Abstract
Background
Diastolic dysfunction refers to an impaired relaxation and an abnormality in a heart’s filling during diastole while left ventricular systolic function is preserved. Diastolic dysfunction is commonly observed in patients with primary hypertension, diabetes and cardiomyopathies such as hypertrophic cardiomyopathy or restrictive cardiomyopathy. We have generated a restrictive cardiomyopathy (RCM) mouse model with troponin mutations in the heart to mimic the human RCM patients carrying the same mutations.
Results
In the present study, we have investigated the ventricular muscle internal dynamics and pressure developed during systole and diastole by inserting a micro-catheter into the left ventricle of the RCM mice with or without treatment of desensitizer green tea extracts catechins. Our results demonstrate that green tea catechin is able to correct diastolic dysfunction in RCM mainly by improving ventricular compliance and reducing the internal muscle rigidity caused by myofibril hypersensitivity to Ca2+.
Conclusion
Green tea extract catechin is effective in correcting diastolic dysfunction and improving ventricular muscle intrinsic compliance in RCM caused by troponin mutations.
Keywords: Diastolic dysfunction, Restrictive cardiomyopathy, Calcium desensitization, Green tea extracts, Pressure-volume relationship, Experimental animals
Background
Diastolic dysfunction refers to an impaired relaxation and an abnormality in a heart’s filling during diastole while left ventricular systolic function is preserved. Diastolic dysfunction is a common sign in elderly population and in patients suffering from primary hypertension and various cardiomyopathies [1, 2]. For example, both hypertrophic cardiomyopathy (HCM) and restrictive cardiomyopathy (RCM) share a common feature of diastolic dysfunction [3, 4]. Diastolic dysfunction is an important clinical syndrome since almost half of heart failure patients have diastolic dysfunction [5–7]. There is a critical need to understand the mechanisms that cause diastolic dysfunction and heart failure and to develop target-based intervention and medications to correct the overt condition and to treat the patients. For the moment, in the absence of such agents and intervention, treatment of diastolic dysfunction is difficult and often ineffective [8].
In our previous studies, we have demonstrated that hypersensitivity of myofibrils to Ca2+ is a key factor associated with impaired relaxation in the heart [9, 10]. Furthermore, we have demonstrated, for the first time that desensitization of Ca2+ hypersensitivity in RCM can correct phenotypes and rescue the RCM mice [11, 12]. In applying green tea extracts catechins into RCM mice, we have further indicated that Ca2+ desensitizer green tea catechins can reverse diastolic dysfunction in RCM mice with troponin mutations [13]. However, it is not clear whether catechins correct the impaired relaxation by altering calcium dynamics or by altering the myofibril protein interactions, i.e. internal cardiac muscle dynamics. In the present study, we have determined the internal ventricular muscle dynamics using pressure-volume measurements in left ventricles in RCM mice with or without treatment of green tea catechins. Furthermore, the calcium handling proteins have been analyzed to exclude their effects on Ca2+ dynamics in RCM myocardial cells. Our results demonstrate that green tea catechin is able to correct diastolic dysfunction in RCM mainly by improving ventricular compliance and reducing the internal muscle rigidity caused by myofibril hypersensitivity to Ca2+.
Methods
Animals
RCM cTnI193His transgenic mice (TG) used in this study were generated in our laboratory by transgenic expression of cTnI193His mutant protein in the heart under the α-myosin heavy chain promoter [9]. Wild-type (WT) C57BL/6 mice were used as controls in the study. Both TG and WT mice were maintained in animal facilities at Florida Atlantic University. This investigation was performed in accordance with NIH guidelines, the Guide for the Care and Use of Laboratory Animals (NIH Pub. N01-OD-4-2139, 8th edition, 2011) and approved by the Institutional Animal Care and Use Committees at Florida Atlantic University.
Treatment of experimental animals
Green tea extract (-)-epigallocatechin-3-gallate (EGCg) was obtained from Sigma-Aldrich (Catalog number E4268). The compound was dissolved freshly in 15 % DMSO (200 mg/ml) as described previously [13]. Both WT and TG mice (2 months old) were injected intraperitoneally daily with EGCg solution (50 mg.kg) (5 days per week) for 3 months. Each group contained eight mice. The control mice were injected with the same amount of 15 % DMSO in a similar manner as described previously [13].
Cardiac function measurement with high resolution echocardiography
Cardiac function in experimental mice was examined and analyzed using a Vevo 770 High-Resolution In Vivo Imaging System (VisualSonics, Toronto, ON, Canada) in our laboratory as described previously [9, 14]. To decrease experimental bias, all of the echocardiography measurements were performed by an examiner blinded to the genotype. Short-axis images were taken to view the left ventricle (LV) or right ventricle (RV) movement during diastole and systole. Transmitral blood flow was observed with Pulse Doppler. All data and images were saved and analyzed with an Advanced Cardiovascular Package Software (VisualSonics, Toronto, ON, Canada) as described previously [9, 14].
Left ventricular pressure–volume analysis
Mice were anesthetized with 3 % isoflurane and were supported by a ventilator with a maintenance dose of 1 % isoflurane after tracheostomy. A research pressure-volume micro-catheter (1F PV catheter, Millar, Inc. Houston, TX) was inserted into the left ventricle through the right carotid artery of the experimental mice. Intra-cardiac pressure (P) and volume (V) were simultaneously measured and real time data were displayed and recorded using a P–V conductance system (MPVS Ultra, AD Instruments, Inc. Houston, TX) coupled to a digital converter (PowerLab, AD Instruments). Heart rate was continuously monitored using either ECG electrodes or direct pressure pulses corresponding to each heartbeat. Hemodynamic parameters were measured under different preloads, which were elicited by transiently compressing the abdominal inferior vena cava. The catheter was advanced into the LV to obtain +dP/dtmax as a measure of systolic function. For diastolic function, LV end-diastolic pressure (LVEDP), −dP/dtmin, and Tau were determined. Tau was determined from fitting to the pressure decay curve from the time when −dP/dtmin occurs using a zero asymptote. At least eight mice were measured for each group. In β-adrenergic stimulation assay, bolus injection of the β-receptor agonist isopretelenol (1.5 mg/kg body wt). Assays were performed on five TG and five WT mice.
Western blotting
Cardiac samples were resolved in NuPAGE 4–12 % gradient BT gels using an Xcell II sure lock Mini Cell gel system from Invitrogen. The proteins were transferred to a nitrocellulose membrane using the Xcell IITM blot module as described previously [9, 10]. The nitrocellulose membrane was blocked with 5 % dry fat milk in TBS-T and incubated with antibodies. The target proteins on immunoblots were visualized by enhanced chemiluminescence (ECL detection kit from GE Healthcare). The following primary antibodies were used: cardiac cTnI 4H6 antibody (1:20000) for cTnI protein. Rb pAb (phospho s16, 1:20000) was used to determine the phosphorylated phospholamban. Ms mAb to SERCA-2 ATPase antibody (1:600) was used to determine the SERCA-2 proteins. Ms mAb to Phosplamban antibody (1:20000) was used to determine phospholamban proteins. The protein bands were scanned by densitometry and quantified among the samples on the same blot.
Statistical analysis
The results are presented as means ± SE. ANOVA followed by post hoc Newman–Keuls (SNK) tests and Student’s t test were used to determine the statistical significance. Statistical significance was set as P <0.05.
Results
Diastolic dysfunction is reversed by EGCg treatment in RCM mice
The data from cardiac function measurements using high resolution echocardiography in experimental mice indicate that enlarged left and right atria are significantly reduced in RCM mice after the treatment of EGCg (Fig. 1 and Table 1). Left ventricular end diastolic dimension (LVEDD) is increased in RCM mice after the treatment. The prolonged left ventricular isovolumentric relaxation time (IVRT) is significantly shortened in RCM mice after the treatment with EGCg (Table 1). These results are consistent with our report in the previous study indicating that green tea extract EGCg is able to reverse diastolic dysfunction and improve cardiac relaxation in RCM mice.
Fig. 1.
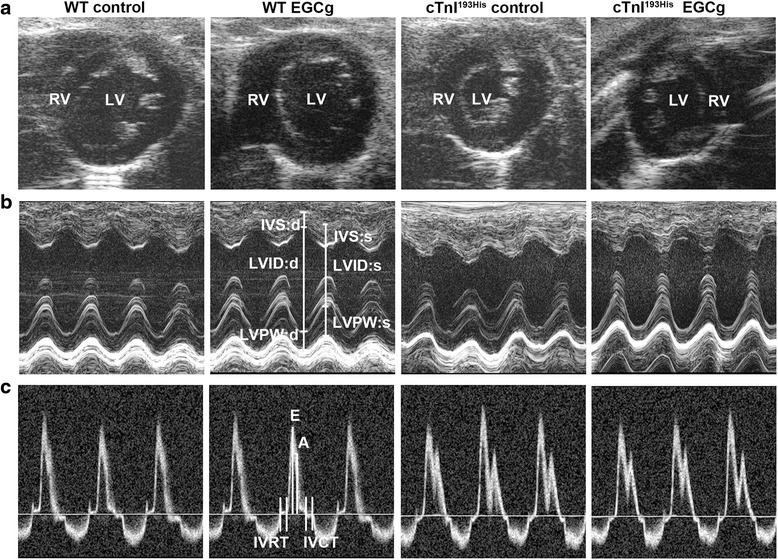
Determination of cardiac function with high resolution echocardiography in WT and RCM TG mice with or without treatment of EGCg. a Representative two-dimensional short axis views obtained from four different groups of the experimental mice. b Representative M-mode images and parameter calculation in experimental mice. c Representative images of pulsed Doppler of mitral inflow obtained from the experimental mice. LV left ventricle, RV right ventricle, E peak velocity of mitral blood inflow in early diastole, A peak velocity of mitral blood inflow in late diastole; E/A ratio; IVRT isovolumic relaxation time; IVCT isovolumetric contraction time, LVID:s left ventricular internal diameter end systole, LVID:d left ventricular internal diameter end diastole
Table 1.
Cardiac function measurements on WT and RCM TG mice
| WT control | WT EGCg | RCM control | RCM EGCg | |
|---|---|---|---|---|
| Parameters | ||||
| Body weight (g) | 31.67 ± 1.53 | 30.67 ± 1.61 | 27.25 ± 1.21* | 28.35 ± 1.28* |
| Heart rate (bpm) | 470.72 ± 38.12 | 481.37 ± 44.15 | 450.75 ± 50.76 | 460.17 ± 49.73 |
| Atria | ||||
| LAEDD (mm) | 2.12 ± 0.02 | 2.13 ± 0.03 | 2.29 ± 0.04** | 2.21 ± 0.05*‡ |
| LAESD (mm) | 1.67 ± 0.03 | 1.69 ± 0.04 | 1.74 ± 0.06 | 1.71 ± 0.05 |
| RAEDD (mm) | 2.11 ± 0.05 | 2.12 ± 0.06 | 2.31 ± 0.11** | 2.26 ± 0.05* |
| RAESD (mm) | 1.71 ± 0.06 | 1.69 ± 0.03 | 1.64 ± 0.04 | 1.68 ± 0.05 |
| LV end diastole | ||||
| IVS (mm) | 0.75 ± 0.04 | 0.76 ± 0.03 | 0.75 ± 0.04 | 0.74 ± 0.03 |
| LVEDD (mm) | 3.88 ± 0.07 | 3.85 ± 0.04 | 3.42 ± 0.0 6* | 3.62 ± 0.03*‡ |
| LV PW (mm) | 0.76 ± 0.02 | 0.75 ± 0.04 | 0.76 ± 0.03 | 0.73 ± 0.03 |
| LV Volume (μl) | 64.67 ± 1.71 | 63.95 ± 2.16 | 46.16 ± 2.24** | 55.92 ± 1.19*‡ |
| LV end systole | ||||
| IVS (mm) | 1.27 ± 0.03 | 1.27 ± 0.04 | 1.24 ± 0.02 | 1.25 ± 0.03 |
| LVESD (mm) | 2.05 ± 0.05 | 2.08 ± 0.06 | 1.58 ± 0.02** | 1.75 ± 0.03*‡ |
| LV PW (mm) | 1.22 ± 0.03 | 1.22 ± 0.04 | 1.21 ± 0.03 | 1.21 ± 0.02 |
| LV Volume (μl) | 13.03 ± 0.43 | 13.42 ± 0.61 | 5.96 ± 0.71 | 9.35 ± 0.80**ǂǂ |
| Ejection Fraction (%) | 79.85 ± 1.41 | 79.01 ± 1.00 | 80.33 ± 1.91 | 80.57 ± 2.16 |
| Fractional Shortening (%) | 46.72 ± 1.22 | 45.38 ± 0.66 | 47.95 ± 1.84 | 47.44 ± 2.33 |
| Mitral Doppler | ||||
| E (mm/s) | 827.61 ± 22.05 | 829.75 ± 24.22 | 740.81 ± 18.93* | 809.04 ± 25.14 |
| A (mm/s) | 657.95 ± 12.34 | 667.35 ± 16.48 | 560.33 ± 33.66 * | 633.57 ± 26.83 |
| E/A | 1.26 ± 0.04 | 1.24 ± 0.02 | 1.32 ± 0.05 | 1.27 ± 0.05 |
| IVRT (ms) | 16.41 ± 0.44 | 16.44 ± 0.46 | 21.43 ± 0.54** | 18.53 ± 0.51*‡ |
| IVCT (ms) | 10.58 ± 0.33 | 11.05 ± 0.29 | 10.36 ± 0.75 | 10.86 ± 0.53 |
Values are expressed as means ± SE for each group. LA left atrium, RA right atrium, LV left ventricle, EDD end diastolic dimension, ESD end systolic dimension, PW posterior wall thickness of LV, IVS intra-ventricular septum, EF ejection fraction of LV, FS fractional shortening of LV, E mitral Doppler E peak velocity, A mitral Doppler A peak velocity, IVRT isovolumetric relaxation time, IVCT isovolumetric contraction time. Statistical significance was determined by ANOVA followed by post hoc Newman-Keuls (SNK) tests
*p <0.05 compared to WT control, **p <0.01 compared to WT control; ǂp <0.05 compared to RCM control; ǂǂp <0.01 compared to RCM control
EGCg corrects diastolic dysfunction by reducing cardiac muscle internal rigidity and increasing ventricular compliance in RCM mice
Mouse left ventricular function was assessed using catheter based P-V loop measurements. The measurements exhibit the P-V loops of each mouse ventricle showing the volume-dependent pressure changes (Fig. 2a and b). These data indicate that the blood filling in the diastole stage is limited in RCM mice and the situation is improved in the RCM mice after the treatment with EGCg (Fig. 2b). Furthermore, we analyzed the pressure volume relationship (PVR) at end systole (upper line) and end diastole (Lower line) in Fig. 2a since the PVR at end systole or end diastole reflects the muscle internal contraction or relaxation status without the influence of the volume changes. Our data indicate that no significant changes are observed in end systole PVR (ESPVR) between WT and RCM mice before and after the treatment of EGCg. However, a significant change, increase, has been observed in RCM mice compared to the WT mice (Fig. 2a). After the treatment of EGCg, the increased end diastole PVR (EDPVR) is significantly reduced (Fig. 2a). These data indicate that increased left ventricular pressure in RCM is caused by the increased ventricular muscle internal pressure and green tea extract EGCg is able to reverse the increased ventricular internal pressure and improve diastolic function in RCM mice. Further analyses of systolic parameters such as ejection fraction (EF)(Fig. 2c), dp/dt max (Fig. 2d) and ESPVR (Fig. 2e) indicate that no significant changes have been observed in systolic function between WT and RCM mice before and after the treatment of EGCg. However, diastolic parameters such as –dp/dt min (Fig. 2f), Tau (Fig. 2g) and EDPVR (Fig. 2h) are significantly altered in RCM mice compared to WT mice and EGCg can correct the changed diastolic parameters in RCM mice.
Fig. 2.
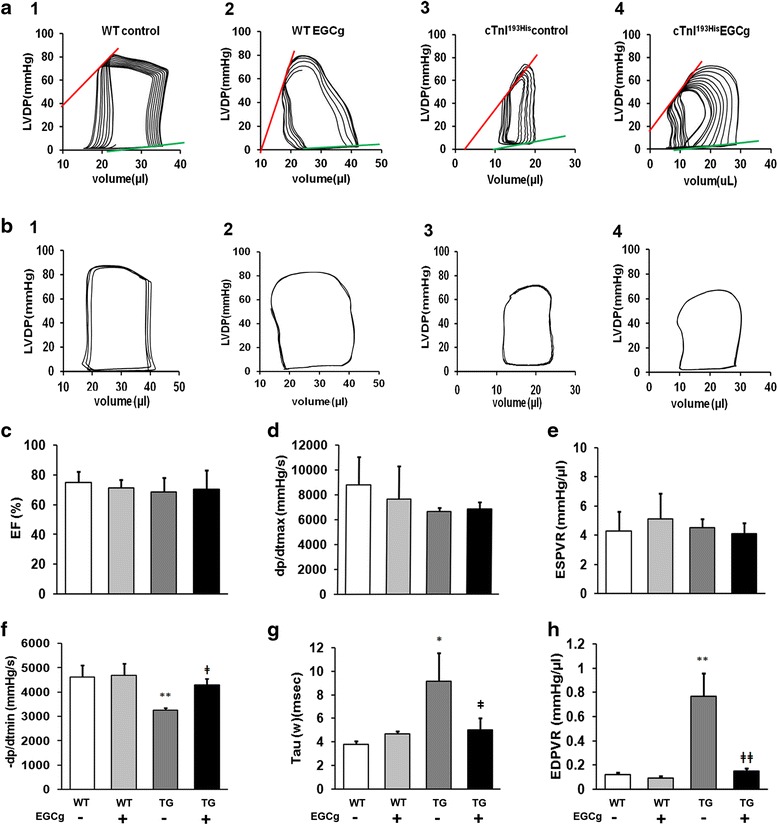
Representative pressure-volume loops obtained from catheter-based left ventricular P-V measurements in experimental mice. a Characteristic changes in left ventricular developmental pressure (LVDP) corresponding to the volume changes. Upper line indicates end systole pressure volume relationship (ESPVR) and the low line indicates end diastole pressure volume relationship (EDPVR) in different groups of mice: 1, WT mice in control group (WT control); 2, WT mice with EGCg treatment (WT EGCg); 3, cTnI193His RCM mice in control (cTnI193His control); 4, cTnI193His RCM with EGCg treatment (cTnI193His EGCg). b Normal baseline PV loops from different groups of mice. Cardiac function parameters are shown in (c), ejection fraction (EF); (d) the maximal rate of contraction (+dP/dt); (e) end-systolic pressure–volume relation slope (ESPVR); (f) the maximal rate of relaxation (−dP/dt); (g) relaxation time constant calculated by Weiss method (τ); (h) end-diastolic pressure–volume relation (EDPVR). Data are presented as means ± SE. *compared between WT and TG mice; ǂ compared between mice with or without treatment. * or ǂ P <0.05; ** or ǂǂ P<0.01
β-adrenergic stimulation cannot change cardiac filling and diastolic function in RCM
We have further tried to determine the effect of β-adrenergic stimulation in RCM mice by injection of isoproterenol (ISO) in tested mice. The data indicate that ISO treatment cannot increase the heart filling and has no effect on LVDP in RCM mice (Fig. 3a, b and c). However, ISO can stimulate heart rate (HR)(Fig. 3d), increase ejection fraction (EF)(Fig. 3e) and maximum contractility (Fig. 3f) in both WT and RCM mice, suggesting that ISO cannot change the internal muscle rigidity and cannot improve diastolic function in RCM mice.
Fig. 3.
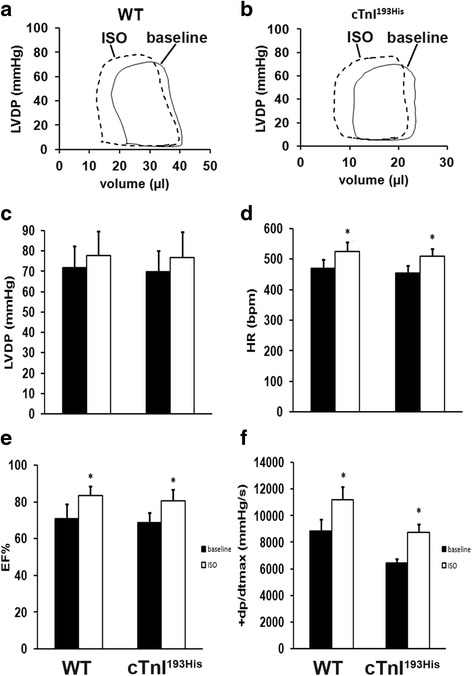
Inotropic responses to β-adrenergic stimulation in WT and cTnI193His RCM mice. Representative pressure-volume loops recorded at baseline (solid curve) and after administration of isoproterenol (ISO; dashed curve) in WT mice (a) and in cTnI193His RCM mice (b). c Left ventricular developed pressure (LVDP); (d) the heart rate (HR); (e) Ejection fraction (EF); (f) the maximal rate of contraction (+dP/dt max). Data are presented as means ± SE. *Significant difference compared between before and after ISO stimulation. * P <0.05
EGCg treatment does not alter Ca2+ handling proteins and their phosphorylation status
Western blotting data indicate that the levels of the tested Ca2+ handling proteins do not show any significant changes both in WT and RCM mice after the treatment of green tea extract EGCg (Fig. 4). These proteins include the sarcoendoplasmic reticulum calcium transport ATPase (SERCA2), phospholamban (PLN) and phosphorylated phospholamban (phosphor-PLN). These data suggest that green tea extract EGCg corrects diastolic dysfunction and improve cardiac relaxation in RCM not via the alteration in Ca2+ handling protein and Ca2+ concentration in myocardial cells.
Fig. 4.
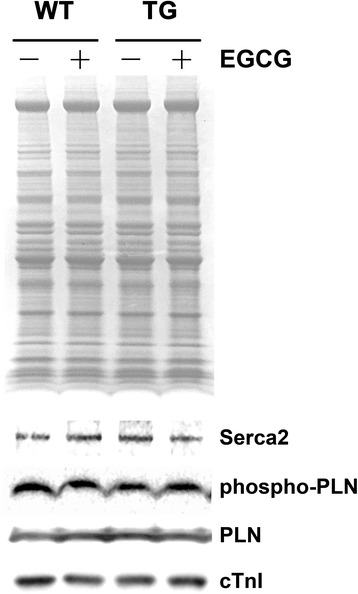
Levels of Ca2+ handling proteins and their phosphorylation status in myocardial cells with or without treatment of EGCg. A representative Western blot showing the levels of Ca2+ handling proteins and their phosphorylation status in WT and TG myocardial cells with or without EGCg treatment
Discussions
Heart failure (HF) is the leading cause of mortality in the United States [1]. In two major types of HF, HF with reduced ejection fraction (HFrEF) and HF with preserved ejection fraction (HFpEF), prevalence of HFpEF is rising, with mortality, morbidity, and healthcare costs now equal to those for HFrEF [2, 5, 15]. However, the pathophysiology of HFpEF is poorly understood, and no medication trials have had positive effects on their primary end-points. A major cause of HFpEF is diastolic dysfunction which is commonly seen in primary hypertension and various cardiomyopathies such as HCM and RCM. Diastolic dysfunction occurs when the ventricles cannot fill normally, and in severe conditions, it can lead to diastolic heart failure (DHF). Recently studies have revealed that cardiac diastolic dysfunction increases with advancing age, approximately 30–40 % of the population (aged 45 or older) had diastolic dysfunction, and nearly half of them were healthy individuals [16–18].
In the present study, we have further confirmed that diastolic dysfunction occurs in RCM mice with troponin mutations in the heart and desensitizing green tea extract EGCg can reverse the diastolic dysfunction in RCM hearts. Using micro-catheter based P-V loop measurements in both WT and RCM TG mice, we have successfully analyzed P-V loops and end diastolic pressure and volume relationship (EDPVR) in the experimental mice. The P/V index reflects the elastance of the left ventricles. The P/V plus dP/dt are common physiologic measurement of cardiac tissues. EDPVR is an index that reveals the internal ventricular muscle dynamics and developed pressure in diastole. Our data indicate that the EDPVR increased significantly in RCM heart compared to the WT hearts, suggesting an increased ventricular muscle internal pressure (force) that is Ca2+ independent in RCM hearts with troponin mutations. The increased ventricular muscle internal pressure (force) causes a rigid ventricle and enlarged left atrium or both atria, which are consistent with what we reported previously of cellular and morphological examination on the same RCM mice [10, 11]. Green tea desensitizing EGCg is able to reverse the diastolic dysfunction in RCM mice because it can correct the increased EDPVR in these mice without any significant effects on Ca2+ dynamics in myocardial cells by altering Ca2+ handling proteins or their phosphorylation status.
This is the first study investigating the ventricular muscle internal dynamics and pressure developed during diastole in RCM hearts with troponin mutations. It is also novel to demonstrate that green tea desensitizing EGCg reverse diastolic dysfunction by correcting the internal EDPVR in the RCM hearts. It is interesting to note that a beta-adrenergic agonist has barely any effect on diastolic function and heart filling although it can increase heart rate and ejection fraction in RCM mice.
The results from this study and our previous studies indicate that myofibril hypersensitivity is a key factor that causes impaired relaxation in HCM or RCM hearts associated with myofibril protein troponin mutations in myocardial cells. Green tea extract EGCg can reverse the impaired relaxation by correcting the hypersensitivity-induced abnormal ventricular muscle internal pressure in RCM hearts. Recently, the desensitization effect of green tea extracts has been further confirmed in cells from a patient with HCM [19]. The effect of green tea extracts occurs in internal ventricular muscle dynamics by interfering with calcium binding to troponin C in myocardial cells [20], and probably not by altering calcium dynamics through changes in calcium handling protein concentrations or their activities. However, one in vitro study using isolated murine cardiomyocytes reported that nanomolar EGCG could increase calcium transients [21]. Further studies are needed to clarify the effects of EGCG on calcium dynamics. In addition, it should be pointed out that diastolic dysfunction covers a vast range of etiology and causes, and green rea desensitization may not be so effective when it is applied to some types of diastolic dysfunction such as those related to fibrosis or amyloidosis.
Conclusion
Green tea extract catechin is effective in correcting diastolic dysfunction and improving ventricular muscle intrinsic compliance in RCM caused by troponin mutations.
Abbreviations
A, mitral Doppler A peak velocity; E, mitral Doppler E peak velocity; EDD, end diastolic dimension; EF, ejection fraction of LV; ESD, end systolic dimension; FS, fractional shortening of LV; HCM, hypertrophic cardiomyopathy; IVCT, isovolumetric contraction time; IVRT, isovolumetric relaxation time; IVS, intra-ventricular septum; LA, left atrium; LV, left ventricle; LVID:d, left ventricular internal diameter end diastole; LVID:s, left ventricular internal diameter end systole; PW, posterior wall thickness of LV; RA, right atrium; RCM, restrictive cardiomyopathy
Acknowledgements
This study was supported by grants from NIH (HL112130-01) and Natural Science Foundation of China (31271218).
Funding
NIH (HL 112130-01) and NSFC (31271218).
Availability of data and materials
The authors wish to share their data in this study. Please contact the corresponding author Dr. Xupei Huang at xhuang@fau.edu.
Authors’ contributions
XW carried out the cardiac function measurements including echocardiography and P-V loop assays. ZZ and CN carried out the Western blotting assays and data analyses. GW and YH participated in the result discussion and part of manuscript writing. XW, WS and XPH participated in experimental design and manuscript drafting. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The experiments using animals in this investigation were performed in accordance with NIH guidelines, the Guide for the Care and Use of Laboratory Animals (NIH Pub. N01-OD-4-2139, 8th edition, 2011) and approved by the Institutional Animal Care and Use Committees (IACUC) at Florida Atlantic University.
Contributor Information
Yimin Hua, Email: Nathan_hua@163.com.
Xupei Huang, Phone: 1-561-2972443, FAX: 1-561-2972221, Email: xhuang@fau.edu.
References
- 1.Braunwald E. The management of heart failure, the past, the present, and the future. Circ Heart Fail. 2008;1:58–62. doi: 10.1161/CIRCHEARTFAILURE.107.752162. [DOI] [PubMed] [Google Scholar]
- 2.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/S0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 3.Dweck D, Sanchez-Gonzalez MA, Chang AN, Dulce RA, Badger CD, Koutnik AP, Ruiz EL, Griffin B, Liang J, Kabbaj M, Fincham FD, Hare JM, Overton JM, Pinto JR. Long term ablation of protein kinase A (PKA)-medicated cardiac troponin I phosphorylation leads to excitation-contraction uncoupling and diastolic dysfunction in a knock-in mouse model of hypertrophic cardiomyopathy. J Biol Chem. 2014;289:23097–111. [DOI] [PMC free article] [PubMed]
- 4.Jean-Charles P., Nan C, Zhang L, Tian J, Huang XP. Cardiac Troponin Mutations : Diastolic Dysfunction and Therapeutic Options. In: Troponin, pp. 259-281, Nova Science Publishers, Inc. 2014.
- 5.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 6.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 7.Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, et al. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation. 2012;126:65–75. doi: 10.1161/CIRCULATIONAHA.111.080770. [DOI] [PubMed] [Google Scholar]
- 8.Mandinov L, Eberli FR, Seiler C, Hess OM. Diastolic heart failure. Cardiovas Res. 2000;45:813–825. doi: 10.1016/S0008-6363(99)00399-5. [DOI] [PubMed] [Google Scholar]
- 9.Du J, Zhang C, Liu J, Sidky C, Huang XP. A point mutation (R192H) in the C-terminus of human cardiac troponin I causes diastolic dysfunction in transgenic mice. Arch Biochem Biophys. 2006;456:143–150. doi: 10.1016/j.abb.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Du J, Liu J, Feng HZ, Houssain MM, Gobara N, Zhang C, Li Y, Jean-Charles P, Jin JP, Huang XP. Impaired relaxation is the main manifestation in transgenic mice expressing a restrictive cardiomyopahty mutation, R193H, in cardiac TnI. Am J Physiol Heart Circ Physiol. 2008;294:H2604–13. [DOI] [PMC free article] [PubMed]
- 11.Li Y, Jean-Charles PY, Nan C, Pinto JR, Wang Y, Liang J, Wu G, Tian J, Feng H, Potter JD, Jin JP, Huang XP. Correcting diastolic dysfunction by Ca2+ desensitizing troponin in a transgenic mouse model of restrictive cardiomyopathy. J Mol Cell Cardiol. 2010;49:402–11. [DOI] [PMC free article] [PubMed]
- 12.Li Y, Zhang L, Jean-Charles P, Nan C, Chen G, Tian J, Jin J-P, Gelb IJ, Huang P. Dose-dependent diastolic dysfunction and early death in a mouse model with cardiac troponin mutations. J Mol Cell Cardiol. 2013;62:227–36. [DOI] [PMC free article] [PubMed]
- 13.Zhang L, Nan C, Chen Y, Tian J, Jean-Charles P, Getfield C, Wang X, Huang XP. Calcium desensitizer catechin reverses diastolic dysfunction in mice with restrictive cardiomyopathy. Arch Biochem Biophys. 2015;573:69–76. doi: 10.1016/j.abb.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, Li Y, Tian J, Zhang L, Jean-Charles P, Gobara N, Nan C, Jin JP, Huang XP. Application of echocardiography on transgenic mice with cardiomyopathies. Biochem Res Intern. 2012 doi: 10.1155/2012/715197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao L, Jollis JG, Anstrom KJ, Whellan DJ, Kitzman DW, Aurigemma GP, et al. Costs for heart failure with normal vs reduced ejection fraction. Arch Intern Med. 2006;166:112–118. doi: 10.1001/archinte.166.1.112. [DOI] [PubMed] [Google Scholar]
- 16.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 17.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 18.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren CM, Karam CN, Wolska BM, Kobayashi T, de Tombe PP, et al. A green tea catechin normalizes the enhanced Ca2+ sensitivity of myofilaments regulated by a hypertrophic cardiomyopathy associated mutation in human cardiac troponin I (K206I) Circ Cardiovasc Genet. 2015 doi: 10.1161/CIRCGENETICS.115.001234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tadano N, Du CK, Yumoto F, Morimoto S, Ohta M, Xie MF, et al. Biological action of green tea catechins on cardiac troponin C. Br J Pharmacol. 2010;161:1034–1043. doi: 10.1111/j.1476-5381.2010.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng W, Hwang HS, Kryshtal DO, Yang T, Padilla IT, Tiwary AK, Puschner B, Pessah IN, Knollmann BC. Coordinated regulation of murine cardiomyocytes contractility by nanomolar (-)-epigallocatechin-3-gallate, the major green tea catechin. Mol Pharmacol. 2012;82(5):993–1000. doi: 10.1124/mol.112.079707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors wish to share their data in this study. Please contact the corresponding author Dr. Xupei Huang at xhuang@fau.edu.


