W. Piper. 2003. Weak organic acid stress inhibits aromatic amino acid uptake by yeast, causing a strong influence of amino acid auxotrophies on the phenotypes of membrane transporter mutants. Eur. J. Biochem. 270:3189-3195. [DOI] [PubMed] [Google Scholar]
- 6.Bracey, D., C. D. Holyoak, and P. J. Coote. 1998. Comparison of the inhibitory effect of sorbic acid and amphotericin B on Saccharomyces cerevisiae: is growth inhibition dependent on reduced intracellular pH? J. Appl. Microbiol. 85:1056-1066. [DOI] [PubMed] [Google Scholar]
- 7.Brul, S., and P. Coote. 1999. Preservative agents in foods. Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 50:1-17. [DOI] [PubMed] [Google Scholar]
- 8.Brul, S., P. Coote, S. Oomes, F. Mensonides, K. Hellingwerf, and F. Klis. 2002. Physiological actions of preservative agents: prospective of use of modern microbiological techniques in assessing microbial behaviour in food preservation. Int. J. Food Microbiol. 79:55-64. [DOI] [PubMed] [Google Scholar]
- 9.Deak, T. 1991. Food borne yeasts. Adv. Appl. Microbiol. 36:179-278. [DOI] [PubMed] [Google Scholar]
- 10.Elmore, S. P., T. Qian, S. F. Grissom, and J. J. Lemasters. 2001. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J. 15:2286-2287. [DOI] [PubMed] [Google Scholar]
- 11.Fleet, G. 1992. Spoilage yeasts. Crit. Rev. Biotechnol. 12:1-44. [DOI] [PubMed] [Google Scholar]
- 12.Guthrie, C., and G. R. Fink (ed.). 1991. Guide to yeast genetics and molecular biology, vol. 194. Academic Press, New York, N.Y.
- 13.Hamasaki, M., T. Noda, and Y. Ohsumi. 2003. The early secretory pathway contributes to autophagy in yeast. Cell Struct. Funct. 28:49-54. [DOI] [PubMed] [Google Scholar]
- 14.Hatzixanthis, K., M. Mollapour, I. Seymour, B. E. Bauer, G. Krapf, C. Schuller, K. Kuchler, and P. W. Piper. 2003. Moderately lipophilic carboxylate compounds are the selective inducers of the Saccharomyces cerevisiae Pdr12p ATP-binding cassette transporter. Yeast 20:575-585. [DOI] [PubMed] [Google Scholar]
- 15.Holyoak, C. D., D. Bracey, P. W. Piper, K. Kuchler, and P. J. Coote. 1999. The Saccharomyces cerevisiae weak-acid-inducible ABC transporter Pdr12 transports fluorescein and preservative anions from the cytosol by an energy-dependent mechanism. J. Bacteriol. 181:4644-4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holyoak, C. D., M. Stratford, Z. McMullin, M. B. Cole, K. Crimmins, A. J. Brown, and P. J. Coote. 1996. Activity of the plasma membrane H+-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl. Environ. Microbiol. 62:3158-3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamada, Y., T. Funakoshi, T. Shintani, K. Nagano, M. Ohsumi, and Y. Ohsumi. 2000. Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J. Cell Biol. 150:1507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, J., and D. J. Klionsky. 2000. Autophagy, cytoplasm-to-vacuole targeting pathway, and pexophagy in yeast and mammalian cells. Annu. Rev. Biochem. 69:303-342. [DOI] [PubMed] [Google Scholar]
- 19.Klionsky, D. 1998. Nonclassical protein sorting to the vacuole. J. Biol. Chem. 273:10807-10810. [DOI] [PubMed] [Google Scholar]
- 20.Klionsky, D. J., J. M. Cregg, W. A. Dunn, Jr., S. D. Emr, Y. Sakai, I. V. Sandoval, A. Sibirny, S. Subramani, M. Thumm, M. Veenhuis, and Y. Ohsumi. 2003. A unified nomenclature for yeast autophagy-related genes. Dev. Cell 5:539-545. [DOI] [PubMed] [Google Scholar]
- 21.Klionsky, D. J., R. Cueva, and D. S. Yaver. 1992. Aminopeptidase I of Saccharomyces cerevisiae is localized to the vacuole independent of the secretory pathway. J. Cell Biol. 119:287-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klionsky, D. J., P. K. Herman, and S. D. Emr. 1990. The fungal vacuole: composition, function, and biogenesis. Microbiol. Rev. 54:266-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs, H. A., D. Wiggins, M. Stubbs, A. Sols, and F. Bedoya. 1983. Studies on the mechanism of the antifungal action of benzoate. Biochem. J. 214:657-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert, R. J., and M. Stratford. 1999. Weak-acid preservatives: modelling microbial inhibition and response. J. Appl. Microbiol. 86:157-164. [DOI] [PubMed] [Google Scholar]
- 25.Ludovico, P., F. Sansonetty, M. T. Silva, and M. Corte-Real. 2003. Acetic acid induces a programmed cell death process in the food spoilage yeast Zygosaccharomyces bailii. FEM Yeast Res. 3:91-96. [DOI] [PubMed] [Google Scholar]
- 26.Ludovico, P., M. J. Sousa, M. T. Silva, C. Leao, and M. Corte-Real. 2001. Saccharomyces cerevisiae commits to a programmed cell death process in response to acetic acid. Microbiology 147:2409-2415. [DOI] [PubMed] [Google Scholar]
- 27.Matsuura, A., M. Tsukada, Y. Wada, and Y. Ohsumi. 1997. Apg1p, a novel protein kinase required for the autophagic process in Saccharomyces cerevisiae. Gene 192:245-250. [DOI] [PubMed] [Google Scholar]
- 28.Mollapour, M., and P. W. Piper. 2001. The ZbYME2 gene from the food spoilage yeast Zygosaccharomyces bailii confers not only YME2 functions in Saccharomyces cerevisiae, but also the capacity for catabolism of sorbate and benzoate, two major weak organic acid preservatives. Mol. Microbiol. 42:919-930. [DOI] [PubMed] [Google Scholar]
- 29.Noda, T., A. Matsuura, Y. Wada, and Y. Ohsumi. 1995. Novel system for monitoring autophagy in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 210:126-132. [DOI] [PubMed] [Google Scholar]
- 30.Pan, X., and D. S. Goldfarb. 1998. YEB3/VAC8 encodes a myristylated armadillo protein of the Saccharomyces cerevisiae vacuolar membrane that functions in vacuole fusion and inheritance. J. Cell Sci. 111:2137-2147. [DOI] [PubMed] [Google Scholar]
- 31.Piper, P., C. O. Calderon, K. Hatzixanthis, and M. Mollapour. 2001. Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 147:2635-2642. [DOI] [PubMed] [Google Scholar]
- 32.Piper, P., Y. Mahe, S. Thompson, R. Pandjaitan, C. Holyoak, R. Egner, M. Muhlbauer, P. Coote, and K. Kuchler. 1998. The pdr12 ABC transporter is required for the development of weak organic acid resistance in yeast. EMBO J. 17:4257-4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Piper, P. W. 1999. Yeast superoxide dismutase mutants reveal a pro-oxidant action of weak organic acid food preservatives. Free Radic. Biol. Med. 27:1219-1227. [DOI] [PubMed] [Google Scholar]
- 34.Restaino, L., L. M. Lenovich, and S. Bills. 1982. Effect of acids and sorbate combinations on the growth of four osmophilic yeasts. J. Food Prot. 45:1138-1141. [DOI] [PubMed] [Google Scholar]
- 35.Rothman, J. E., and G. Warren. 1994. Implications of the SNARE hypothesis for intracellular membrane topology and dynamics. Curr. Biol. 4:220-233. [DOI] [PubMed] [Google Scholar]
- 36.Sikorski, R. S., and P. Hieter. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens, T., B. Esmon, and R. Schekman. 1982. Early stages in the yeast secretory pathway are required for transport of carboxypeptidase Y to the vacuole. Cell 30:439-448. [DOI] [PubMed] [Google Scholar]
- 38.Stratford, M., and P. A. Anslow. 1996. Comparison of the inhibitory action on Saccharomyces cerevisiae of weak acid preservatives, uncouplers, and medium-chain fatty acids. FEMS Microbiol. Lett. 142:53-58. [DOI] [PubMed] [Google Scholar]
- 39.Stratford, M., and P. A. Anslow. 1998. Evidence that sorbic acid does not inhibit yeast as a classic ′weak acid preservative.' Lett. Appl. Microbiol. 27:203-206. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi, T., Y. Nakakita, J. Watari, and K. Shinotsuka. 2000. Application of a bioluminescence method for the beer industry: sensitivity of MicroStar-RMDS for detecting beer-spoilage bacteria. Rapid Microbe detection system. Biosci. Biotechnol. Biochem. 64:1032-1037. [DOI] [PubMed] [Google Scholar]
- 41.ter Steeg, P. F., J. C. Hellemons, and A. E. Kok. 1999. Synergistic actions of nisin, sublethal ultrahigh pressure, and reduced temperature on bacteria and yeast. Appl. Environ. Microbiol. 65:4148-4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas, D. S. 1993. Yeasts as spoilage organisms in beverages, p. 517-561. In A. H. Rose and J. S. Harrison (ed.), The yeasts: yeast technology, 2nd ed., vol. 5. Academic Press, London, United Kingdom. [Google Scholar]
- 43.Thorsness, P. E., and E. R. Weber. 1996. Escape and migration of nucleic acids between chloroplasts, mitochondria, and the nucleus. Int. Rev. Cytol. 165:207-234. [DOI] [PubMed] [Google Scholar]
- 44.Thumm, M. 2002. Hitchhikers guide to the vacuole—mechanisms of cargo sequestration in the Cvt and autophagic pathways. Mol. Cell 10:1257-1258. [DOI] [PubMed] [Google Scholar]
- 45.Tolkovsky, A. M., L. Xue, G. C. Fletcher, and V. Borutaite. 2002. Mitochondrial disappearance from cells: a clue to the role of autophagy in programmed cell death and disease? Biochimie 84:233-240. [DOI] [PubMed] [Google Scholar]
- 46.Tsukada, M., and Y. Ohsumi. 1993. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 333:169-174. [DOI] [PubMed] [Google Scholar]
- 47.Tudor, E. A., and R. G. Board. 1993. Food-spoilage yeasts, p. 435-516. In A. H. Rose and J. S. Harrison (ed.), The yeasts: yeast technology, 2nd ed., vol. 5. Academic Press, London, United Kingdom. [Google Scholar]
- 48.Veiga, A., and A. Madeira-Lopes. 2000. Effects of weak acid preservatives on the growth and thermal death of the yeast Pichia membranifaciens in a commercial apple juice. Int. J. Food Microbiol. 56:145-151. [DOI] [PubMed] [Google Scholar]
- 49.Viljakainen, S., A. Visti, and S. Laakso. 2002. Concentrations of organic acids and soluble sugars in juices from nordic berries. Acta Agric. Scand. 52:101-109. [Google Scholar]
- 50.Visti, A., S. Viljakainen, and S. Laakso. 2003. Preparation of fermentable lingonberry juice through removal of benzoic acid by Saccharomyces cerevisiae yeast. Food Res. Int. 36:597-602. [Google Scholar]
- 51.Wang, Y. X., N. L. Catlett, and L. S. Weisman. 1998. Vac8p, a vacuolar protein with armadillo repeats, functions in both vacuole inheritance and protein targeting from the cytoplasm to vacuole. J. Cell Biol. 140:1063-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]