Abstract
Background
Adventitious root development is a complex process regulated through a variety of signaling molecules. Hydrogen gas (H2) and nitric oxide (NO), two new signaling molecules are both involved in plant development and stress tolerance.
Results
To investigate the mechanism of adventitious root development induced by hydrogen-rich water (HRW), a combination of fluorescence microscopy and molecular approaches was used to study cell cycle activation and cell cycle-related gene expression in cucumber (Cucumis sativus ‘Xinchun 4’) explants. The results revealed that the effect of HRW on adventitious root development was dose-dependent, with maximal biological responses at 50 % HRW. HRW treatment increased NO content in a time-dependent fashion. The results also indicated that HRW and NO promoted the G1-to-S transition and up-regulated cell cycle-related genes: CycA (A-type cyclin), CycB (B-type cyclin), CDKA (cyclin-dependent kinase A) and CDKB (cyclin-dependent kinase B) expression. Additionally, target genes related to adventitious rooting were up-regulated by HRW and NO in cucumber explants. While, the responses of HRW-induced adventitious root development and increase of NO content were partially blocked by a specific NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt, NO synthase (NOS)-like enzyme inhibitor NG –nitro-L-arginine methylester hydrochloride, or nitrate reductase inhibitors tungstate and NaN3. These chemicals also partially reversed the effect of HRW on cell cycle activation and the transcripts of cell cycle regulatory genes and target genes related adventitious root formation.
Conclusions
Together, NO may emerge as a downstream signaling molecule in H2-induced adventitious root organogenesis. Additionally, H2 mediated cell cycle activation via NO pathway during adventitious root formation.
Keywords: Hydrogen-rich water, Nitric oxide, Cell cycle, Cell cycle-related genes, Adventitious root formation, Cucumber
Background
Adventitious rooting is a key step in the vegetative propagation of plants. Understanding the mechanism of adventitious rooting is of significant importance to strategize breeding efforts to maximizing its marketable yield [1]. Adventitious root development is a complex process regulated by several lines of environmental and endogenous factors. In recent years, there has been increasing interest in the field of signal transduction during adventitious rooting. Untill now, nitric oxide (NO) [2, 3], Ca2+ ions, calmodulin (CaM) [4], Ca2+-dependent protein kinase activities (CDPK) [5], cyclic guanosinemonophosphate (cGMP) [6], ethylene [7], mitogen-activated protein kinase [2], carbon monoxide [8], polyamines [9], hydrogen peroxide [10], hydrogen sulfide [11] and hydrogen gas (H2) [12] have been suggested to be involved in adventitious rooting process. However, the complex network of signaling molecule associated with adventitious rooting remains unclear. A better understanding of the regulation of initiation of adventitious rooting by signaling molecules will advance our understanding of the molecular mechanisms regulating adventitious root development.
The gaseous compound NO is a redox-active small signaling molecule which may regulate almost all biotic and abiotic stress responses [13, 14]. Previous studies have also demonstrated the involvement of NO in plant various physiological processes such as maturation and senescence [15, 16], seed germination or dormancy [17, 18], floral transition [19] and stomatal movement [20]. NO is also required for root organogenesis, including lateral root formation [21], root hair formation [22], and adventitious rooting [3, 23]. Although numerous studies have demonstrated the involvement of NO in adventitious roots formation, there is currently little information on its mechanism.
H2, a colorless, odorless and tasteless gas is the structurally simplest gas in nature and known as an inert gas. Previous studies have demonstrated that H2 is a potential therapeutic medical gas [24]. It has received worldwide attention because it could selectively reduce hydroxyl radical and peroxynitrite in cell [25]. Subsequently, accumulated evidence from a variety of animal experiments and clinical tests indicated that H2 may act as an anti-inflammatory, anti-apoptotic, and anti-allergic agent [26]. More recently, there have been some reports indicating that H2 may play critical roles in plant stress response including salinity [27–29], drought [29, 30], paraquat-induced oxidative stress [30], cadmium toxicity [31], aluminum stress [32], mercury toxicity [33], and UV-A irradiation [34]. Hydrogen-rich water also could delay postharvest ripening and senescence of kiwifruit during storage by regulating the antioxidant defence [35]. Notably, Lin et al. [12] found that H2 might regulate cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. The author suggested that exogenous HRW treatment might be a good option to induce plant root organogenesis. However, the mechanism of H2 on regulating adventitious root development needs to be fully investigated.
Thus, H2 and NO have been considered as signaling modulators with multiple biological functions in plants. Little information is available, however, about the connection between H2 and NO in regulating physiological process. It was found that H2 inhibited LPS/IFNγ-induced NO production through modulation of signal transduction in macrophages and ameliorates inflammatory arthritis in mice [36]. It provided the molecular basis for H2 effects on inflammation and a functional interaction between H2 and NO. The interaction effect between H2 and NO was also found in alfalfa. Recently, HRW was reported to alleviate aluminum-induced inhibition of root elongation via decreasing NO production [32]. More recently, the crosstalk between H2 and NO was reported to play central roles in the ABA signaling cascade during stomatal movement [37].
The cell cycle consists of two major events, cell development which is the critical driving forces in completion of the ontogenic program during plants life cycle. All phases of cell cycle are regulated by the heterodimeric complexes of highly conserved proteins, cyclin-dependent kinase (CDK) and cyclin [38]. In higher eukaryotes, the activation of CYCD and CDKA leads to produce a repressor protein kinase that phosphorylates the retinoblastoma protein at G1-to-S phase transition [39]. In the next checkpoint, B-type CKDs and A-and B-type cyclins are involved in G2-to-M phase transition [40]. In addition, CDK/cyclin complexes are inactivated by a class of CDK-inhibitory proteins [41].
It was suggested that H2 and NO have positive effects on adventitious root formation. Our previous results showed that 50 μM NO donor sodium nitroprusside (SNP) had significant effect on adventitious root [10]. However, the crosstalk of NO and H2 in promoting adventitious rooting and its mechanism are still puzzled. In the study, molecular and pharmacological approaches were used to investigate the influence of H2 and NO on the adventitious root development in cucumber (Cucumis sativus ‘Xinchun 4’) explants, as well as cell cycle activation and cell cycle- and adventitious rooting related genes in hypocotyls. Therefore, the objectives of this study are to determine the role of NO in H2-regulated cell cycle during adventitious rooting.
Results
HRW promoted adventitious root development in a dose-dependent manner
To understand the effect of HRW on adventitious root development, cucumber explants were treated with different concentrations of HRW (0, 1 %, 10 %, 50 %, 100 %). As compared with the control (distilled water), HRW had significant effects on adventitious rooting (Fig. 1). Compared with the control, 1 and 10 % HRW had no significant difference in root number and root length, but the effects were significantly lower than treatments with 50 and 100 % HRW. Among the different concentrations, the maximum root number (10.13) and root length (7.84 cm) were observed with 50 % HRW treatment (Fig. 1). Therefore, the promotions of root development were maximal at 50 % HRW, and this concentration was used further for studies during rooting process.
Fig. 1.
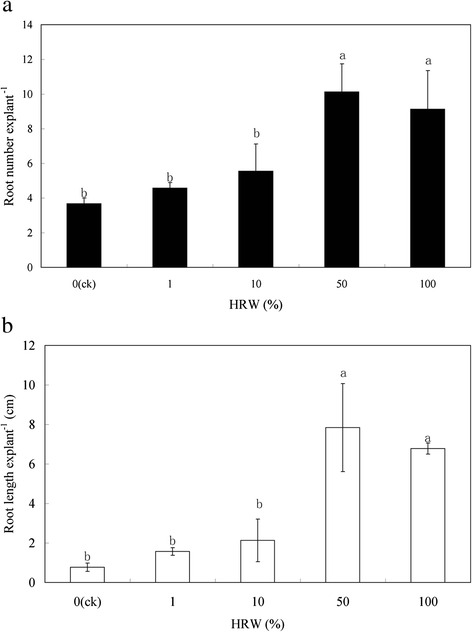
Effect of different concentrations of HRW on the induction of adventitious root development in cucumber explants. The primary root system was removed from hypocotyls of 5-day-old germinated cucumber. Explants were incubated with distilled water or different concentrations of HRWas indicated for 5 days. Adventitious root numbers (a) and length (b) were expressed as mean ± SE (n = 3) 10 explants was used in an independent experiment. Bars not sharing the same letters were significantly different by Duncan’s test (P < 0.05)
The HRW-induced adventitious root development was reversed by NO elimination
SNP induced adventitious root development, however, cPTIO reduced the positive effect of SNP. To further investigate the involvement of NO in H2-induced adventitious root development, the effects of NO scavenger cPTIO, NOS inhibitor L-NAME and NR inhibitor NaN3 and tungstate on adventitious root development of explants treated with HRW were determined. HRW -induced rooting was partially reversed by cPTIO, L-NAME tungstate or NaN3. Compared with the control, explants treated HRW plus with cPTIO, L-NAME or NaN3 resulted in a significant decrease root number and length (Fig. 2). Furthermore, other by-products of SNP decomposition had no significant promotion on adventitious root development and it was significantly lower than treatments with the active SNP. The above results demonstrated that NO may act as a downstream signaling molecular in HRW-induced adventitious root development.
Fig. 2.
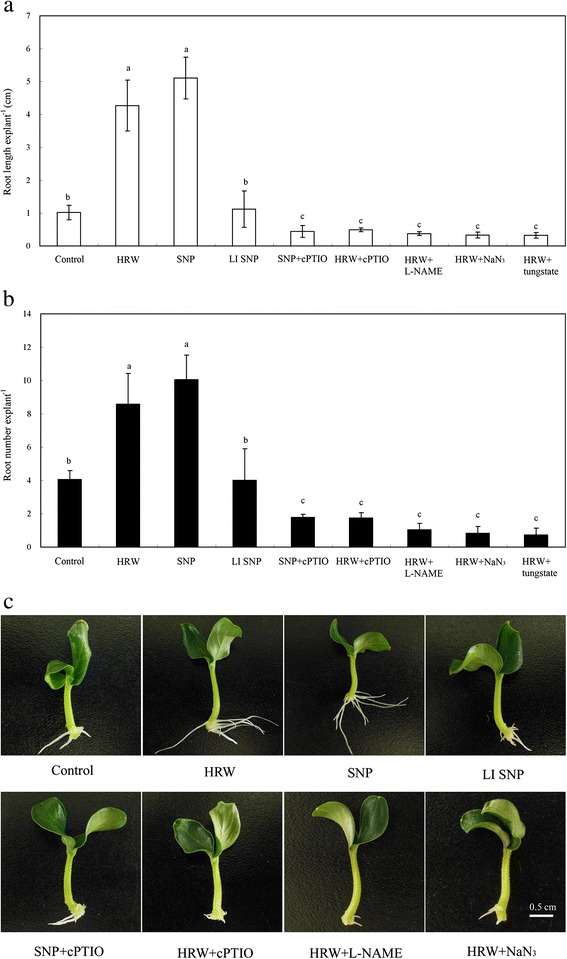
Effect of HRW, SNP, LI SNP, cPTIO, L-NAME and NaN3 on adventitious root development in cucumber explants. The primary root system was removed from hypocotyls of 5-day-old germinated cucumber. Explants of cucumber were incubated with 50 % HRW, 50 μM SNP, 50 μM LI SNP, light-inactivated sodium nitroprusside, 200 μM cPTIO, 30 μM L-NAME or 10 μM NaN3 as indicated for 5 days. Adventitious root number (a) and length (b) were expressed as mean ± SE (n = 3). 10 explants was used in an independent experiment. Bars not sharing the same letters were significantly different by Duncan’s test (P < 0.05). Photographs (c) were taken after 5 days of treatment. Bar = 5 cm
The production of endogenous NO was involved in HRW-induced of adventitious root formation
Since NO plays a key role in regulating adventitious root development, we assayed whether H2 affects NO production in hypocotyls treated with HRW or with distilled water. As shown in Fig. 3, 50 % HRW induced NO production in a time-dependent manner. After 6 h of HRW treatment, an increase in NO content was observed, reaching a maximum at 24 h of treatment. While, NO content was weakly detected in the control explants, which was significant lower than that in HRW-treated explants at 18 and 24 h (Fig. 3).
Fig. 3.
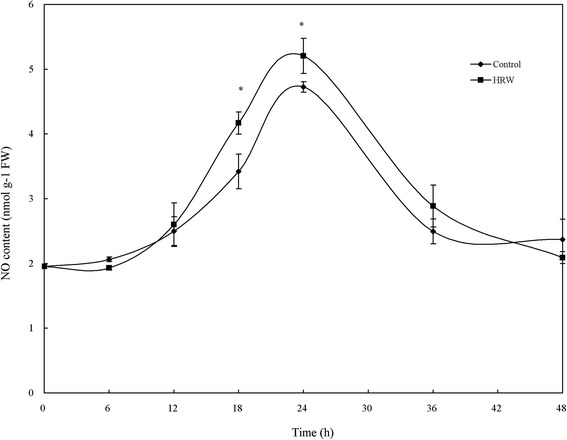
Effect of HRW on the production of endogenous NO during adventitious rooting. The primary root system was removed from the hypocotyls of 5-d-old, germinated cucumber seedlings. NO levels of hypocotyls were determined by Greiss reagent in explants treatment with distilled water (control) or 50 % HRW for 24 h. Values (means ± SE) are the averages of three independent experiments (n = 3). Asterisks indicate that mean values are significantly different between the treatments of HRW and Control (P < 0.05) according to Duncan’s multiple test
NO may be involved in HRW-induced cell cycle activation during adventitious root development
To gain insight into the mechanism of HRW-induced adventitious root development, whether NO might be involved in HRW functioning in the induction of cell-division activity was analyzed. Representative pictures of nuclei (DAPI panels), together with DNA histograms obtained from DAPI staining (HCA panels), were shown in Fig. 4a. Compared to the control samples, treatments with 50 % HRW or 50 μM SNP showed much more pronounced foci (Fig. 4a), thereby indicating more cells were at G1-to-S transition phase. However, cPTIO, L-NAME, or NaN3 reduced pronounced foci induced by HRW. DNA histograms obtained from DAPI staining, together with representative pictures of nuclei, were shown in Fig. 4a (HCA panels). DNA histograms of asynchronous cells clearly display a S peak in HRW and SNP treatment and a diploid (G1) peak in the control, HRW + cPTIO, HRW + L-NAME and HRW + NaN3 treatments.
Fig. 4.
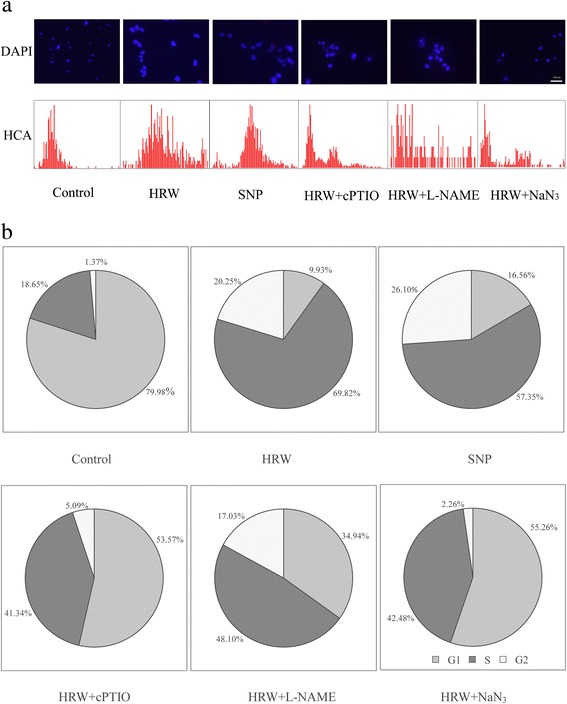
Effect of HRW, SNP, cPTIO, L-NAME and NaN3 on cell cycle phase distribution during adventitious rooting. The primary root system was removed from hypocotyls of 5-day-old germinated cucumber. Explants of cucumber were incubated with 50 % HRW, 50 μM SNP, 200 μM cPTIO, 30 μM L-NAME or 10 μM NaN3 as indicated for 6 h. Cucumber hypocotyls were fixed by paraformaldehyde and nuclei were extracted by chopping in Galbraith buffer. The nuclei were resulted with DAPI, and then visualized by fluorescent microscopy; the same samples were processed for multiparametric cell cycle high-content analysis (HCA) (a). Bar = 100 μm. The relative fluorescence intensity of nuclei was analyzed by Image Pro software (b)
The highest percentage of cells in G1 phase was found in the control, which were 700.50 % and 634.20 % higher than that in HRW and SNP treatments, respectively (Fig. 4b). However, the percentage of total cell population in HRW and SNP treatments was higher than that in the control (Fig. 4b). Thus, H2 and NO promoted G1 to S transition phase, suggesting that a subpopulation of cells in G1 phase was induced to enter a new cell cycle in a synchronous manner. If cPTIO, L-NAME, or NaN3 was added to the HRW solution simultaneously, the percentage of cells in G1 phase was increased by 43.64, 25.01 and 45.33 %, but the percentage of cells in S phase was decreased by 28.48, 21.72 and 27.3 % (Fig. 4b).
NO participates in HRW-mediated transcript levels of cell cycle regulatory genes during adventitious root development
To examine the molecular mechanism of HRW-induced phase changes during adventitious root development, the changes in the expression of two cyclins (CycA and CycB) and two CDK (CDKA and CDKB) genes were analyzed by qRT-PCR. As shown in Fig. 5a, HRW and SNP increased the expression of CycA by 15.14 and 26.28 % in comparison with the control, respectively. However, compared with HRW treatment, the expression of CycA was significantly reduced by 83.00, 67.70 and 98.90 % in the treatments of HRW + cPTIO, HRW + L-NAME and HRW + NaN3 (Fig. 5a). Figure 5b showed that HRW and SNP treatments increased the expression of CycB to 138.83 and 148.11 % of the control. Subsequent observation showed that the applications of cPTIO, L-NAME and NaN3 were able to down-regulate HRW-mediated expression of CycB by 64.97, 54.50 and 96.30 %, respectively (Fig. 5b). The transcript level of CDKA in explants treated with HRW and SNP increased and reached a maximum 145.38 and 164.21 % increase compared to the control. However, when, cPTIO, L-NAME and NaN3 were administered to HRW-treated explants, it resulted 26.96, 20.44, and 23.51 % reduction in the expression of CDKA (Fig. 5c). Meanwhile, the higher transcript levels of CDKB was observed in the HRW and SNP treatments, which were 143.72 and 163.95 % higher than that of controls. In addition, cPTIO, L-NAME and NaN3 decreased the transcription levels of the CDKB in HRW treatment (Fig. 5d). These findings suggested that NO might be involved in H2-induced the expression of cell cycle regulatory genes.
Fig. 5.
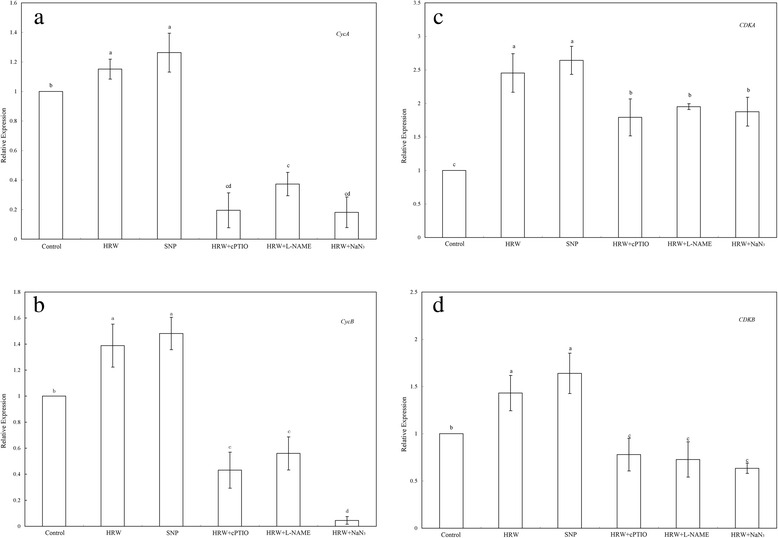
Effect of HRW, SNP, cPTIO, L-NAME and NaN3 on the expression of cell cycle-related genes in adventitious rooting. The primary root system was removed from hypocotyls of 5-day-old germinated cucumber. Explants of cucumber were incubated with 50 % HRW, 50 μM SNP, 200 μM cPTIO, 30 μM L-NAME or 10 μM NaN3 alone, or in the combination treatments as indicated for 6 h. Then, CycA (a), CycB (b), CDKA (c) and CDKB (d) expression levels were analyzed by real-time RT-PCR. The expression levels of the genes were presented as values relative to water treatment (Con). Values (means ± SE) are the averages of three independent experiments. Bars with different letters were significantly different in comparison with the control at P < 0.05 according to Duncan’s multiple test
NO was responsible for HRW-induced the expression profiles of CsDNAJ-1,CsDPK1 and CsCDPK5 during adventitious root development
Furthermore, CsDNAJ-1, CsCDPK1 and CsCDPK5 gene expression was used as a molecular probe to investigate the molecular mechanism of HRW-induced adventitious rooting. Compared with the control, HRW and SNP treatment were able to induce higher expression of the CsDNAJ-1, CsCDPK1 and CsCDPK5 during the first 24 h period of treatments (Fig. 6). These expressions were well matched with the number and length of adventitious root observed after another 4 days of treatment. It was interesting to note that the expression of CsDNAJ-1 induced by HRW and SNP was 252.67 and 236.35 % higher than the control treatment (Fig. 6a). The CsCDPK1 and CsCDPK5 transcript level was induced by HRW and SNP also higher than that the control (Fig. 6 a and b). The co-treatment of HRW and cPTIO down-regulated the transcript levels of CsDNAJ-1, CsCDPK1 and CsCDPK5 by 27.46, 33.56 and 54.20 %, respectively (Fig. 6). Meanwhile, if L-NAME was administered to HRW-treated explants, it also resulted in a reduction in these genes expression. Compared with HRW treatment, HRW + NaN3 treatment significantly decreased the transcript levels of these genes (Fig. 6). Thus, the HRW-induced expression of CsDNAJ-1, CsCDPK1 and CsCDPK5 genes was significantly inhibited by cPTIO, L-NAME and NaN3. The above results further strengthened the hypothesis that NO might be, at least partially, involved in HRW-induced adventitious root development.
Fig. 6.
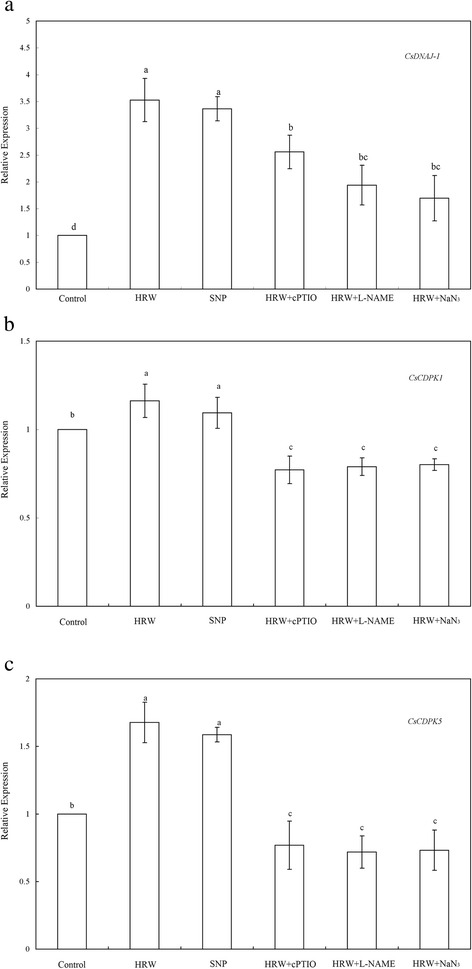
Effect of HRW, SNP, cPTIO, L-NAME and NaN3 on the expression profiles of CsDNAJ-1(a), CsDPK1(b), CsCDPK5(c) in the induction of adventitious rooting. The primary root system was removed from hypocotyls of 5-day-old germinated cucumber. Explants of cucumber were incubated with 50 % HRW, 50 μM SNP, 200 μM cPTIO, 30 μM L-NAME or 10 μM NaN3 alone, or in the combination treatments as indicated for 24 h. Then, relative gene expression was analyzed by real-time RT-PCR. The expression levels of the genes were presented as values relative to water treatment (Con). Values (means ± SE) are the averages of three independent experiments. Bars with different letters were significantly different in comparison with the control at P < 0.05 according to Duncan’s multiple test
Discussion
Adventitious roots play important roles in nutrient and water uptake in plant; and their formation is widely used for plant clonal propagation. Previous studies have shown the role and relationship between H2 and NO in plant stress response [32] and stomatal closure [37]. However, there is little research in the crosstalk between H2 and NO during adventitious rooting. Here, we focus on the involvement of NO H2-induced in cell cycle activation during adventitious rooting.
H2 has aroused worldwide attention because of its selective reduction [25]. Accumulating evidence indicates that H2 is a new signaling molecular and plays important roles in plants. In the present study, we illustrated that HRW, when applied exogenously, enhanced the number and length of adventitious root in a dose-dependent manner (Fig. 1). These findings are also consistent with a previous report showing that exogenous H2 was able to regulate cucumber adventitious root development [12]. Much research has focused on the physiological roles of H2 in plant responses against salt stress [27, 28], cadmium toxicity [31], mercury toxicity [33], paraquat-induced oxidative stress [30] and aluminum stress [32]. Ample evidences have shown that NO classified as a gasotransmitter was able to induce adventitious rooting [23]. We have shown previously that NO, when applied exogenously, also promoted the formation of adventitious root in marigold [10]. NO was found to promote adventitious root development in Panax ginseng by the generation of O2− [42]. In order to ascertain whether NO signaling pathway is involved in H2-induced adventitious root, NO scavenger cPTIO and inhibitors L-NAME tungstate and NaN3 were used in the experiment. We found that cPTIO, L-NAME tungstate and NaN3 caused partial inhibition of HRW-induced adventitious root development (Fig. 2). In view of the inhibitory roles of NO-scavenger, NOS-inhibitor and NR-inhibitor in HRW-induced response, NO might be an essential gas signaling molecule in H2-mediated adventitious rooting. Until now, the relationship between H2 and NO both in animals and plants still remains to be elucidated. Although H2 could not inhibit intracellular NO production, it significantly suppressed NO-induced cytotoxicity in PC12 cells [43]. In alfalfa, H2 alleviated aluminum stress via decreasing NO production [32]. Xie et al. [37] demonstrated that NO production was contributed to H2-promoted stomatal closure in Arabidopsis. Our results also showed that when NO production was blocked, the promotive roles of HRW in adventitious rooting were reversed (Fig. 2). These results indicate that NO may act as a downstream signaling molecule involved in H2-induced adventitious root development. However, further studies are needed to determine the crosstalk between H2 and NO in different physiological processes.
Previous animal researches showed that there is a relationship between H2 and NO in some situations [36, 43]. In plants, Xie et al. [37] discovered that exposure of Arabidopsis to HRW resulted in an increase in NO content. Additionally, the effect of H2 in alleviating aluminum-induced inhibition of root elongation in alfalfa may derive from the decrease of NO [32]. In addition, an increase in H2-mediated NO production catalyzed by NOS-like protein and NR might be required and be part of the molecular events involved in H2 action. Here, our evidence supports the possibility that NOS-like- and NR-dependent NO production contributes to H2-promoted adventitious root development in cucumber (Fig. 3). Coincidently, it has been demonstrated that NO levels are apparent during the H2-induced stomatal closure [37]. It has also been found opposite observation that H2 decreased NO production in aluminum-induced inhibition of root elongation [32]. Therefore, present researches indicate that there is a complex interaction between H2 and NO in some physiological processes.
Previous studies have shown that cell cycle regulation in the xylem pericycle may play crucial roles in root organogenesis [44, 45]. Cell cycle regulation occurred in the xylem pericycle, in which cells proceed to G2 phase, whereas the rest of pericycle remained at G1 phase [46]. Here, our analyses indicated that that H2- and NO-induced an accumulation of cells in the S phase during adventitious rooting, indicating that H2- and NO-induced cell cycle activation contributed to rooting (Fig. 4). It has been previously reported that cell cycle induction played a key function in adventitious root growth [45] and lateral root formation [47]. This is the first report to show that H2 and NO are involved in cell cycle progression during adventitious root development. Interestingly, cPTIO, L-NAME, and NaN3 all partially reduced H2-induced accumulation of cells in S phase and then inhibited the adventitious root development (Fig. 4). It is generally accepted that plant hormones play a central role in the reactivation of the cell cycle during root development [48]. The inducible effect of NO on cell cycle activation has been reported in tomato lateral formation [47]. The authors noted that auxin-dependent cell cycle gene regulation might be dependent on NO. Thus, our pharmacological evidence supports the possibility that, at least in our experimental condition, H2 and NO might form a linear signaling pathway in regulating cell cycle activation during adventitious rooting.
Cell-cycle regulatory genes have been shown to be involved in regulating cell division in internode growth [49] and root meristem induction [45]. To understand the mechanisms of NO involved in H2-regulated cell cycle that resulting in root initiation, detailed molecular studies were conducted in this study. The genes involved in the transition of G1 to S, CycA, CycB, CDKA and CDKB were significantly up-regulated by NO and H2 treatments (Fig. 5), which consistent with the phase change of cell cycle. Otvos et al. [50] reported that NO promoted cell division and embryogenic cell formation in leaf protoplast-derived cells of alfalfa. NO transiently induced CyCA2;1 and CYCD3;1 mRNA expression in cell suspensions. These results correspond well with the expression of CycA and CycB in our experimental condition. Previous study also found that NO mediated the induction of the CYCD3;1 gene at the beginning of lateral root primordial formation [47]. Thus, these results validate the involvement of NO in regulating cell cycle-related genes during lateral and adventitious rooting. It is noteworthy that the expression of CDKA was markedly up-regulated by H2 and NO, suggesting that A-type CDKs may play a main role in G1-to-S transition. Himanen et al. [44] also found that the transcription level of CDKA was induced in auxin-mediated cell cycle activation during early lateral root initiation. The level of the CDKA transcript remained at high levels in NO treatment during lateral rooting indicating that pericycle cells may be competent for cell division in tomato [47]. Additionally, our data showed that the transcript level of CDKB was less than that of CDKA after H2 treatment. It may because B-type CDKs regulates cell cycle progression to the mitotic phase, G2 to M [51]. This study further showed that the transcript levels of CycA, CycB, CDKA and CDKB induced by H2 were partially inhibited by the NO scavenger cPTIO, NOS inhibitor L-NAME and NR inhibitor NaN3 (Fig. 5). Thus, the transcript profile presented here allows a simple model to be proposed that NO may be involved in cell cycle activation during H2-induced adventitious rooting. The evidence provided here further confirms that H2 and NO may specifically regulate cell cycle activation and adventitious root formation. Furthermore, NO may be downstream signal molecule during H2 –induced adventitious rooting, present in competent pericycle cells, which finally activates cell cycle-related genes.
It has been observed that the target genes of adventitious root development, CsDNAJ-1, CsCDPK1 and CsCDPK5 could be induced by auxin, CO [8], and hydrogen sulfide [11]. All DnaJ-like proteins characterized by a J domain regulated interactions with Hsp70 in protein folding and the assembly and disassembly of protein complexes. It has illustrated that the genes of DnaJ-like protein (s) and calcium-dependent protein kinases (CDPKs) were involved in the initiation and development of adventitious root [8, 52]. Additionally, IAA and NO induced the activity of CDPK that associated with cell differentiation, division, and /or differentiation during the formation of adventitious root [5]. In this study, the molecular evidence showed that H2 and NO treatments both induced high expression of the CsDNAJ-1, CsCDPK1 and CsCDPK5 genes (Fig. 6), which were consistent with the number of adventitious roots observed. Bai et al. [52] found that IAA, 3-O-C10-HL and H2O2 increased the expression of CDC2, ARC2, and CDPK as well as the Aux/IAA gene family members AUX22c, AUX22d, and AUX22e in mung bean. Recently, Lin et al. [12] also reported that H2 induced the expression of target genes related to adventitious root formation in a heme oxygenase-1/carbon monoxide-dependent manner during cucumber adventitious rooting. Application of NO scavenger cPTIO, NOS inhibitor L-NAME and NR inhibitor NaN3 were able to down-regulate H2-induced transcription levels of CsDNAJ-1, CsCDPK1 and CsCDPK5 (Fig. 6). Thus, we deduced that H2-induced CsDNAJ-1, CsCDPK1 and CsCDPK5 expression might be regulated by NO production. These findings might suggest that CsDNAJ-1, CsCDPK1 and CsCDPK5 expression at the earlier state might be required for H2-induced adventitious root development in a NO-independent manner.
Conclusion
Taken together, our results suggested that both H2was involved in adventitious root development and NO might be downstream signal molecules in the H2 signalling cascade. Meanwhile, the evidence presented here indicated that H2 activated cell cycle and up-regulated cell cycle-related genes and target genes related to adventitious rooting via NO pathway (Fig. 7). However, the network responsible for adventitious root development induced by H2 and NO may be very complex. Therefore, considerably more work will be done for further investigation of the molecular mechanism underlying H2- and NO-induced adventitious rooting.
Fig. 7.
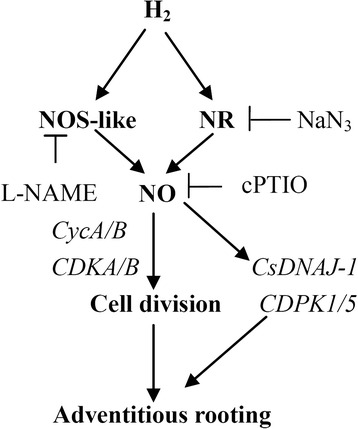
Schematic model of the signaling networks involving H2 and NO during adventitious root development in cucumber. The H2 triggers a transient NO accumulation. In turn, NO induces the division of cell and the expression of cell cycle-related gens, CycA/B and CDKA/B. The above pathway might be mediated by the expression of CsDNAJ-1 and CDPK1/5 genes. T bars, inhibition
Methods
Plant material and growth conditions
Cucumber (Cucumis sativus ‘Xinchun 4’) seeds were kindly supplied by Gansu Academy of Agricultural Sciences, Lanzhou, China. Selected identical seeds were germinated in Petri dishes on filter papers soaked in distilled water, then transferred to an illuminating incubator and maintained at 25 ± 1 °C for 5d with a 14-h photoperiod (photosynthetically active radiation = 200 μmol m−2 s−1). Primary roots of 5-d-old seedlings were removed and the cucumber explants were then maintained under the same temperature and photoperiod conditions described above for another 5 d in the presence of different media as indicated below. Then, records were taken of root number per explants, root length. Meanwhile, corresponding photographs were taken.
Treatments of explants, chemicals
After primary roots were removed, every ten cucumber explants were put into a Petri dish containing 6 ml of distilled water, different concentration of hydrogen-rich water (HRW) as indicated in Fig. 1. 30 μM N-nitro-L-arginine methyl ester (L-NAME, Sigma, USA) or 200 μM 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO, Sigma, USA), or 100 μM tungstate or 10 μM NaN3 were added together with optimum concentration of HRW. 200 μM cPTIO was added together with optimum concentration of SNP. Inactivated SNP (50 μM) had previously been exposed to light to drive off NO about 24 h. The concentration of these chemicals was selected based on the results of preliminary experiment and previous experiment [10]. The solutions were prepared in complete darkness, and immediately diluted to the demanded concentrations at pH values of 6.5. Unless stated otherwise, the remaining chemicals were of analytical grade from Chinese companies.
Preparation of hydrogen-rich water (HRW)
Purified H2 gas (99.99 %, v/v) generated from a hydrogen gas generator (QL-300, Saikesaisi Hydrogen Energy Co., Ltd., China) was bubbled into 2 l distilled water at a rate of 300 ml min−1 for 30 min. Then, the corresponding HRW was rapidly diluted to required saturations (1, 10, 50 %, [v/v]). In our experimental conditions, H2 concentration in freshly prepared HRW determined with a “Dissolved hydrogen portable meter” (Trustlex Co., Led, ENH-1000, Japan) was 0.45 mM, and maintained at a relative constant level in 25 °C for at least 12 h.
Determination endogenous NO content
NO content was determined using the Greiss reagent method [3] with some modifications. Samples of cucumber hypocotyl (0.2 g) were frozen in liquid nitrogen, then ground in a mortar and pestle in 4 mL of 50 mM ice-cold acetic acid buffer, pH 3.6, containing 4 % (w/v) zinc diacetate. The homogenates were centrifuged at 10,000 × g for 15 min at 4 °C, and the supernatants were collected. For each sample, 0.1 g charcoal (Shanghai Chemical Reagent Co. Ltd.) was added. After vortex mixing and filtration, the filtrate was leached and collected. A mixture of 1 mL of filtrate and 1 mL of Greiss reagent was incubated at room temperature for 30 min to concert nitrite into a purple azo-dye. The absorbance was then assayed at 540 nm. NO content was calculated by comparison to a standard curve of NaNO2.
Hypocotyl cell nucleus extracts
Cucumber hypocotyls were excised about 0.5 cm and fixed in 4 % formaldehyde (0.1 M phosphate buffer, pH 7.4) for 15 min. Then the hypocotyls were cut up with blade in Galbraith butter (45 mM MgCl2, 30 mM sodium citrate, 20 mM 4-morpholinopropanesulfonic acid). Finally, cell nucleus suspension was collected with 30 μm mesh screen in 5 ml tube.
Fluorescence microscopic image analysis of the cell cycle
Microscopy was conducted as described by [53] with modifications. Cell nucleus suspension added fluorochrome DAPI (1:100000, Molecular probe), 0.1 % Triton-X 100 and 50 μg mL−1 Rnase1 (DNase-free, Qiagen) was observed and taken photos with fluorescence microscope (Leica 400×, Planapo, Wetzlar, Germany). Microscopic photography conditions setting value was fixed and no excessive exposure of the nucleus. Each sample was used to analysis at least 1000 the fluorescence microscopic images of nucleus. The fluorescence intensity of nucleus (grey value) was measured with the help of Image Pro software (Media Cyberntics, USA).
RNA extraction
Total RNA was extracted from about 200 mg (fresh-weight) excised cucumber hypocotyl (5 mm) 6 h and 24 h after treatment using TRIZOL reagent (Sangon, China) according to the manufacturer’s instructions.
Transcript level estimation with qRT-PCR
Quantitative Real-time PCR (qRT-PCR) reactions were performed using an ABI StepOne Plus system (Applied Biosystems, Carlsbad, CA) along with Qiagen Quantifast SYBR Green PCR Kit (Huaxia Ocean Science and Technology Con., Ltd., China). Gene-specific primers of cell cycle-related genes (at 6 h after treatment) and target genes (at 24 h after treatment) responsible for adventitious rooting for qRT-PCR were amplified using the primers in Table 1. Each reaction (20 μl total volume) consisted of 10 μl iQ SYBR Gree Supermix, 1 μl of diluted cDNA and 0.4 μl of forward and reserve primers. PCR cycling conditions were as follows: 5 min at 95 °C followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C with data collection at the annealing step. After the 40 cycles, we included a dissociation/melting curve stage with 15 s at 95 °C, 60 s at 60 °C, and 15 s at 95 °C. The cucumber actin gene was used as an internal control. The calculation of relative gene expression was conducted as described by Livak and Schmittgen [54].
Table 1.
Primers used for qPT-PCR assays
| Gene | Accession no. | Primer pairs |
|---|---|---|
| CycA | EW968279 | F: 5′- GCCTCTGCTGTAACAACACTCAT-3′ |
| R: 5′- TGTGCTGGCTGTATTTTTCTCTG -3′ | ||
| CycB | EW968280 | F: 5′- AATGAGGGCTATTTTGGTGGA-3′ |
| R: 5′- TATCCGAAAGGCACACAAAGTC-3′ | ||
| CDKA | EW968281 | F: 5′- ATCTAAAACCCCAAAATCTGCT-3′ |
| R: 5′- CAAATGCTCTTGCCAGTCC -3′ | ||
| CDKB | EW968282 | F: 5′-CAATCCCTCTATGTCGTTCG-3′ |
| R: 5′- CAAATGCTCTTGCCAGTCC-3′ | ||
| CsDNAJ-1 | X67695 | F: 5′- GACCACTCTCCACGATGTCAAC-3′ |
| R: 5′- ATCAATGTGTTATGGCGGTAGC-3′ | ||
| CDPK1 | AJ312239 | F: 5′- GGAGTTGGAAGGAGGACGATG-3′ |
| R: 5′- TGAGATTTAGCAGTAAGGACGC-3′ | ||
| CDPK5 | AY02785 | F: 5′- ATGAGGAAAGGCAATCAGGAAT-3′ |
| R: 5′- AAAGAAGCACATAAAATCAAGCAGA | ||
| actin | DQ641117 | F: 5′-CCCATCTATGAGGGTTACGCC-3′ |
| R: 5′-TGAGAGCATCAGTAAGGTCACGA-3′ |
Statistical analysis
Where indicated, results were expressed as the mean values ± SE of at least three independent experiments. Statistical analysis was performed using the Statistical Package for Social Sciences for Windows (version 13.00; SPSS, Inc., Chicago, IC, USA). For statistical analysis, Duncan’s multiple test (P < 0.05) was chose as appropriate.
Abbreviations
CDKA, cyclin-dependent kinase A; CDKB, cyclin-dependent kinase B; CycA, A-type cyclin; CycB, B-type cyclin; DAPI, 4′, 6-diamidino-2-phenylindole; H2, hydrogen gas; H2O2, hydrogen peroxide; HRW, hydrogen-rich water; LI SNP, Light-inactivated SNP; L-NAME, NO synthase (NOS)-like enzyme inhibitor NG –nitro-L-arginine methylester hydrochloride; NO, nitric oxide; SNP, souium nitroprusside; cPTIO, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt
Acknowledgments
The authors are grateful to the editors and the anonymous reviewers for their valuable comments and help.
Funding
This research was supported by the National Natural Science Foundation of China (no. 31160398, 31560563), the Post Doctoral Foundation of China (nos. 20100470887, 2012t50828), the Key Project of Chinese Ministry of Education (no. 211182), the Research Fund for the Doctoral Program of Higher Education (no. 20116202120005), and the Natural Science Foundation of Gansu Province, China (no. 1308RJZA179, 1308RJZA262).
Availability of data and materials
Raw data could be obtained by request to the corresponding author.
Authors’ contributions
YZ and WL designed research; YZ, MW and LN performed research; YZ, WL and ZM contributed analyzed data; and YZ and WL wrote the paper. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interest.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Contributor Information
Yongchao Zhu, Email: 519158840@qq.com.
Weibiao Liao, Email: liaowb@gsau.edu.cn.
Lijuan Niu, Email: 502345778@qq.com.
Meng Wang, Email: 1002310717@qq.com.
Zhanjun Ma, Email: mazhanjun@gsau.edu.cn.
References
- 1.de Klerk GJ, Ter Bruge J, Marinova S. Review the formation of adventitious roots: New concepts, new possibilities. In Vitro Cell Dev-Pl. 1999;35:189–199. doi: 10.1007/s11627-999-0076-z. [DOI] [Google Scholar]
- 2.Pagnussat CG, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol. 2004;135:279–286. doi: 10.1104/pp.103.038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao WB, Xiao HL. Nitric oxide and hydrogen peroxide are involved in indole-3-butyric acid-induced adventitious root development in marigold. J Hortic Sci and Biotech. 2011;86:159–165. doi: 10.1080/14620316.2011.11512742. [DOI] [Google Scholar]
- 4.Liao WB, Zhang ML, Huang GB, Yu JH. Ca2+ and CaM are involved in NO-and H2O2-induced adventitious root development in marigold. J Growth Regul. 2012;31:253–264. doi: 10.1007/s00344-011-9235-7. [DOI] [Google Scholar]
- 5.Lanteri ML, Pagnussat GC, Lamattina L. Calcium and calcium-dependent protein kinases are involved in nitric oxide- and auxin-induced adventitious root formation in cucumber. J Exp Bot. 2006;57:1341–1351. doi: 10.1093/jxb/erj109. [DOI] [PubMed] [Google Scholar]
- 6.Pagnussat CG, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan R, Wang JX, Tian XS. Influence of ethylene on adventitious root formation in mung bean hypocotyls cuttings. Plant Growth Regul. 2002;36:135–139. doi: 10.1023/A:1015051725089. [DOI] [Google Scholar]
- 8.Xuan W, Zhu FY, Xu S, Huang BK, Ling TF, Qi JY, Ye MB, Shen WB. The heme oxygenase/carbon monoxide system is involved in the auxin-induced cucumber adventitious rooting process. Plant Physiol. 2008;148:881–893. doi: 10.1104/pp.108.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biondi S, Diaz T, Iglesias I, Gamberini G, Bagni N. Polyamines and ethylene in relation to adventitious root formation in Prunus avium shoot cultures. Physiol Plantarum. 1990;78:474–483. doi: 10.1111/j.1399-3054.1990.tb09066.x. [DOI] [Google Scholar]
- 10.Liao WB, Xiao HL, Zhang ML. Role and relationship of nitric oxide and hydrogen peroxide in adventitious root development of marigold. Acta Physiol Plant. 2009;31:1279–1289. doi: 10.1007/s11738-009-0367-3. [DOI] [Google Scholar]
- 11.Lin YT, Li MY, Cui WL, Lu W, Shen WB. Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J Plant Growth Regul. 2012;31:519–528. doi: 10.1007/s00344-012-9262-z. [DOI] [Google Scholar]
- 12.Lin YT, Zhang W, Qi F, Cui WT, Xie YJ, Shen WB. Hydrogen-rich water regulates cucumber adventitious root development in a heme oxygenase-1/carbon monoxide-dependent manner. J Plant Physiol. 2014;171:1–8. doi: 10.1016/j.jplph.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Cantrel C, Vazquez T, Puyaubert, Reze N, Lesch M, Kaiser W, Dutillcul C, Guillas I, Zachowski A, Baudouin E. Nitric oxide participates in cold-responsive phosphosphingolipid formation and gene expression in Arabidopsis thaliana. New Phytol. 2011;189:415–427. doi: 10.1111/j.1469-8137.2010.03500.x. [DOI] [PubMed] [Google Scholar]
- 14.Camejo D, Romero-Puertas Mdel C, Rodriguez-Serrano M, Sandalio LM, Lazaro JJ, Jimenez A, Sevilla F. Salinity-induced changes in S-nitrosylation of pea mitochondrial proteins. J Proteomics. 2013;79:87–99. doi: 10.1016/j.jprot.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Guo FQ, Crawford NM. Arabidopsis nitric oxide synthasel is trageted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell. 2005;17:3436–3450. doi: 10.1105/tpc.105.037770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao WB, Zhang ML, Yu JH. Role of nitric oxide in delaying senescence of cut rose flowers and its interaction with ethylene. Sci Hortic. 2013;155:30–38. doi: 10.1016/j.scienta.2013.03.005. [DOI] [Google Scholar]
- 17.Bethke PC, Libourel IG, Aoyama N, Chung YY, Still DW, Jones RL. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 2007;143:1173–1788. doi: 10.1104/pp.106.093435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libourel IG, Bethke PC, De Michele R, Jones RL. Nitric oxide gas stimulates germination of dormant Arabidopsis seeds: use of a flow-through apparatus for delivery of nitric oxide. Planta. 2006;223:813–820. doi: 10.1007/s00425-005-0117-8. [DOI] [PubMed] [Google Scholar]
- 19.He Y, Tang RH, Hao Y, Stevens RD, Cook CW, Ahn SM, Jing L, Yang Z, Chen L, Guo F, Fiorani F, Jackson RB, Crawford NM, Pei Z. Nitric oxide represses the Arabidopsis floral transition. Science. 2004;205:1968–1971. doi: 10.1126/science.1098837. [DOI] [PubMed] [Google Scholar]
- 20.Guo FQ, Okamoto M, Crawford NM. Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science. 2003;302:100–103. doi: 10.1126/science.1086770. [DOI] [PubMed] [Google Scholar]
- 21.Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a cnetral role in determining lateral root development in tomato. Planta. 2004;218:900–905. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- 22.Lombardo MC, Graziano M, Polacco JC, Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signa Behav. 2006;1:28–33. doi: 10.4161/psb.1.1.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagnussat GC, Simontacchi M, Puntarulo S, Lamattina L. Nitric oxide is required for root organogenesis. Plant Physiol. 2002;129:954–956. doi: 10.1104/pp.004036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dole M, Wilson FR, Fife WP. Hyperbaric hydrogen therapy: a possible treatment for cancer. Science. 1975;190:152–154. doi: 10.1126/science.1166304. [DOI] [PubMed] [Google Scholar]
- 25.Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Kastsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- 26.Zheng XF, Sun XJ, Xia ZF. Hydrogen resuscitation, a new cytoprotective approach. Clin ExpPharmacol P. 2011;38:155–163. doi: 10.1111/j.1440-1681.2011.05479.x. [DOI] [PubMed] [Google Scholar]
- 27.Xie YJ, Mao Y, Lai D, Zhang W, Shen WB. H2 enhances Arabidopisis salt tolerance by manipulating ZAT10/12-mediated antioxidant defence and controlling sodium exclusion. Plos One. 2012. 10. 1371/journal. pone. 004800. [DOI] [PMC free article] [PubMed]
- 28.Xu S, Zhu SS, Jiang YL, Wang N, Wang R, Shen WB, Yang J. Hydrogen-rich water alleviates salt stress in rice during seed germination. Plant Soil. 2013;370:47–57. doi: 10.1007/s11104-013-1614-3. [DOI] [Google Scholar]
- 29.Zeng JQ, Zhang MY, Sun XJ. Molecular hydrogen is involved in phytohormone signaling and stress responses in plants. Plos One. 2013 doi: 10.1371/journal.pone.0071038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin QJ, Zhu KK, Cui WT, Xie YJ, Han B, Shen WB. Hydrogen gas acts as a novel bioavitve molecule in enhacning plant tolerance to paraquat-induced oxidative stress via the modulating of heme oxygenase-1 signaling system. Plant Cell Environ. 2013;36:956–969. doi: 10.1111/pce.12029. [DOI] [PubMed] [Google Scholar]
- 31.Cui WT, Gao CY, Fang P, Lin GQ, Shen WB. Alleviation of cadmium toxicity in medicago sativa by hydrogen-rich water. J Hazard Mater. 2013;260:715–724. doi: 10.1016/j.jhazmat.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 32.Chen M, Cui W, Xie Y, Zhang C, Shen W. Hydrogen-rich water alleviates aluminum-induced inhibition of root elongation in alfalfa via decreasing nitric oxide production. J Hazard Mater. 2013;267:40–47. doi: 10.1016/j.jhazmat.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 33.Cui WT, Fang P, Zhu KK, Mao Y, Gao CY, Xie YJ, Wang J, Shen WB. Hydrogen-rich water confers plant tolerance to mercury toxicity in alfalfa seedings. Ecotox Environ Safe. 2014;105:103–111. doi: 10.1016/j.ecoenv.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Su NN, Wu Q, Liu YY, Cai JT, Shen WB, Xia K, Cui J. Hydrogen-rich water reestablishes ROS homeostasis but exerts differential effects on anthocyanin synthesis in two varieties of radish sprouts under UV-A irradiation. J Agr Food Chem. 2014;62:6454–6462. doi: 10.1021/jf5019593. [DOI] [PubMed] [Google Scholar]
- 35.Hu HL, Li PX, Wang YN, Gu RX. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem. 2014;156:100-–9. doi: 10.1016/j.foodchem.2014.01.067. [DOI] [PubMed] [Google Scholar]
- 36.Itoh T, Hamada N, Terazawa R, Ito M, Ohno K, Ichihara M, Nozawa Y, Ito M. Molecular hydrogen inhibits lipopolysaccharide/interferon γ-induced nitric oxide production through modulation of signal transduction in macrophages. Biochem Bioph Res Co. 2011;411:143–149. doi: 10.1016/j.bbrc.2011.06.116. [DOI] [PubMed] [Google Scholar]
- 37.Xie YJ, Mao Y, Zhang W, Lai DW, Wang QY, Shen WB. Reactive oxygen species-dependent nitric oxide production contributes to hydrogen-promoted stomatal closure in Arabidopsis. Plant Physiol. 2014 doi: 10.1104/pp.114.237925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inzé D, De Veylder L. Cell cycle regulation in plant development. Ann Rev Genet. 2006;40:77–105. doi: 10.1146/annurev.genet.40.110405.090431. [DOI] [PubMed] [Google Scholar]
- 39.Boniotti MB, Gutierrez C. A cell-cycle regulated kinase activity phsphorylates plant retinoblastoma protein and contains, in Arabidopsis, a CDKA/cyclin D complex. Plant J. 2001;28:341–350. doi: 10.1046/j.1365-313X.2001.01160.x. [DOI] [PubMed] [Google Scholar]
- 40.Joubès J, Chevalier C, Dudits D, Heberle-Bors E, Inzé D, Umeda M, Renaudin JP. CDK-related protein kinases in plants. Plant Mol Biol. 2000;43:607–620. doi: 10.1023/A:1006470301554. [DOI] [PubMed] [Google Scholar]
- 41.De Veylder L, Beeckman T, Beemster GTS, Krols L, Terras F, Landrieu I, Van Der Schueren E, Maes S, Naudts M, Inzé D. Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell. 2001;13:1653–1667. doi: 10.1105/tpc.13.7.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tewari RK, Hahn EJ, Paek KY. Function of nitric oxide and superoxide anion in the adventitious root development and antioxidant defence in Panax ginseng. Plant Cell Rep. 2008;27(3):563–573. doi: 10.1007/s00299-007-0448-y. [DOI] [PubMed] [Google Scholar]
- 43.Kashiwagi T, Yan HX, Hamasaki T, Kinjo T, Nakamichi N, Teruya K, Kabayama S, Shirahata S. Electrochemically reduced water protects neural cells from oxidative damage. Oxid Med and Cell Longev. 2014 doi: 10.1155/2014/869121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Himanen K, Boucheron E, Vanneste S, de Almeida EJ, Inzé D, Beeckman T. Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell. 2002;14:2339–2351. doi: 10.1105/tpc.004960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorbiecke R, Sauter M. Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiol. 1999;119:21–29. doi: 10.1104/pp.119.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beeckman T, Burssens S, Inzé D. The peri-cell-cycle in Arabidopsis. J Exp Bot. 2001;52:403–411. doi: 10.1093/jexbot/52.suppl_1.403. [DOI] [PubMed] [Google Scholar]
- 47.Correa-Aragunde N, Graziano M, Chevalier C, Lamattina L. Nitric oxide modulates the expression of cell cycle regualtory genes during lateral root formation in tomato. J Exp Bot. 2006;3:581–588. doi: 10.1093/jxb/erj045. [DOI] [PubMed] [Google Scholar]
- 48.Casimiro I, Marchant A, Bhalerao BP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, Bennett M. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13:483–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sauter M, Mekhedov SL, Kende H. Gibberellin promotes histone H1 kinase activity and the expression of cdc2 and cyclin genes during the induction of rapid growth in deepwater rice internodes. Plant J. 1995;7:622–632. doi: 10.1046/j.1365-313X.1995.7040623.x. [DOI] [PubMed] [Google Scholar]
- 50.Otvos K, Pasternak TP, Miskolczi P, Domoki M, Dorjotov D, Szucs A, Bottka S, Dudits D, Feher A. Nitric oxide is required for, and promotes auxin-mediated activation of, cell division and embryogenic cell formation but does not influence cell cycle progression in alfalfa cell cultures. Plant J. 2005;43:849–860. doi: 10.1111/j.1365-313X.2005.02494.x. [DOI] [PubMed] [Google Scholar]
- 51.Boudolf V, Rombatus S, Naudts M, Inzé D, De Veylder L. Identification of novel cyclin-dependent kinases interacting with the CKS1 protein of Arabisopsis. J Exp Bot. 2001;52:1381–1382. doi: 10.1093/jexbot/52.359.1381. [DOI] [PubMed] [Google Scholar]
- 52.Bai XG, Todd CD, Desikan R, Yang YP, Hu XY. N-3-oxo-decanoyl-L-homoserinelactone activates auxin-induced adventitious root formation via hydrogen peroxide-and nitric oxide-dependent cyclin GMP signaling in mung bean. Plant Physiol. 2012;158:725–36. doi: 10.1104/pp.111.185769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gasparri F, Cappella P, Galvanl A. Multiparametric cell cycle analysis by automated microscopy. J Biomol Screen. 2006;11:586–598. doi: 10.1177/1087057106289406. [DOI] [PubMed] [Google Scholar]
- 54.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data could be obtained by request to the corresponding author.


