Abstract
Background
The elimination of malaria requires high-quality surveillance data to enable rapid detection and response to individual cases. Evaluation of the performance of a national malaria surveillance system could identify shortcomings which, if addressed, will improve the surveillance program for malaria elimination.
Methods
Case-level data for the period 2005–2014 were extracted from the China National Notifiable Infectious Disease Reporting Information System and Malaria Enhanced Surveillance Information System. The occurrence of cases, accuracy and timeliness of case diagnosis, reporting and investigation, were assessed and compared between the malaria control stage (2005–2010) and elimination stage (2011–2014) in mainland China.
Results
A total of 210 730 malaria cases were reported in mainland China in 2005–2014. The average annual incidence declined dramatically from 2.5 per 100 000 people at the control stage to 0.2 per 100 000 at the elimination stage, but the proportion of migrant cases increased from 9.8 % to 41.0 %. Since the initiation of the National Malaria Elimination Programme in 2010, the overall proportion of cases diagnosed by laboratory testing consistently improved, with the highest of 99.0 % in 2014. However, this proportion was significantly lower in non-endemic provinces (79.0 %) than that in endemic provinces (91.4 %) during 2011–2014. The median interval from illness onset to diagnosis was 3 days at the elimination stage, with one day earlier than that at the control stage. Since 2011, more than 99 % cases were reported within 1 day after being diagnosed, while the proportion of cases that were reported within one day after diagnosis was lowest in Tibet (37.5 %). The predominant source of cases reporting shifted from town-level hospitals at the control stage (67.9 % cases) to city-level hospitals and public health institutes at the eliminate stage (69.4 % cases). The proportion of investigation within 3 days after case reporting has improved, from 74.6 % in 2010 to 98.5 % in 2014.
Conclusions
The individual case-based malaria surveillance system in China operated well during the malaria elimination stage. This ensured that malaria cases could be diagnosed, reported and timely investigated at local level. However, domestic migrants and overseas populations, as well as cases in the historically malarial non-endemic areas and hard-to-reach area are new challenges in the surveillance for malaria elimination.
Electronic supplementary material
The online version of this article (doi:10.1186/s40249-016-0163-4) contains supplementary material, which is available to authorized users.
Keywords: Malaria, Surveillance, Evaluation, Elimination, China
Multilingual abstracts
Please see Additional file 1 for translations of the abstract into the six official working languages of the United Nations.
Background
Malaria is considered one of the most significant tropical diseases of humans, being a vector borne plasmodial infection transmitted via the bites of the female Anopheles mosquito [1]. According to the latest global estimates from the World Health Organization (WHO), a total of 214 million cases of malaria and 438 000 deaths occurred in 2015 [2]. Significant progress has been made towards malaria control over the past decade [3–5]. As of December 2014, of the 106 countries with sustained transmission of malaria in 2000, 19 countries are in the pre-elimination or elimination phase, and seven are in the prevention of malaria reintroduction phase [2]. To achieve the goal of elimination, a sustained and well-operated malaria surveillance system is considered as a critical measure [6]. WHO launched Global Malaria Programme’s new initiative of 3T, Test, Treat, and Track in 2012, which supports malaria-endemic countries in their effort to achieve universal coverage with diagnostic testing and antimalarial treatment, as well as in strengthening malaria surveillance [7]. This program and the implementation of the 3T is contingent on the provision of timely and accurate surveillance data to monitor performance and identify threats to malaria control and elimination.
A national malaria elimination program (NMEP) was launched in China in 2010, with the goal of nationwide elimination of malaria by 2020 [8]. The elimination stage is different from the control stage, and requires monitoring and responding to each individual malaria infection, and to ultimately stop local malaria transmission [6, 9, 10]. China developed a case-based malaria surveillance system to collect information required for diagnoses and investigations, and to facilitate a rapid response to individual cases [9, 10]. For the elimination of malaria, it is essential to understand the strengths and limitations of the program by quantitatively evaluating the performance and efficiency of NMEP [11].
In this study, we compare the critical components of malaria surveillance for elimination, including diagnosis, reporting and investigation of cases between control stage and elimination stage, to evaluate the operational performance of malaria surveillance system in China, and to further improve the surveillance for malaria elimination.
Methods
National malaria surveillance system
In the People’s Republic of China, malaria is a notifiable infectious disease; case definitions are listed in the unified criteria issued by the Chinese National Health and Family Planning Commission [12]. It is mandatory that all suspected, probable and laboratory-confirmed cases should be reported to the malaria surveillance system. A laboratory-confirmed case is defined as a case with: malaria parasites confirmed by microscopy, a positive rapid diagnostic test (RDT), a positive polymerase chain reaction test (PCR), or a case presentation with or without typical malaria symptoms. All other cases with malaria-like symptoms and a history of travel to a malaria endemic area during malaria transmission season, or a history of blood transfusion in past 2 weeks, but without positive laboratory test results, were classified as suspected or probable cases [12]. Both of these kinds of cases were regarded as non-laboratory confirmed malaria cases in this study.
In China, the national malaria surveillance program consists of two systems: National Notifiable Infectious Disease Reporting Information System (NIDRIS) and Malaria Enhanced Surveillance Information System (MESIS), both of which were developed by the Chinese Center for Disease Control and Prevention (China CDC) [10, 13–15]. NIDRIS was established in 2004, through which 39 notifiable infectious diseases were reported. Individual case information is reported by physicians in clinics and hospitals. Reported information includes demographic information, date of onset of symptoms, date of diagnosis, date of reporting, and the reporting institute. Since the initiation of the malaria elimination stage in 2010, MESIS was developed to collect detailed epidemiological information pertaining to malaria cases to aid in malaria elimination in China. MESIS collects data on the course of case diagnosis, history of travel, date of case investigation, treatment, classification (autochthonous or imported case), and the results of case verification by city-level CDC and province-level CDC. All information in MESIS is reported by the staff of local county CDCs. Information in the MESIS and NIDRIS systems can be linked by use of unique patient identifiers.
The ‘1-3-7’strategy has been designed to monitor and respond to individual malaria cases during the elimination stage in China. ‘1-3-7’refers to reporting cases within 1 day after diagnosis, investigating cases within 3 days after reporting, and completing the response within 7 days after reporting [9]. The reporting requirement necessitates all hospitals and healthcare institutes across the country to report individual case information to the NIDRIS within 1 day after case diagnosis. Since 2010, if any malaria case is reported to NIDRIS, a notification is automatically sent by short text message to the staff’s cell phone in the county CDC; the staffs in the county CDC are responsible for verifying the malaria data in NIDRIS [13]. Then, staffs in the local county CDC are required to conduct an epidemiological investigation within three days after case report and to enter the investigation information into MESIS. The local area with occurrence of malaria cases would be identified as a focus, and risk assessment on local transmission would be performed by county CDC staff. Then, control measures would be taken and should be completed within seven days after reporting (Fig. 1).
Fig. 1.
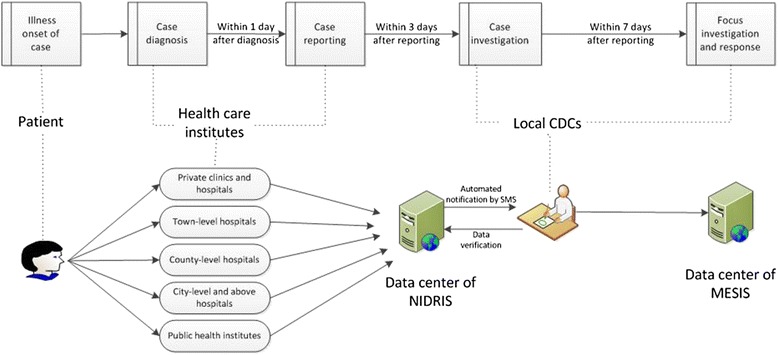
Diagram of malaria diagnosis, reporting and investigation in China (NIDRIS: National Notifiable Infectious Disease Reporting Information System; MESIS: Malaria Enhanced Surveillance Information System; CDCs: Centers for Disease Control and Prevention; SMS: Short Message Service)
Data analysis
This study included all malaria cases recorded in NIDRIS from Jan 1, 2005, to Dec 31, 2014, and those in MESIS from Jan 1, 2011 to Dec 31, 2014. As NMEP was launched in May of 2010, the period from 2005 to 2010 was taken as the control stage, and the period from 2011 to 2014 was designated as the elimination stage in this study. The timeliness rate of reporting was calculated by dividing the number of cases reported within 1 day by the number of all reported cases. The timeliness rate of investigation was calculated by dividing the number of cases investigated within 3 days after reporting by the total number of cases investigated. The interval time from illness onset to diagnosis, diagnosis to report, and report to investigation, and the proportion of lab-confirmed cases were calculated, and comparisons between the control and the elimination stages conducted. All analyses were further stratified by the residence, locations of case, reporting hospitals or institutes, mosquito species and origin.
Each case was classified as either a local or migrant case. If the reporting institute and residential address of a case were located in the same county, the case was classified as a local case otherwise the case was classified as a migrant case. Among all 31 provinces in mainland China, the province where the case located was categorized as either an endemic or non-endemic province, and was listed in NMEP according to the historical epidemiological information of malaria in that province [8]. Non-endemic provinces included seven provinces (Beijing, Tianjin, Inner Mongolia, Heilongjiang, Jilin, Qinghai and Ningxia), the remaining 24 provinces in mainland China were regarded as endemic provinces. The reporting institutes were classified into five categories, based on their population coverage: private clinics and hospitals; town-level hospitals; county-level hospitals; city-level hospitals; and public health institutes. The city-level hospitals included the prefecture-level hospitals and provincial-level hospitals. Public health institutes covered all levels of CDCs, and Bureaus of Entry-Exit Inspection and Quarantine. Imported case was defined as patient who had a travel history to a malaria-endemic country within 1 month prior to illness onset [16]. Otherwise, the case was classified as autochthonous case.
Results
Occurrence of cases
A total of 210 730 malaria cases were reported during the period 2005 to 2014, with an annual average of 32 887 cases in the control stage (2005–2010), and 3 352 cases per year in the elimination stage (2011–2014). The annual incidence rate was 3.1 cases per 100 000 in 2005 and 4.7 per 100 000 in 2006, then decreased each year until 2012 (0.2 cases per 100 000 people), rose to 0.3 cases per 100 000 people in 2013. The average incidence from 2005 to 2010 was 2.5 cases per 100 000 people, this rate was more ten times than that of the period from 2011 to 2014 (0.2 cases per 100 000 people; Table 1). The majority of cases during the control stage were attributed to P. vivax infection (78.4 %), while P. falciparum infection predominated during the elimination stage (55.7 %), increasing from 9.4 % in 2005 to 63.1 % in 2014.
Table 1.
Characteristics of malaria cases at control stage and elimination stage in China
| Characteristics | Control stage (2005–2010) | Elimination stage (2011–2014) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | Average per year | 2011 | 2012 | 2013 | 2014 | Average per year | |
| Number of cases | 40,226 | 61,204 | 47,380 | 26,727 | 14,278 | 7506 | 32,887 | 4127 | 2453 | 3905 | 2924 | 3352 |
| Incidence rate (per 100 000) | 3.1 | 4.7 | 3.6 | 2.0 | 1.1 | 0.6 | 2.5 | 0.3 | 0.2 | 0.3 | 0.2 | 0.2 |
| Cases by species | ||||||||||||
| P. vivax | 30,692 | 48,169 | 38,768 | 21,322 | 10,747 | 4956 | 25,776 | 2432 | 1021 | 897 | 851 | 5201 |
| (%) | (76.3) | (78.7) | (81.8) | (79.8) | (75.3) | (66.0) | (78.4) | (58.9) | (41.6) | (23.0) | (29.1) | (38.8) |
| P. falciparum | 3771 | 2872 | 1691 | 1025 | 1043 | 1304 | 1951 | 1442 | 1354 | 2825 | 1844 | 7465 |
| (%) | (9.4) | (4.7) | (3.6) | (3.8) | (7.3) | (17.4) | (5.9) | (35.0) | (55.2) | (72.3) | (63.1) | (55.7) |
| Others | 5763 | 10,163 | 6921 | 4380 | 2488 | 1246 | 5160 | 253 | 78 | 183 | 229 | 743 |
| (%) | (14.3) | (16.6) | (14.6) | (16.4) | (17.3) | (16.6) | (15.7) | (6.1) | (3.2) | (4.7) | (7.8) | (5.5) |
| Cases by residence | ||||||||||||
| Local | 36,009 | 55,999 | 43,489 | 24,180 | 12,413 | 5849 | 29,657 | 2639 | 1325 | 2413 | 1538 | 1979 |
| (%) | (89.5) | (91.5) | (91.8) | (90.5) | (86.9) | (77.9) | (90.2) | (63.9) | (54.0) | (61.8) | (52.6) | (59.0) |
| Migrant | 4217 | 5205 | 3891 | 2547 | 1865 | 1657 | 3230 | 1488 | 1128 | 1492 | 1386 | 1373 |
| (%) | (10.5) | (8.5) | (8.2) | (9.5) | (13.1) | (22.1) | (9.8) | (36.1) | (46.0) | (38.2) | (47.4) | (41.0) |
| Cases by locationa | ||||||||||||
| 24 endemic provinces | 40,182 | 61,130 | 47,312 | 26,659 | 14,221 | 7436 | 32,823 | 4033 | 2386 | 3801 | 2837 | 3264 |
| (%) | (99.9) | (99.9) | (99.7) | (99.8) | (99.6) | (99.1) | (99.8) | (97.7) | (97.3) | (97.3) | (97.0) | (97.4) |
| 7 non-endemic provinces | 44 | 74 | 68 | 68 | 57 | 70 | 64 | 94 | 67 | 104 | 87 | 88 |
| (%) | (0.1) | (0.1) | (0.3) | (0.2) | (0.4) | (0.9) | (0.2) | (2.3) | (2.7) | (2.7) | (3.0) | (2.6) |
| Cases by reporting institutes | ||||||||||||
| Private clinics and hospitals | 321 | 334 | 540 | 347 | 193 | 182 | 320 | 62 | 41 | 69 | 57 | 57 |
| (%) | (0.8) | (0.6) | (1.1) | (1.3) | (1.3) | (2.4) | (1.0) | (1.5) | (1.7) | (1.8) | (2.0) | (1.7) |
| Town-level hospitals | 24,433 | 44,923 | 34,466 | 17,870 | 8767 | 3449 | 22,318 | 1379 | 298 | 174 | 129 | 495 |
| (%) | (60.7) | (73.4) | (72.7) | (66.8) | (61.4) | (46.0) | (67.9) | (33.4) | (12.2) | (4.4) | (4.4) | (14.8) |
| County-level hospitals | 5400 | 5764 | 4919 | 3278 | 1742 | 865 | 3661 | 403 | 327 | 711 | 448 | 472 |
| (%) | (13.4) | (9.4) | (10.4) | (12.3) | (12.2) | (11.5) | (11.1) | (9.8) | (13.3) | (18.2) | (15.3) | (14.1) |
| City-level hospitals | 2562 | 2878 | 2490 | 1840 | 1352 | 1333 | 2076 | 1186 | 923 | 1342 | 1363 | 1204 |
| (%) | (6.4) | (4.7) | (5.3) | (6.9) | (9.5) | (17.8) | (6.3) | (28.7) | (37.6) | (34.4) | (46.6) | (35.9) |
| Public health institutes | 7510 | 7305 | 4965 | 3392 | 2224 | 1677 | 4512 | 1097 | 864 | 1609 | 927 | 1124 |
| (%) | (18.7) | (11.9) | (10.5) | (12.7) | (15.6) | (22.3) | (13.7) | (26.6) | (35.2) | (41.2) | (31.7) | (33.5) |
| Cases by originb | ||||||||||||
| Autochthonous cases | – | – | – | – | – | – | – | 1507 | 253 | 92 | 67 | 480 |
| (%) | – | – | – | – | – | – | – | (36.5) | (10.3) | (2.4) | (2.3) | (14.3) |
| Imported cases | – | – | – | – | – | – | – | 2620 | 2200 | 3813 | 2857 | 2872 |
| (%) | – | – | – | – | – | – | – | (63.5) | (89.7) | (97.6) | (97.7) | (85.7) |
aEndemic provinces include Liaoning, Xinjiang, Hebei, Shanxi, Shaanxi, Shandong, Henan, Jiangsu, Shanghai, Zhejiang, Anhui, Hubei, Hunan, Jiangxi, Fujian, Guangdong, Hainan, Guangxi, Yunnan, Sichuan, Guizhou, Chongqing, Tibet, and Gansu; non-endemic provinces include Beijing, Tianjin, Inner Mongolia, Heilongjiang, Jilin, Qinghai, Ningxia, according to the national malaria elimination program [3]
bOnly the origins of cases occurring within the period 2011–2014 were included in this study, because each malaria case was required to be classified as either autochthonous or imported since 2010, according to the national malaria elimination program [3]
Migrant cases accounted for only 10.5 % of cases in 2005; these increased to 47.4 % in 2014. Overall, the proportion of migrant cases at the elimination stage (41.0 %) was significantly higher than that at the control stage (9.8 %; X2 = 11699.8, P < 0.001). Geographically, cases predominately occurred in the 24 historically endemic-provinces (99.7 %). The proportion of cases occurring in the seven non-endemic provinces increased over the course of the study, from 0.1 % in 2005 to 3.0 % in 2014. During the elimination stage, the proportion of autochthonous cases declined dramatically, from 36.5 % in 2011 to 2.3 % in 2014. The proportion of cases reported by town-level hospitals reduced sharply from 67.9 % during the control period to only 14.8 % in the elimination stage. In contrast, during the period from 2011 to 2014, city-level hospitals and public health institutes became the predominant sources of case reporting (67.4 %). The proportion of cases reported by both city-level hospitals and public health institutes showed an increasing trend, from 28.7 % in 2011 to 46.6 % in 2014, and from 26.6 % in 2011 to 31.7 % in 2014, respectively.
Case diagnosis
The majority of malaria cases (66.2 %; 139 498) were laboratory confirmed. The proportion of cases that were laboratory confirmed (PLab) in the elimination stage (91.1 %) was higher than for the control stage (64.5 %; Fig. 2a). Furthermore, during the elimination stage, PLab showed an increasing yearly trend, from 75.9 % in 2011 to 99.0 % in 2014 (Fig. 2b). PLab in malaria-endemic provinces (91.4 %) was significantly higher than that in the non-endemic provinces (79.0 %; X2 = 65.8, P < 0.001) for the period 2011 to 2014. The number of provinces with PLab greater than 90 % increased from two provinces (2005 and 2010) to 18 provinces (2011 to 2014). The seven non-endemic provinces were among the 13 provinces with PLab lower than 90 % during 2011–2014 (Fig. 3).
Fig. 2.
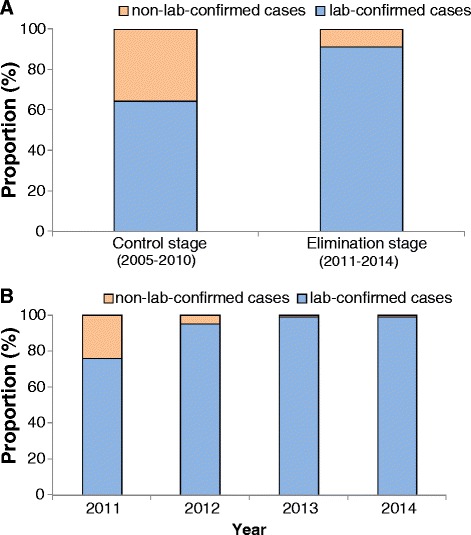
Proportion of lab-confirmed malaria cases during 2005–2014 in China (a proportion of cases between control stage and elimination stage; b proportion of cases by year during elimination stage)
Fig. 3.

Proportion of lab-confirmed malaria by province during 2005–2014 in China (a control stage [2005–2010]; b elimination stage [2011–2014])
There were five provinces with PLab < 70 % during 2011–2014: Tibet (8/24, 33.3 %), Guizhou (72/211, 34.1 %), Ningxia (8/14, 57.1 %), Tianjin (32/48, 66.7 %) and Qinghai (9/13, 69.2 %). The average PLab of these five provinces was 14.0 % in 2011 and 54.1 % in 2012, improved markedly in 2013 and 2014 (87.5 % and 89.1 %, respectively). During the period 2011 to 2014, 114 cases were reported by town-level hospitals in five provinces and their PLab (9/114) was 7.9 % which was lower than that from other reporting institutes (120/196, 61.2 %). Among the 105 non-laboratory-confirmed cases, 104 cases were from Guizhou Province.
During 2005–2014, the median interval from onset of illness to diagnosis (Tdiag) was 4 days (IQR: 2–6). The median Tdiag was 3 days during the elimination stage (IQR: 1–6), one day earlier than the control stage (Fig. 4a).
Fig. 4.
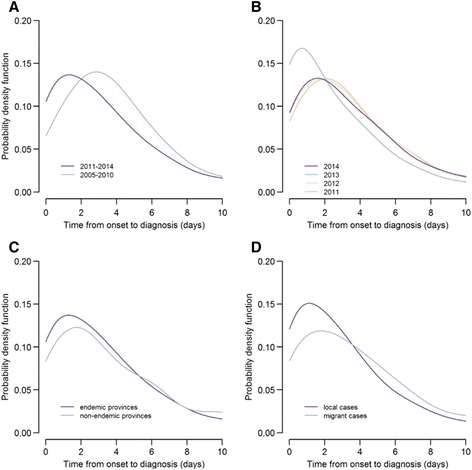
Distributions of time from illness onset to diagnosis of malaria cases in China (a by stage; b by year; c by case geographical distribution of cases during 2011–2014; d by case migration during 2011–2014)
The timeliness of diagnosis was stable during the period from 2011 to 2013, with the median Tdiag being 2 days in 2013, and 3 days during the other 3 years (Fig. 4b). There was no difference of median Tdiag between P. vivax and P. falciparum cases during the period 2011 to 2014. The proportion of cases with Tdiag < 4 days was higher for endemic provinces (65.0 %) than for non-endemic provinces (56.5 %; X2 = 10.7, P = 0.001). The proportion of cases with Tdiag < 4 days was also higher for local cases (69.9 %) than migrant cases (57.3 %; X2 = 224.7, P < 0.001; Fig. 4c and d). The reporting institutes were associated with Tdiag, where the proportion of cases reported from city and higher-level hospitals with Tdiag ≤4 days (58.9 %) was lower than that from other hospitals or public health institutes (70.9 %) (X2 = 209.1, P < 0.001).
Case reports
For the period 2005 to 2014, 86.1 % cases were reported within one day after diagnosed. There was a significant increase in the proportion of notifications within one day over the course of the study, from 85.2 % at the control stage to 99.7 % in elimination stage (X2 = 2115.0, P < 0.001). From 2011, more than 99 % of cases were reported within 24 h after diagnosis (Table 2). From 2011 to 2014, reporting of 40 cases occurred more than 1 day after diagnosis; these came from 16 provinces; most occurred in Tibet (15 cases) and Anhui province (6 cases). The proportion of cases that were reported within one day after diagnosis was lowest in Tibet (37.5 %) from 2011 to 2014. Reporting rates less than a day (from diagnosis) was over 95 % in all the other provinces. Of the 40 instances of delayed reporting, 50 % (20 cases) were reported by public health institutes and 35 % (14 cases) were reported by city-level hospitals.
Table 2.
The reports and investigations for malaria cases in China, 2011–2014
| Item | 2011 | 2012 | 2013 | 2014 | Total |
|---|---|---|---|---|---|
| Number of case reports | 4094 | 2433 | 3793 | 2519 | 12,839 |
| Median interval time from diagnosis to report (day) | 0 | 0 | 0 | 0 | 0 |
| IQR of interval time from diagnosis to report (day) | 0–1 | 0–1 | 0–1 | 0–1 | 0–1 |
| Percentage of cases reported within 1 day after diagnosis (%) | 99.9 | 99.5 | 99.8 | 99.4 | 99.7 |
| Number of case investigations | 3293 | 2007 | 3203 | 2564 | 11,067 |
| Median interval time from report to investigation (day) | 1 | 0 | 0 | 0 | 0 |
| IQR of interval time from report to investigation (day) | 0–4 | 0–2 | 0–1 | 0–1 | 0–1 |
| Percentage of investigations made within 3 days after reports (%) | 74.6 | 88.6 | 99.3 | 98.5 | 89.9 |
Case investigations
During the period August 2012 to December 2014, there were 14,295 short messages alerts automatically sent to the staffs of local CDCs; 99.0 % of which were acted upon appropriately. The median interval from the alert to data verification was 0.84 h (IQR: 0.18–4.4 h); 65.0 % of alerts were verified by the local CDCs within 2 h of the message being sent.
During the malaria elimination stage (2011–2014), the median interval from report to investigation was less than one day (IQR: 0–2 days). For the period 2011 to 2014, 89.9 % (9 944/11 067) of cases were investigated within 3 days after case reported, with an increasing trend from 74.6 % in 2010 to 98.5 % in 2014 (Table 2). The timeliness of case investigation for 2013–2014 (99.0 %) was higher than for 2011–2012 (80.1 %). Amongst migrant cases, 9.2 % (418/4 549) cases were not investigated within three days of reporting during 2011 to 2014, which was slightly lower than that for local cases (10.8 %, 705/6 518, X2 = 7.8, P = 0.005). There was no significant difference in the proportion of cases investigated within 3 days between endemic and non-endemic provinces (X2 = 0.89, P = 0.35).
Discussion
Over the past 10 years, with the effective control of the malaria epidemic, China has shifted it approach to malaria from control (2005–2010) to an elimination phase (2011–2014) [17–22]. This study described changes in the source of malaria cases over this period and reports on the accuracy and timeliness of malaria diagnosis, reporting and investigation.
One of the key features of a malaria surveillance system, in the elimination phase, is to be sufficiently sensitive to detect all malaria infections and facilitate early treatment to prevent secondary cases [3, 6]. All suspected cases of malaria should receive a parasitological test at the elimination stage [16]. This study demonstrated that the proportion of laboratory-confirmed cases has increased significantly from the control stage (64.5 %) to the elimination stage (91.1 %) adding diagnostic certainty to existing epidemiological data. This finding concurs with the findings of other studies [23]. A study of the diagnosis and reporting of malaria cases in China during 2005–2008 concluded that the capacity for laboratory diagnosis needed to be further strengthened, especially in the local medical institutes [24]. This appears to have occurred in the majority of provinces, however, PLab was still <70 % in five provinces from 2011–2014. Furthermore, the PLab in the town-level hospitals (7.9 %) was lower than that for other reporting institutes. This indicates that the capacity for diagnosis in town-level hospitals remains a challenge for malaria elimination programmes, especially in the five provinces with low capacity of laboratory testing. The proportion of laboratory-confirmed cases occurring in non-endemic provinces was also found to be significantly lower than for endemic provinces. This may be a function of a lack of perceived risk, experience, skills or due to poor personal training in the facilities of hospitals and CDCs in these regions; regardless, this represents another area that needs to be strengthened.
Timeliness is one of important attributes of surveillance system evaluation [25, 26]. In this study, the timeliness of case reporting has maintained at higher proportions (above 99 %) during 2011 to 2014, which might indicate the improvement of the internet-based reporting approach of NIDRIS. However, our analysis found that the PLab and the proportion of cases reported in 1 day was the lowest in Tibet during 2011 to 2014 due to the poor capacity of malaria control and prevention in the local CDC of Tibet, and the low accessibility of healthcare services by inconvenient transport [27, 28]. With an aim to achieve a nationwide elimination goal, China’s “1-3-7” surveillance and response strategy should also supported and focus on those remote areas with malaria reported [29].
There are a number of challenges that need to be addressed if China is to successfully eliminate malaria. Population movement has the potential to spread malaria from endemic areas to non-endemic areas or to reintroduce malaria to regions where it has been eliminated [30]. In our study, one apparent characteristic of malaria cases was the increasing proportion of migrant cases in the elimination phase; the need to address this issue has been identified by other researchers [31]. The time from onset to diagnosis for migrant cases was longer than that for local cases, and the timeliness of migrant case investigation was relatively poor. These features might be associated with the characteristics of migrant population, including living in the areas with high malaria endemicity or high risk season, poor accommodation without suitable vector prevention, lack of knowledge and awareness of malaria prevention, poor awareness to seek treatment, and limited accessibility of healthcare service [32, 33]. Equitable provision of diagnosis and treatment, as well as investigation services for this population, are challenges should be taken into account during elimination stage [34]. It is imperative that the issues affecting migrant populations are addressed in national programme, such as through active screening of returning workers especially in areas with high risk of local transmission, if malaria eradication is to be achieved [31].
There were some limitations to this study. Complete data on foci investigation in MESIS during 2011 to 2014 was not available and, as such, we could not analyze the performance of foci investigation and disposal within 7 days. According to the new surveillance project issued by China CDC [35], the data on foci investigation will be collected in MESIS from 2015 onwards. This will allow the whole profile of Chinese “1-3-7” malaria strategy to be better analyzed. Moreover, only the accuracy and timeliness of reporting of surveillance system were evaluated in this study, the other attributes such as the data completeness and validity, and sensitivity and specificity for the malaria surveillance system need to be further assessed in the future.
Conclusions
Our study found that the source of malaria case detection has changed greatly from the control to elimination stage, and the individual case-based malaria surveillance system in China generally operated well during the malaria elimination stage. However, China still faces many challenges, including the changing epidemiology of malaria cases among the domestic migrant and foreign populations, and those who are in the historically non-endemic areas, and hard-to-reach populations.
Abbreviations
CDC, Center for Disease Control and Prevention; IQR, Interquartile Range; MESIS, Malaria Enhanced Surveillance Information System; NIDRIS, National Notifiable Infectious Disease Reporting Information System; NMEP, National Malaria Elimination Programme; PCR, Polymerase Chain reaction Test; PLab, Proportion of Lab-confirmed cases; RDTs, Rapid Diagnostic Tests; Tdiag, the median time from illness onset to diagnosis; WHO, World Health Organization
Acknowledgements
This study was supported by grants from the Ministry of Science and Technology of China (2012ZX10004-201, 2012ZX10004- 220, 2014BAI13B05) and the Ministry of Health of China (No. 201202006). We appreciated Dr. Gabriel J Milinovich from the Queensland University of Technology, Australia for the valuable comments on the manuscript.
Authors’ contributions
JS, SZ, QG, ZL, WH, AC and SL designed the analysis. JS, SZ, QZ, ZZ and CZ collected and cleared the data. JS and ZL wrote the first draft of the paper. All authors contributed to the interpretation of the results, and to the revision and final preparation of the paper submission. All authors read and approved the final manuscript.
Authors’ information
Junling Sun is an epidemiologist and the Head of the Branch of Parasitic Diseases, Division of Infectious Diseases, Key Laboratory of Surveillance and Early-warning on Infectious Disease, Chinese Center for Disease Control and Prevention, whose major research interests are on parasitic disease and epidemiology.
Competing interests
The authors declare that they have no competing interests. The funding bodies had no role in the study design, data collection and analysis, preparation of the manuscript, or the decision to publish.
Additional file
Multilingual abstracts in the six official working languages of the United Nations. (PDF 258 kb)
References
- 1.Zofou D, Nyasa RB, Nsagha DS, Ntie-Kang F, Meriki HD, Assob JC, Kuete V. Control of malaria and other vector-borne protozoan diseases in the tropics: enduring challenges despite considerable progress and achievements. Infect Dis Poverty. 2014;3:1. doi: 10.1186/2049-9957-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . World Malaria Report 2015. Geneva: WHO Press; 2015. [Google Scholar]
- 3.WHO. From malaria control to malaria elimination: a manual for elimination scenario planning. Geneva: WHO Press; 2014.
- 4.Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, Gueye CS, Fullman N, Gosling RD, Feachem RG. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–11. doi: 10.1016/S0140-6736(13)60310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tambo E, Adedeji AA, Huang F, Chen JH, Zhou SS, Tang LH. Scaling up impact of malaria control programmes: a tale of events in Sub-Saharan Africa and People's Republic of China. Infect Dis Poverty. 2012;1:7. doi: 10.1186/2049-9957-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Disease surveillance for malaria elimination: operational manual. Geneva: WHO Press; 2012.
- 7.WHO. T3-Test. Treat. Track- scaling up diagnostic testing, treatment and surveillance for malaria. Geneva: WHO Press; 2012.
- 8.China NHFPC. Action plan of China malaria elimination (2010-2020). 2012. http://www.nhfpc.gov.cn/jkj/s5873/201005/f84f1c4b0f32420990d23b65a88e2d87.shtml. Accessed 30 Oct, 2015. (in Chinese).
- 9.Cao J, Sturrock HJ, Cotter C, Zhou S, Zhou H, Liu Y, Tang L, Gosling RD, Feachem RG, Gao Q. Communicating and monitoring surveillance and response activities for malaria elimination: China's "1-3-7" strategy. PLoS Med. 2014;11:e1001642. doi: 10.1371/journal.pmed.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng XY, Xia ZG, Vong S, Yang WZ, Zhou SS. Surveillance and response to drive the national malaria elimination program. Adv Parasitol. 2014;86:81–108. doi: 10.1016/B978-0-12-800869-0.00004-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhou XN, Bergquist R, Tanner M. Elimination of tropical disease through surveillance and response. Infect Dis Poverty. 2013;2:1. doi: 10.1186/2049-9957-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.China NHFPC. Diagnostic criteria for malaria (WS 259-2006).2006. (in Chinese) http://www.nhfpc.gov.cn/zwgkzt/s9499/201410/d29f0a078dd143f8b6374ed23dc40400.shtml. Accessed 30 Oct, 2015.
- 13.Yang W, Li Z, Lan Y, Wang J, Ma J, Jin L, Sun Q, Lv W, Lai S, Liao Y, Hu W. A nationwide web-based automated system for outbreak early detection and rapid response in China. Western Pac Surveill Response J. 2011;2:10–15. doi: 10.5365/WPSAR.2010.1.1.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Wang Y, Jin S, Wu Z, Chin DP, Koplan JP, Wilson ME. Emergence and control of infectious diseases in China. Lancet. 2008;372:1598–605. doi: 10.1016/S0140-6736(08)61365-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang FZ, Yap P, Zhang SY, Xie HG, Ouyang R, Lin YY, Chen ZY. Surveillance and response strategy in the malaria post-elimination stage: case study of Fujian province. Adv Parasitol. 2014;86:183–203. doi: 10.1016/B978-0-12-800869-0.00007-X. [DOI] [PubMed] [Google Scholar]
- 16.China CDC. Chinese technical scheme of malaria elimination. Beijing; 2011.
- 17.Cao J, Zhou SS, Zhou HY, Yu YB, Tang LH, Gao Q. Malaria from control to elimination in China: transition of goal, strategy and interventions. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2013;25:439–43. [PubMed] [Google Scholar]
- 18.Feng J, Xia ZG. Analysis of trends in cases of malaria reported from 2004 to July 2013 in the People’s Republic of China. Zhongguo Bing Yuan Sheng Wu Xue Za Zhi. 2014;9:442–446. [Google Scholar]
- 19.Feng J, Xiao H, Xia Z, Zhang L, Xiao N. Analysis of malaria epidemiological characteristics in the People's Republic of China, 2004-2013. Am J Trop Med Hyg. 2015;93:293–99. doi: 10.4269/ajtmh.14-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Lai S, Zheng C, Zhang H, Zhou S, Hu W, Clements AC, Zhou XN, Yang W, Hay SI, Yu H, Li Z. The epidemiology of Plasmodium vivax and Plasmodium falciparum malaria in China, 2004-2012: from intensified control to elimination. Malar J. 2014;13:419. doi: 10.1186/1475-2875-13-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou S, Li Z, Cotter C, Zheng C, Zhang Q, Li H, Zhou S, Zhou X, Yu H, Yang W. Trends of imported malaria in China 2010–2014: analysis of surveillance. Malar J. 2016;15:39. doi: 10.1186/s12936-016-1093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin JH, Zhou SS, Xia ZG, Wang RB, Qian YJ, Yang WZ, Zhou XN. Historical patterns of malaria transmission in China. Adv Parasitol. 2014;86:1–19. doi: 10.1016/B978-0-12-800869-0.00001-9. [DOI] [PubMed] [Google Scholar]
- 23.Zhou SS, Zhang SS, Zhang L, Rietveld AE, Ramsay AR, Zachariah R, Bissell K, Van den Bergh R, Xia ZG, Zhou XN, Cibulskis RE. China's 1-3-7 surveillance and response strategy for malaria elimination: Is case reporting, investigation and foci response happening according to plan? Infect Dis Poverty. 2015;4:55. doi: 10.1186/s40249-015-0089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng C, Zhang Q, Li H. Analysis on the diagnosis and report of malaria cases in China during 2005-2008. Zhonghua Ji Bing Kong Zhi Za Zhi. 2010;14:507–09. [Google Scholar]
- 25.Calba C, Goutard FL, Hoinville L, Hendrikx P, Lindberg A, Saegerman C, Peyre M. Surveillance systems evaluation: a systematic review of the existing approaches. BMC Public Health. 2015;15:448. doi: 10.1186/s12889-015-1791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN, Birkhead GS. Update guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep. 2001;50(RR-13):1–35. [PubMed] [Google Scholar]
- 27.Zhou X, Zhang S, Xu J, Xia Z, Wang R, Qian Y, Zhou S, Yang W. Risk Assessment for Malaria Elimination in P. R. China. Zhong Guo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Xue Za Zhi. 2014;32:414–18. [PubMed] [Google Scholar]
- 28.Zhang L, Zhou S, Feng J, Fang W, Xia Z. Malaria situation in the People’s Republic of China in 2014. Zhong Guo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Xue Za Zhi. 2015;33:319–26. [PubMed] [Google Scholar]
- 29.Zhou S, Yan J, Xia Z, Feng J, Hu T, Tang L, et al. Opportunities, challenges and strategies for malaria elimination in the Tibet Autonomous Region of China. Zhong Guo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Xue Za Zhi. 2015;33:425–28. [PubMed] [Google Scholar]
- 30.Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B. Malaria resurgence: a systematic review and assessment of its causes. Malar J. 2012;11:122. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu G, Zhou S, Horstick O, Wang X, Liu Y, Muller O. Malaria outbreaks in China (1990-2013): a systematic review. Malar J. 2014;13:269. doi: 10.1186/1475-2875-13-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Yang Y, Xiao N, Zhou S, Lin K, Wang D, Zhang Q, Jiang W, Li M, Feng X, Yu J, Ren X, Lai S, Sun J, Fang Z, Hu W, Clements AC, Zhou X, Yu H, Yang W. Malaria imported from Ghana by returning gold miners, China, 2013. Emerg Infect Dis. 2015;21:864–67. doi: 10.3201/eid2105.141712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Hsiang MS, Zhou H, Wang W, Cao Y, Gosling RD, Cao J, Gao Q. Malaria in overseas labourers returning to China: analysis of imported malaria in Jiangsu Province, 2001–2011. Malar J. 2014;13:29. doi: 10.1186/1475-2875-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens P, Hall L. Malaria on the move: human population movement and malaria transmission. Emerg Infect Dis. 2000;6:103–09. doi: 10.3201/eid0602.000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.China CDC. Malaria elimination surveillance Project (2015 edition). 2015. (in Chinese).


