Abstract
The yield of human alpha 2b interferon in Escherichia coli was optimized by replacement of low-usage arginine codons located in the mRNA 5′ end. The differences observed among the various gene variants suggest that codon usage, Shine-Dalgarno-like sequences, and mRNA secondary structure contribute to the performance of E. coli translation machinery.
Human alpha interferons (IFN-α) belong to a family of homologous proteins that are known to display antiviral, antiproliferative, and immunomodulatory activities (3, 11). IFN-α2b is one of the most studied IFN-α, and its intronless sequence encodes a nonglycosylated protein containing 165 amino acid residues (3, 10).
The expression of heterologous proteins in Escherichia coli is often hampered by the presence of arginine low-usage codons, AGG and AGA. This is probably due to the low abundance of the cognate tRNA in bacteria (2, 4, 6, 9, 16). The inhibition of translation is more pronounced when these low-usage codons are located in clusters near the mRNA 5′ end (1, 5, 7, 13), which can mimic the Shine-Dalgarno (SD) sequence even in the absence of an associated cognate initiation codon (1, 8, 12). The presence in mRNA of stable secondary structures that can block initiation has also been correlated with preventing translation in E. coli (14, 15).
This work aims to study the effect of rare arginine codons, single and clustered, located at the mRNA 5′ end, as well as the effect of the inherent secondary structures, on the yield of human IFN-α2b (hu-IFN-α2b) in E. coli. Site directed mutagenesis was used to construct a series of hu-IFN-α2 gene variants. Some variants comprised the replacement of arginine clusters (Arg12Arg13 and Arg22Arg23), while others included the replacement of an isolated codon (Arg33). The evaluation of hu-IFN-α2b yield from these gene variants suggests the contribution of several factors, namely codon usage, SD-like sequences, and mRNA secondary structure, to gene expression in E. coli.
A 520-bp fragment coding for hu-IFN-α2b was amplified from human genomic DNA by PCR, using the sense, 5′-TTGAATTCATATGTGTGATCTGCCTCAAACCCACAGC-3′,and the antisense, 5′-CCTAGGTAATAAGGAAGAAGAATTTGAAAGAACG-3′, oligonucleotides. This fragment was restricted and cloned into BamHI/EcoRI-digested pUC19. Subsequently, the hu-IFN-α2b fragment was cut from pUC-IFNα and cloned into a BamHI/NdeI-digested pET-9a expression vector (Promega). A series of hu-IFN-α2b gene variants (Fig. 1) was constructed by site-directed mutagenesis (QuickChange kit; Stratagene). Both IFN wild-type and variant genes were sequenced by Replicon (Berlin, Germany).
FIG. 1.
Nucleotide sequences of hu-IFN-α2b gene variants. The nucleotide substitutions are shown underlined, and SD-like sequences are highlighted in grey. (For amino acid position numbering we have not considered the first methionine, which is found only in the recombinant protein.)
E. coli JM109(DE3) cells harboring either pET9-IFNα or pET9-IFN-MRx were grown at 37°C and at 250 rpm in a shake flask containing 100 ml of Luria-Bertani medium plus kanamycin (30 μg/ml). The host strain contains, integrated into its chromosome, a single copy of the gene for the T7 RNA polymerase under the control of the isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible lacUV5 promoter. Consequently, upon IPTG induction, large amounts of T7 RNA polymerase are produced, which leads to the expression of genes positioned downstream of the T7 promoter, as is the case of the hu-IFN-α2b gene. Protein expression was induced at mid-exponential phase (optical density [OD] at 600 nm of ∼1) with 1 mM IPTG. No significant differences were observed between maximal growth rates (0.57 ± 0.07 h−1) of cells expressing the different gene variants.
Total bacterial proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Immunodetection was performed through an overnight incubation with a monoclonal antibody against hu-IFN-α2b, produced by MT4/E4 hybridoma cells (European Collection of Cell Cultures), followed by incubation with a goat anti-mouse immunoglobulin G alkaline phosphatase conjugate and then with an alkaline conjugate substrate (Bio-Rad). Western blots were photographed in the Eagle Eye System (Stratagene) and analyzed by densitometry with the TotalLab v1.11 software (Phoretix, Newcastle upon Tyne, United Kingdom). Total hu-IFN-α was quantified using a calibration curve on each Western blot constructed with 50, 100, 200, and 400 ng of a pharmaceutical IFN-α2b standard, Intron A (Schering-Plough Corp.).
Both amino-terminal and internal amino acid sequencing (carried out by Eurosequence, Groningen, The Netherlands) confirmed, respectively, the hu-IFN-α2b sequences Asp2-Thr6 and Ser150-Leu161, indicating that the correct protein was expressed.
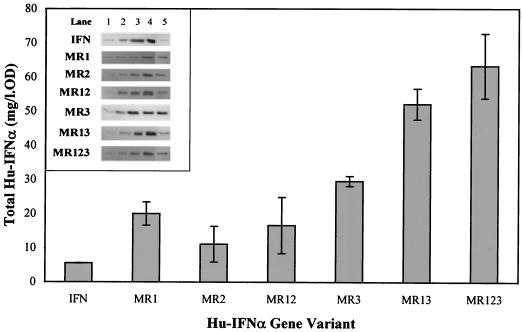
Recombinant protein expression was detected through sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the hu-IFN-α2b yield was evaluated by performing densitometry on Western blots. Yields were compared at 5 h postinduction (mid-exponential phase). At this stage, hu-IFN-α2b yields were maximal in all constructs except for MR13 and MR123 (∼75% of maximal yield).
The gene variants constructed by replacing the low-usage arginine codons (AGG and AGA) with the major-usage codon (CGC) were found to express higher levels of hu-IFN-α2b than the nonmutated clone. However, a correlation between the number of replaced codons and protein yield was not found.
Hu-IFN-α2b yields of variants MR1 and MR2 increased 3.6-fold and 2.0-fold, respectively, relative to the control gene (Fig. 2), which has two rare tandem arginine codons, Arg12Arg13 (AGGAGG) and Arg22Arg23 (AGGAGA) (Fig. 1). Due to the similarity with the SD sequence, these dual clusters of arginine codons often have been called artificial translation initiators. The presence of those sequences in a gene which is to be expressed in E. coli is known to originate incorrect translation events, such as frameshifting and dissociation of the translation complexes (13). This results from the competition with the functional SD sequence (8, 12) and thus leads to a decrease in protein yield. The improved hu-IFN-α2b yield obtained upon replacement of the artificial translational initiators (MR1 and MR2 variants) can be associated to a decrease in the frequency of these incorrect translational events, corroborating previous findings (17). However, since the number of rare arginine codons replaced in these two constructs was the same (two codons), the differences in protein yield observed between them was unexpected. Apparently the potential role of these tandem arginine codons as translational initiators, due to their similarity with the SD sequence, is less important the farther away they are from the initiation codon. In variant MR2, which has the tandem arginine 63 nucleotides downstream from the initiation codon, the replacement effect is weaker than in MR1, which contains the tandem arginine (33 nucleotides) closer to the initiation codon, and thus, hu-IFN-α2b yield increases less than with the nonmutated gene.
FIG. 2.
Hu-IFN-α2b yield obtained from each gene variant at 5 h postinduction. In the insert, the first four lanes in the Western blots correspond to the standard hu-IFN-α, 50, 100, 200, and 400 ng, respectively. Lane 5 corresponds to the 5-h sample of each gene variant: IFN (5 μl; OD = 1.96), MR1 (2 μl; OD = 2.58), MR2 (10 μl; OD = 1.50), MR12 (4 μl; OD = 1.37), MR3 (5 μl; OD = 1.50), MR13 (1 μl; OD = 1.80) and MR123 (1 μl; OD = 2.72) l., liter.
Gene variant MR12 has both rare tandem arginine codons (Arg12Arg13 and Arg22Arg23) replaced and expressed 16.6 mg of hu-IFN/liter of a bacterial culture with an OD of 1, a figure that corresponds to a threefold increase relative to the nonmutated clone. Therefore, hu-IFN-α2b yield from MR12 is probably regulated not only by codon usage but also by a particular feature in the mRNA secondary structure which will be discussed later.
The more intriguing result was the comparatively high hu-IFN-α2b yield, 29.6 mg of hu-IFN/liter of a bacterial culture with an OD of 1 (Fig. 2), attained by the single replacement of Arg33 (gene variant MR3). This was further confirmed by introducing additional mutations, which resulted in the MR13 and MR123 variants (Fig. 1). These clones displayed high levels of hu-IFN-α2b yield which correspond to a 9.5-fold and 11.5-fold increase relative to the nonmutated clone (Fig. 2).
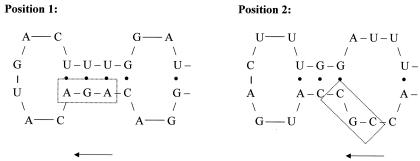
The reason behind such a strong replacement effect was thought to be related to some feature in the mRNA secondary structure. An in silico analysis of the mRNA secondary structures of each transcript variant (677 nucleotides), as predicted by MFOLD (17) (http://www.bioinfo.rpi.edu/applications/mfold/old/rna/ [18 February 2003, last date accessed]), showed no significant differences between folding energies in all gene variants (E value of ≥−231.6 kcal/mol and ≤−227.5 kcal/mol). Regions with a high number of base pairs, such as stems, are less favorable for ribosome progression than unpaired regions, such as loops, and thus decrease the translation rate. However, a relationship between the total number of base pairs in the transcript and the protein yield was not found. Nevertheless, a detailed analysis of the predicted mRNA secondary structures of each construct shows a distinct location for the Arg33 codon. Figure 3 revealed that the nonreplaced Arg33 codon (IFN, MR1, MR2, and MR12) is placed in a stem (position 1). The highest hu-IFN-α2b producers (MR3, MR13, and MR123) contain the replaced Arg33 codon in a 3-3 nucleotide loop (position 2). Regardless of the codon replacement effect, the hu-IFN-α2b yield increases with the decrease in the number of base pairs located in the neighboring regions of the Arg33 codon. This suggests that the easiness of the ribosome progression through a specific secondary and tertiary structure of the mRNA might have some contribution to the efficiency of the translational machinery.
FIG. 3.
Close-up of the positions of the Arg33 codon found in the mRNA secondary structures. The small arrows indicate the direction of translation.
Independently of the regulating effects of translation, the described replacement of arginine codons showed a cumulative effect upon hu-IFN-α2b yield: MR13 ≈ MR1 + MR3; MR123 ≈ MR1 + MR2 + MR3 ≈ MR2 + MR13. However, this cumulative replacement effect is not clearly seen in the MR12 variant, probably because the mRNA secondary and tertiary structure negative effect overrides the benefits of replacing the less-rare codons.
In conclusion, this work highlights several aspects of protein translation in E. coli. Codon usage, SD-like sequences, and mRNA secondary and tertiary structures were found to contribute to the overall efficiency of the bacterial translational system.
Acknowledgments
This work was supported by the Portuguese Ministry of Science and Technology FCT/ADI PRAXIS XXI project Protexpress and a Ph.D. grant, BD/16962/97.
We thank A. Kelly for his assistance in the design of oligonucleotides.
REFERENCES
- 1.Alexandrova R., M. Eweida, F. Georges, B. Dragulev, M. AbouHaidair, and I. Ivanov. 1995. Domains in human interferon alpha-1 gene containing tandems of arginine codons AGG play the role of translational initiators in Escherichia coli. Int. J. Biochem. Cell Biol. 27:469-473. [DOI] [PubMed] [Google Scholar]
- 2.Bonekamp, F., and K. F. Jensen. 1988. The AGG codon is translated slowly in E. coli even at very low expression levels. Nucleic Acids Res. 16:3013-3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bordens, R., S. E. Grossberg, P. P. Trotta, and T. L. Nagabbushan. 1997. Molecular and biologic characterization of recombinant interferon-a2b. Semin. Oncol. 24:S9-41-S9-51. [PubMed] [Google Scholar]
- 4.Brinkmann, U., R. E. Mattes, and P. Buckel. 1989. High-level expression of recombinant genes in Escherichia coli is dependent on the availability of the dnaY gene product. Gene 85:109-114. [DOI] [PubMed] [Google Scholar]
- 5.Chen, G. F., and M. Inouye. 1990. Suppression of the negative effect of minor arginine codons on gene expression; preferential usage of minor codons within the first 25 codons of the Escherichia coli genes. Nucleic Acids Res. 18:1465-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia, O. L., B. González, A. Menéndez, A. E. Sosa, J. R. Fernández, H. Santana, and N. Meneses. 1996. The argU gene product enhances expression of the recombinant human alpha 2-interferon in Escherichia coli. Ann. N. Y. Acad. Sci. 782:79-86. [DOI] [PubMed] [Google Scholar]
- 7.Goldman, E., A. H. Rosenberg, G. Zubay, and F. W. Studier. 1995. Consecutive low-usage leucine codons block translation only when near the 5′ end of a message in Escherichia coli. J. Mol. Biol. 245:467-473. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov, I. G., R. Alexandrova, B. Dragulev, A. Saraffova, and M. AbouHaidar. 1992. Effect of tandemly repeated AGG triplets on the translation of CAT-mRNA in Escherichia coli. FEBS Lett. 307:173-176. [DOI] [PubMed] [Google Scholar]
- 9.Jeong, W., and H. C. Shin. 1998. Supply of the ArgU gene product allows high-level expression of recombinant human interferon-alpha-2a in Escherichia coli. Biotechnol. Lett. 20:19-22. [Google Scholar]
- 10.Kaluz, S., A. Gibadulinová, and P. Kontsek. 1993. Interferon alpha 2b but not interferon alpha 2a detected in human genomic DNA. Acta Virol. 37:97-100. [PubMed] [Google Scholar]
- 11.Lydon, N. B., C. Favre, S. Bove, O. Neyret, S. Benureau, A. M. Levine, G. F. Seelig, T. L. Nagabhushan, and P. P. Trotta. 1985. Immunochemical mapping of α-2 interferon. Biochemistry 24:4131-4141. [DOI] [PubMed] [Google Scholar]
- 12.Mawn, M. V., M. J. Fournier, D. A. Tirrell, and T. L. Mason. 2002. Depletion of free 30S ribosomal subunits in Escherichia coli by expression of RNA containing Shine-Dalgaro-like sequences. J. Bacteriol. 184:494-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenberg, A. H., E. Goldman, J. J. Dunn, F. W. Studier, and G. Zubay. 1993. Effects of consecutive AGG codons on translation in Escherichia coli, demonstrated with a versatile codon test system. J. Bacteriol. 175:716-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tessier, L. H., P. Sondermeyer, T. Faure, D. Dreyer, A. Benavente, D. Villeval, M. Courtney, and J. P. Lecocq. 1984. The influence of mRNA primary and secondary structure on human IFN-γ. Nucleic Acids Res. 12:7663-7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, H., S. Miao, D. Chen, L. Wang, and S. S. Koide. 1999. Assignment of chromosomal locus and evidence for alternatively spliced mRNAs of a human sperm membrane protein (hSMP-1). Biochim. Biophys. Acta 1447:119-124. [DOI] [PubMed] [Google Scholar]
- 16.Zahn, K. 1996. Overexpression of an mRNA dependent on rare codons inhibits protein synthesis and cell growth. J. Bacteriol. 178:2926-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]