Abstract
Importance
Optimization of glycemic control is critical to reduce diabetes related complications, but long-term success is challenging. Although vision loss is among the greatest fears of individuals with diabetes, comprehensive personalized diabetes education and risk assessments are not consistently employed in ophthalmology settings.
Objective
To determine whether point-of-care measurement of HbA1c and personalized diabetes complication risk assessments performed during retinal ophthalmology visits improve glycemic control as assessed by HA1c.
Design/Setting
Ophthalmologist office based clinical trial where investigators from 42 sites were randomly assigned to provide either study-prescribed augmented diabetes assessment and education, or usual care.
Participants
Adults with type 1 or 2 diabetes enrolled into two cohorts: “more frequent” than annual follow-up (502 control and 488 intervention participants) and “annual” follow-up (368 and 388 participants).
Intervention(s)
Point-of-care measurement of HbA1c, blood pressure, and retinopathy severity; individualized estimate of retinopathy progression risk derived from the visit findings; structured comparison and review of past and current clinical findings; and structured education with immediate assessment and feedback regarding participant understanding. Intervention was performed at enrollment and routine ophthalmic follow-up visits scheduled at least 12 weeks apart.
Main Outcome Measure(s)
Mean change in HbA1c from baseline to 1 year. Secondary outcomes included body mass index, blood pressure, and diabetes self-management practices and attitudes surveys.
Results
In the “more frequent” cohort, mean (SD) change in HbA1c at 1 year was −0.1% (1.5%) in the control group and −0.3% (1.4%) in the intervention group (adjusted mean difference −0.09%, 95% confidence interval −0.29% to +0.12%, P=0.35). In the “annual” cohort, mean (SD) change in HBA1c was 0.0% (1.1%) and −0.1% (1.6%), respectively (mean difference −0.05%, 95% confidence interval −0.27% to +0.18% P=0.63). Results were similar for all secondary outcomes.
Conclusions and Relevance
Long-term optimization of glycemic control is not achieved by a majority of individuals with diabetes. Addition of personalized education and risk assessment during retinal ophthalmology visits, as provided in this study, did not result in HbA1c improvement compared with usual care over 1 year. These data suggest that optimizing glycemic control remains a substantive challenge requiring interventional paradigms other than those examined in this study.
Introduction
Multiple randomized clinical trials have established that intensive blood glucose control can reduce the onset and progression of microvascular complications in individuals with diabetes.1–4. The Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications study (EDIC) underscored the benefit of closely monitored intensive glycemic control in type 1 diabetes on retinopathy and other diabetic complications.5, 6 Preventative therapy is a fundamental tenant to reduce the numerous ocular and systemic complications of diabetes.
Despite the benefits, optimal glycemic control, as assessed by HbA1c, is notoriously difficult to achieve, especially long-term.7–9 Forty-one percent of US adults with diabetes who participated in the 2005–2010 National Health and Nutrition Examination Surveys (NHANES) did not have anHbA1c of less than the American Diabetes Association suggested goal of 7%.10 Obstacles to achieving optimal glycemic control include lack of awareness of potential organ damage, mistrust of the medical system, cultural beliefs, financial constraints, poor access to care, depression, denial, lack of motivation and lack of perseverance.11
One potential strategy to improve glycemic control is to leverage factors known to be highly motivating for people with diabetes, including fear of vision loss. Results from a nationwide poll by the American Foundation for the Blind demonstrated that Americans fear blindness more than HIV/AIDS, cancer, stroke or heart attacks.12 Other studies have shown similar results where fear of vision loss even exceeds fear of dying prematurely.13 Studies suggest that when patients with diabetes received personalized discussions of their retinal images during an endocrinology visit, HbA1c over the following 3 months improved more than endocrinology evaluation alone.14
An annual dilated eye examination is the minimum recommendation for individuals with diabetes. At this ophthalmic visit physicians have an opportunity to personalize glycemic control and eye disease education. With knowledge of current key factors for an individual, the risk for onset or progression of retinopathy and kidney disease can be estimated. Although not performed in most ophthalmology practices, obtaining and tracking point-of-careHbA1c over time and recording blood pressure, in addition to retinopathy status, can allow for personalized education and potentially provide patients with motivation for achieving improved systemic control. Individualized risk reports might have a greater impact than recommendations based on population averages. Assessments may also be shared with primary care providers.
Given the large and growing worldwide diabetic population,15 closing the gap between the known benefits of intensive glycemic control, and the ability to achieve this goal, is of great public health importance. Thus, a trial to ascertain whether combining personalized risk assessments and diabetes education at an ophthalmology visit can improve glycemic control for individuals with diabetes was warranted.
Methods
This randomized, multi-center clinical trial (www.clinicaltrials.gov - NCT01323348) was conducted by the Diabetic Retinopathy Clinical Research Network (DRCR.net) at 42 clinical sites in the United States (protocol available at www.drcr.net). The study adhered to the tenets of the Declaration of Helsinki. The protocol and Health Insurance Portability and Accountability Act compliant informed consent forms were approved by multiple institutional review boards and study oversight was provided by an independent data and safety monitoring committee. Each study participant gave written informed consent prior to participation in the study. Key aspects of the protocol pertinent to this manuscript are summarized herein.
Study Population
Eligible participants were >18 years old with type 1 or type 2 diabetes and expected routine retina specialist follow up required at least annually. Principal exclusion criteria were: (1) knownHbA1c <7.5% within the prior 6 months (participant report or available records at the time of enrollment), (2) prior complete panretinal photocoagulation, (3) initiation of insulin treatment within 3 months from the date of enrollment, (4) visual acuity loss in both eyes which prohibits ability to read study materials, and (5) significant renal disease including use of erythropoietin or a history of chronic renal failure requiring dialysis or kidney transplant. Subjects with knownHbA1c <7.5% were excluded since these better controlled patients would have less room forHbA1c improvement, less risk overall for diabetes complications, and reduced diabetic retinopathy progression.
Synopsis of Study Design
Sites chose to be randomized by site [34 sites (88 ophthalmologists)] or by ophthalmologist [8 sites (35 ophthalmologists)] to “usual care” or “intervention”; participants were assigned according to the enrolling site or ophthalmologist. The study included private practices (57%) and institutions (43%). Randomization was stratified by site-reported race/ethnicity. Follow-up was planned for 2 years with the primary outcome at one year. However, based on the one-year results, after DSMC approval, the study was ended prior to the planned 2-year follow-up.
Participants in the “usual care” group were only required to complete an annual study visit, although if they returned for a standard care visit 9 to 17 weeks after their baseline visit a 3-month study visit was documented. Participants in this group did not receive any additional diabetes education care beyond the ophthalmologist’s standard visit approach. Participants in the “intervention” group received the following intervention at each standard of care visit, but not more often than every 12 weeks: 1) a point-of-care measurement ofHbA1c, blood pressure and retinopathy severity, 2) a graph showing personalized risk for worsening retinopathy based on the participant’sHbA1c and diabetes type (eAppendix 1A and 1B), 3) a report summarizing personalized risk assessment for renal disease and retinopathy based on the participant’sHbA1c (eAppendix 1C), 4) a graph plotting the participant’s previous and currentHbA1c results (eAppendix 1D), 5) an email with instructions for access to their individualized risk assessment findings, and 6) supplemental diabetes management education materials (baseline only). Materials were reviewed by the ophthalmologist via a script, given to the participant, and mailed to their primary care provider (eAppendix 1F). Each participant completed an assessment of their understanding of key diabetes control issues and immediate feedback and supplemental education was given if the assessment was not answered 100% correctly (eAppendix 1E).
Examination Procedures
Baseline testing included: visual acuity (obtained from a usual care assessment within the past 3 months), ocular examination of both eyes including dilated fundus examination, blood pressure measurement, collection of a blood sample for central labHbA1c measurement, measurement ofHbA1c using the DCA Vantage point-of-care device (intervention group only), measurement of height and weight, and completion of the Problem Areas in Diabetes (PAID) Questionnaire and the Self-Care Inventory (SCI-2) Questionnaire. The PAID Questionnaire is a validated self-administered questionnaire consisting of 20 items scored 0–4 (“Not a problem” to “Serious Problem”) covering a range of emotional problems frequently reported in persons with diabetes. 16, 17 The SCI-2 Questionnaire is a validated self-administered questionnaire that consists of 17 items scored 1–5 (“Never” to “Always”) that measure perceived adherence to diabetes self-care recommendations. 18–20 Testing at the 1-year visit included all of the tests performed at the baseline visit.
Diabetes and ophthalmic management was left to the participant in partnership with the study participant’s medical care providers. Adverse event reporting was limited to severe hypoglycemia, as defined by DCCT criteria.21
Statistical Methods
The study was designed to evaluate intervention versus control participants within two separate cohorts. Participants were included in the “more frequent” cohort if at least one retinal visit occurred between baseline and 1 year, otherwise they were included in the “annual” cohort. Assuming a difference between treatment groups in 1-year change in HbA1c of 0.5% for each cohort, standard deviation of change of 1.8%, intra-cluster (i.e., within site) correlation coefficient of 0.03, type 1 error rate of 0.05, and power of 90%, the sample size was estimated to be 800 participants with a baseline central laboratoryHbA1c value of ≥6.0% for each cohort for a total of 1600 participants equally distributed among 25 cluster units per treatment group. To account for potential 10% loss to follow up, the target sample size was increased to 900 per cohort. The recruitment goal was met in the “more frequent” cohort, however the recruitment goal in the “annual” cohort could not be met in a timely manner and recruitment was discontinued after 756 participants were enrolled.
All analyses included only participants with a baseline central laboratoryHbA1c value of ≥6.0%. The primary outcome was the change inHbA1c from baseline to 1 year. Treatment group comparisons were made within each cohort using a repeated measures analysis of covariance model (ANCOVA) to adjust for baselineHbA1c and correlation of participants within the same site. The primary analysis included only participants with baseline and 1-year central lab values and was an intent-to-treat analysis with study participants analyzed in their assigned group (intervention or control) regardless of whether the education intervention was actually received.
Secondary outcomes of body mass index and blood pressure, and PAID and SCI-2 composite scores, were compared between intervention groups within each cohort using linear mixed ANCOVA to adjust for the baseline value and correlation of participants within the same site. For PAID and SCI-2 surveys, 0–100 point composite scores were calculated for each participant at each visit.20, 22 History of medical conditions (including occurrence of severe hypoglycemic episodes, myocardial infarction, stroke, renal failure) were collected at baseline and annual visits. There were no identifiable relationships between these events and treatment group, for either cohort (data not shown).
Parallel analyses were performed on all outcomes for the data collected at 3 months and 2 years. Results were similar to 1 year and are not included in this report.
All P values are 2-sided. SAS version 9.4 (SAS Inc, Cary, NC) was used for all analyses.
Results
There were 25 clusters (ophthalmologists or sites) randomly assigned to each group. The control group included 502 and 368 participants in the “more frequent” and “annual” cohorts (range 11–31 and 1–25 participants/cluster), respectively and the intervention group included 488 and 388 participants in the “more frequent” and “annual” cohorts (range 7–29 and 3–24 participants/cluster), respectively, enrolled April 2011 through January 2013.
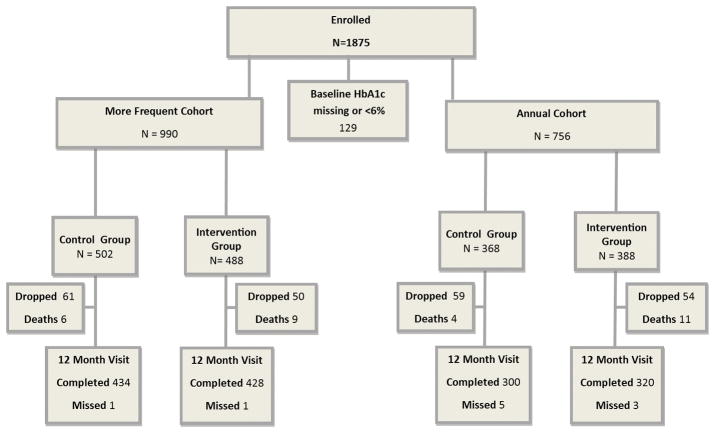
Follow-up completion is summarized in Figure 1. The 1-year visit completion rate (excluding deaths) for the “more frequent” cohort was 88% and 89% in the control and intervention groups, respectively; for the “annual” cohort it was 82% and 85%, respectively. Baseline characteristics were similar when comparing 1-year completers versus non-completers (data not shown), with the exception of a higher mean central labHbA1c in the non-completers of each cohort/group (8.7% versus 8.4% in non-completers versus completers, overall).
Figure 1. Follow-up Flowchart.
Of 42 sites, 34 were randomized on the site level (at 17 sites all investigators were assigned to Intervention, at 17 sites all investigators were assigned to Control) and 8 were randomized on the investigator level.
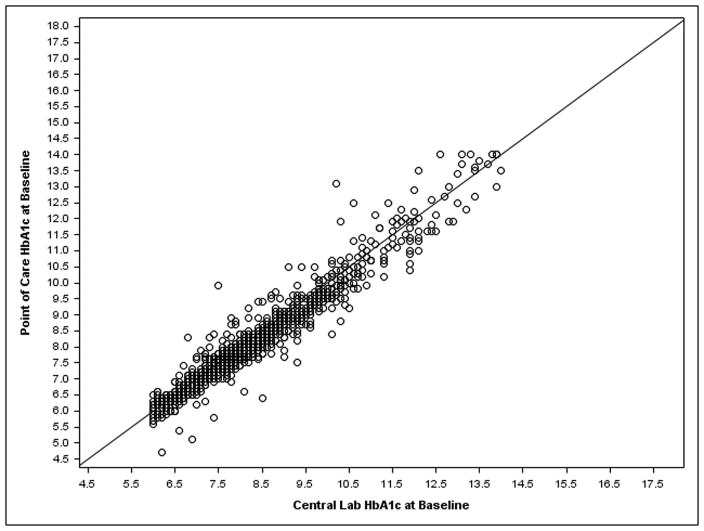
Baseline characteristics by patient were similar between treatment groups and between cohorts (eTable 1). The mean central lab HbA1c for the “more frequent” cohort was 8.3% and 8.6% in the control and intervention groups, respectively; and 8.3% and 8.4% for the “annual” cohort, respectively. The correlation between participant’s last knownHbA1c and baseline central labHbA1c was r=0.71 (among the 631 participants across both cohorts and both treatment groups for whom the last known date was within the prior 6 months) (Figure 2A). The correlation between point of careHbA1c and baseline central labHbA1c was r=0.96 (among 867 participants across both cohorts in the intervention group, excluding 9 cases where labHbA1c>14%, the maximum possible point of care value) (Figure 2B).
Figure 2. HbA1c: Central Lab Value versus a) Patient Knowledge at Baseline and b) Point of Care.
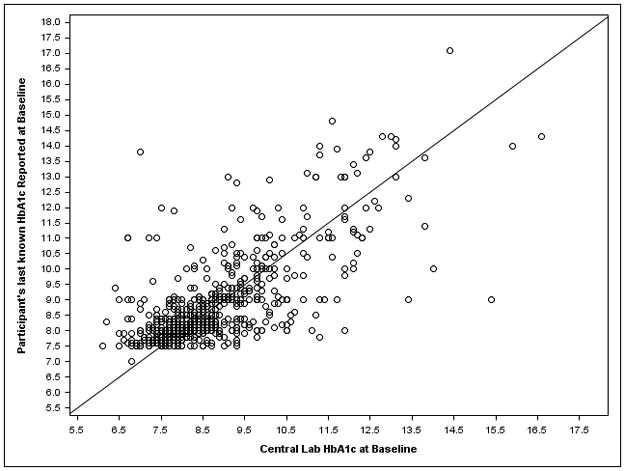
a) Both cohorts and treatment groups with last known HbA1c within the prior 6 months. B) Both cohorts in the intervention group
“More Frequent” Cohort Outcomes
Of participants who completed a 1-year visit in the “more frequent” cohort, the median number of retina visits was 3 and 2 for the control and intervention groups, respectively (Table 1). Prior to the annual visit, only a single follow-up visit occurred in 34% and 40%, respectively, while 35% and 24% had 5 or more follow-up visits. Within the intervention group, 22% received no interventions after baseline.
Table 1.
Number of Follow-up Retina Visits and Interventions Prior to 1-Year – “More Frequent” Cohort only
| Control N = 424** | Intervention N = 423** | |
|---|---|---|
|
| ||
| Number of Follow Up Retina Visits* Prior to 1-Year Visit | ||
|
| ||
| 1 | 145 (34%) | 169 (40%) |
| 2 | 54 (13%) | 68 (16%) |
| 3 | 49 (12%) | 51 (12%) |
| 4 | 28 (7%) | 33 (8%) |
| 5 or more | 148 (35%) | 102 (24%) |
|
| ||
| Median (25th, 75th percentile) | 3 (1, 6) | 2 (1, 4) |
| Mean ± SD | 4 ± 4 | 3 ± 3 |
|
| ||
| Number of Interventions*** Received After Baseline and Prior to 12 Month Visit | ||
|
| ||
| 0 | NA | 91 (22%) |
| 1 | NA | 176 (42%) |
| 2 | NA | 110 (26%) |
| 3 | NA | 46 (11%) |
Case report form captures this information for clinical center visits at the DRCR.net site that enrolled the participant.
Includes only participants who completed the 1-Year primary outcome visit and had a non-missing central lab value (i.e., participants in the primary analysis).
A visit was counted as an intervention if point of care HbA1c was captured at the visit.
The 1-year, mean change (SD) inHbA1c was −0.1% (1.5%) in the control group and −0.3% (1.4%) in the intervention group (mean difference adjusting for baselineHbA1c −0.09%, 95% confidence interval −0.29% to +0.12%, P=0.35; adjusted for additional potential confounders [race, income, and education level] P=0.29) (Table 2). Results were similar when the “annual” cohort was pooled with the “more frequent” cohort. A significant effect of intervention number was not detected (eTable 2).
Table 2.
Primary analysis of HbA1c* at 1 Year
| More Frequent Cohort | Annual Cohort | |||
|---|---|---|---|---|
|
| ||||
| Control N=424 | Intervention N=423 | Control N=292 | Intervention N=312 | |
| Baseline HbA1c (%) | ||||
| Median (25th, 75th percentile) | 8.0 (7.2, 9.0) | 8.2 (7.3, 9.3) | 8.0 (7.1, 9.1) | 8.0 (7.2, 9.3) |
| Mean ± SD | 8.3 ± 1.6 | 8.5 ± 1.7 | 8.2 ± 1.5 | 8.4 ± 1.7 |
| One Year HbA1c† (%) | ||||
| Median (25th, 75th percentile) | 7.9 (7.0, 9.0) | 8.0 (7.0, 9.1) | 8.0 (7.0, 9.2) | 7.9 (7.1, 9.2) |
| Mean ± SD | 8.2 ± 1.7 | 8.2 ± 1.6 | 8.2 ± 1.6 | 8.3 ± 1.8 |
|
| ||||
| Change in HbA1c from baseline (%) | ||||
| Median (25th, 75th percentile) | −0.1 (−0.6, +0.5) | −0.1 (−0.8, +0.5) | 0.0 (−0.6, +0.5) | −0.1 (−0.6, +0.5) |
| Mean ± SD | −0.1 ± 1.5 | −0.3 ± 1.4 | 0.0 ± 1.1 | −0.1 ± 1.6 |
| Mean Difference (Intervention – Control) ± Standard Error‡ | −0.09 ± 0.09 | −0.05 ± 0.09 | ||
| 95% Confidence Interval‡ | (−0.29, + 0.12) | (−0.27, +0.18) | ||
| P-value‡ | 0.35 | 0.63 | ||
|
| ||||
| % with HbA1c < 7.0% | 97 (23%) | 97 (23%) | 70 (24%) | 69 (22%) |
| % with HbA1c > 10.0% | 47 (11%) | 61 (14%) | 34 (12%) | 44 (14%) |
| % with relative decrease in HbA1c ≥ 10% | 90 (21%) | 106 (25%) | 50 (17%) | 64 (21%) |
| % with absolute decrease in HbA1c ≥ 0.5% | 131 (31%) | 154 (36%) | 83 (28%) | 92 (29%) |
| % with absolute increase in HbA1c ≥ 0.5% | 112 (26%) | 110 (26%) | 76 (26%) | 78 (25%) |
From central lab measurement.
- †1 year HbA1c missing for 10 control, 5 intervention
- ‡From a repeated measures model adjusting for baseline HbA1c and correlation between patients within site, assuming the correlation is the same for any pair of patients at a given site, and the within site correlation is the same at all sites. P-value from same model adjusting for additional potential confounders (race, education level, income) = 0.29
- †1 year HbA1c missing for 8 control, 8 intervention
- ‡From a repeated measures model adjusting for baseline HbA1c and correlation between patients within site, assuming the correlation is the same for any pair of patients at a given site, and the within site correlation is the same at all sites. Pvalue from same model adjusting for additional potential confounders (race, education level, income) = 0.44
Other sensitivity analyses were consistent with the primary analysis results, including, using all available measurements (point-of-care measurement or primary care physician-reported values) when central lab value was not available, excluding N = 48 (3%) cases where the central lab indicated that an abnormal hemoglobin variant was observed, evaluating only the subset of 1-year data occurring within the +/− 1 month protocol window, or imputing for missing 1-year data (data not shown). Results were also consistent when stratified by predefined baseline subgroups of interest (based onHbA1c, last knownHbA1c, annual income, diabetes type, diabetic retinopathy severity, visual acuity, and diabetic macular edema), and when the ANCOVA model was repeated separately for participants where randomization was at the site level and for those where randomization was at the investigator level (no statistically significant interactions, data not shown). An interaction between site and treatment group was not identified (P=0.11) within the 8 sites where investigators were randomized within site (132 participants in the control group and 127 in the intervention group).
Secondary outcomes were similar between randomization groups at 1 year (Table 3). Mean (SD) change in mean arterial blood pressure was −1 (12) mmHg for both randomization groups, and body mass index was 0.0 (3.8) kg/m2 in the control group and −0.1 (3.6) kg/m2 in the intervention group. PAID and SCI-2 survey results are summarized in eTable 3.
Table 3.
Secondary Outcomes at 1 Year
| More Frequent Cohort | Annual Cohort | |||
|---|---|---|---|---|
|
| ||||
| Control | Intervention | Control | Intervention | |
|
| ||||
| Change in Mean Arterial Blood Pressure* (mmHg) | ||||
|
| ||||
| Overall | N = 432 | N=428 | N=297 | N=320 |
| Median (25th, 75th percentile) | −1 (−8, +6) | −1 (−8, +6) | −1 (−8, +5) | 0 (−7, +7) |
| Mean ± SD | −1 ± 12 | −1 ± 12 | −1 ± 11 | 0 ± 12 |
| <140/80 at baseline | N=282 | N=276 | N=195 | N=221 |
| Median (25th, 75th percentile) | +2 (−5, +9) | +3 (−5, +9) | +1 (−4, +7) | +2 (−5, +9) |
| Mean ± SD | +3 ± 11 | +2 ± 11 | +2 ± 10 | +3 ± 12 |
| ≥140/80 at baseline | N=150 | N=152 | N=102 | N=99 |
| Median (25th, 75th percentile) | −8 (−13, 0) | −7 (−15, 0) | −6 (−14, 0) | −5 (−13, +1) |
| Mean ± SD | −7 ± 11 | −8 ± 11 | −7 ± 10 | −6 ± 11 |
|
| ||||
| Change in Body Mass Index† (kg/m2) | ||||
|
| ||||
| Overall | N=419 | N=424 | N=293 | N=319 |
| Median (25th, 75th percentile) | 0.0 (−1.1, +1.1) | −0.1 (−1.3, +0.9) | 0.0 (−1.2, +1.1) | −0.2 (−1.3, +0.9) |
| Mean ± SD | +0.0 ± 3.8 | −0.1 ± 3.6 | −0.1 ± 3.4 | −0.3 ± 3.2 |
| <25 at baseline | N=40 | N=57 | N=36 | N=37 |
| Median (25th, 75th percentile) | +0.2 (−0.7, +1.3) | +0.3 (−0.4, +1.8) | +0.2 (−0.8, +0.5) | 0.0 (−0.6, +0.7) |
| Mean ± SD | +0.7 ± 2.3 | +0.9 ± 2.1 | +0.2 ± 1.5 | +0.2 ± 2.4 |
| ≥25 at baseline | N=379 | N=367 | N=257 | N=282 |
| Median (25th, 75th percentile) | 0.0 (−1.3, +1.1) | −0.3 (−1.3, +0.9) | 0.1 (−1.4, +1.2) | −0.2 (−1.4, +0.9) |
| Mean ± SD | 0.0 ± 3.9 | −0.2 ± 3.8 | −0.1 ± 3.6 | −0.4 ± 3.3 |
Missing for 2 Control Group participants in the More Frequent cohort and 3 Control Group participants in the Annual cohort
Missing for 13 Control Group and 4 Intervention Group participants in the More Frequent cohort and 7 Control Group and 1 Intervention Group participants in the Annual cohort. 2 Control Group participants in the More Frequent cohort were excluded as extreme outliers.
Annual Cohort Outcomes
In the “annual” cohort, the 1-year mean change (SD) in HbA1c was 0.0% (1.1%) in the control group and −0.1% (1.6%) in the intervention group (mean difference adjusting for baseline HbA1c −0.05%, 95% confidence interval −0.27% to +0.18%, P=0.63) (Table 2). Results were consistent when evaluating parallel sensitivity analyses as performed in the “more frequent” cohort described above (data not shown) and when evaluating secondary outcomes including mean arterial blood pressure, BMI and survey data (Table 3, eTable 3).
Discussion
In this multicenter study, individualized assessment of diabetes mellitus complication risks during ophthalmology visits based on point of careHbA1c, blood pressure and retinopathy severity, combined with personalized and standardized education delivered directly by each participant’s ophthalmologist, did not alter either short or long term glycemic control among participants with diabetes as compared with usual care. Although the data supporting the beneficial effects of improved diabetes control of are overwhelming, these results emphasize the difficulty of changing personal behavior and treatment paradigms through participant education,23 even when motivated by fear of possible future ocular and renal complications.
In this study, the control group received standard care that likely varied between sites. Thus, the similarity ofHbA1c between intervention and control groups at one year should not be interpreted to imply that educational interventions aimed at improving systemic control are not worthwhile. Rather, it emphasizes the enormous challenge and the critical need for additional approaches other than those employed in this study to identify effective strategies to improve glycemic control. Possible alternatives include (1) personalized retinal imaging and review to directly visualize the individual’s current eye disease, (2) displaying images of severe diabetic complications such as blinding stages of eye disease, dialysis, or amputations, (3) exposure to individuals who have experienced these debilitating complications, (4) more frequent educational interaction or (5) additional communication with primary diabetes care providers. Although results of the personalized assessments were mailed to each participant’s primary care providers, the protocol did not require further communication to ensure non-ophthalmic follow-up. Whether such attempts to improve communication between treating ophthalmologists and primary care physicians helps reinforce participant behavior remains unknown.
Only 36% and 40% (more frequent and annual groups, respectively) of intervention participants provided an email address to receive intervention materials, suggesting that a many did not utilize computers for communications or were uncomfortable providing this information. The 277 participants who provided an email address were younger, 70–74% never accessed the web link to their individualized risk assessment findings and only 12–19% accessed it 2 or more times during the study. Such limited utilization highlights the need for other novel approaches to engage persons with diabetes.
The lack of intervention effect in this study could reflect the standard care given by this specialized investigator group which is highly attuned to evidence-based retinal care for individuals with diabetes, and possibly already providing patient education at a level where the prescribed intervention would not add incremental benefit. Nevertheless, in this study 13–18% had poorly controlled diabetes (baselineHbA1c >10%) and approximately half hadHbA1c >8.0%, suggesting that there was room for improvement. Although the annual cohort enrollment did not meet calculated sample size and thus a negative finding must be interpreted with caution, the more frequent cohort did meet sample size requirements there was no intervention difference despite the potentially greater effect in this group. There was also no evidence of a dose-response effect in a secondary analysis pooling both cohorts.
Of potential importance for future studies, these data demonstrated excellent correlation between point of careHbA1c obtained in a retina specialist office andHbA1c obtained in a central lab. We are unable to assess whether the more frequent group became desensitized to the intervention over time. National trends suggest that glycemic control has improved among Americans with diabetes over the last few decades.10 However, this study and other recent epidemiologic assessments demonstrate that despite improvements in educational efforts and greater awareness of the importance of health status, there still exists a sizable population that remains poorly controlled.
Data supporting the multiple beneficial effects of systemic control of diabetes are overwhelming and there is a critical need to encourage intensive blood glucose control among individuals with diabetes in order to prevent long-term development and progression of vasculopathy across multiple organ systems. Although the addition of personalized education and risk assessment during ophthalmology visits in this study did not improve glycemic control, long-term optimization of glycemic control is still a cornerstone of diabetes care. These results suggest that optimizing glycemic control requires more extensive interventional paradigms than examined in this study and further research into new technologies and models of behavioral change. In the meantime, ophthalmologists and all other diabetes care providers should continue their efforts to maximize education, assessment, systemic control and treatment of complications for patients with diabetes.
Supplementary Material
Acknowledgments
Funding/Support: Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, U.S. Department of Health and Human Services EY14231, EY23207, EY18817.
Footnotes
Previous Presentations: None
Allison Ayala had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Disclaimer: Dr. Neil Bressler is the JAMA Ophthalmology Editor-in-Chief but was not involved in the review process or the acceptance of the manuscript.
This article contains online-only material. eTables 1–3, eAppendix 1 a–f, eAppendix 2
Author Contributions: Study concept and design: Aiello, Ayala, Arnold-Bush, N. Bressler, Glassman, Jampol, Melia,
Acquisition of data: Aiello, Ayala, Antoszyk, Arnold-Bush, Baker, N. Bressler, Elman, Glassman, Jampol, Melia, Nielsen, Wolpert
Analysis and interpretation of data: Aiello, Ayala, Antoszyk, Arnold-Bush, Baker, N. Bressler, Elman, Glassman, Jampol, Melia, Nielsen, Wolpert
Drafting of the manuscript: Aiello, Ayala, Arnold-Bush, Melia.
Critical revision of the manuscript for important intellectual content: Aiello, Ayala, Antoszyk, Arnold-Bush, Baker, N. Bressler, Elman, Glassman, Jampol, Melia, Nielsen, Wolpert
Obtained funding: N. Bressler, Glassman, Jampol.
Administrative, technical, and material support: Ayala, Arnold-Bush, N. Bressler, Glassman, Jampol, Melia.
A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net.
Role of the Sponsor: The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study, nor in the collection, management, analysis, or interpretation of the data, or in the preparation of the manuscript or the decision to submit the manuscript for publication.
References
- 1.Davis M, Fisher M, Gangnon R, et al. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: ETDRS report #18. Invest Ophthalmol Vis Sci. 1998;39:233–52. [PubMed] [Google Scholar]
- 2.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2):156–63. doi: 10.1007/s001250051594. [DOI] [PubMed] [Google Scholar]
- 3.The Diabetes Control and Complication Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complication in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complication Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342(6):381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643–53. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasker RD. The diabetes control and complications trial. Implications for policy and practice. N Engl J Med. 1993;329(14):1035–6. doi: 10.1056/NEJM199309303291410. [DOI] [PubMed] [Google Scholar]
- 7.Shaw KM. Overcoming the hurdles to achieving glycemic control. Metabolism. 2006;55(5 Suppl 1):S6–9. doi: 10.1016/j.metabol.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Dagogo-Jack S, Funnell MM, Davidson J. Barriers to achieving optimal glycemic control in a multi-ethnic society: a US focus. Curr Diabetes Rev. 2006;2(3):285–93. doi: 10.2174/157339906777950606. [DOI] [PubMed] [Google Scholar]
- 9.Borus JS, Laffel L. Adherence challenges in the management of type 1 diabetes in adolescents: prevention and intervention. Curr Opin Pediatr. 2010;22(4):405–11. doi: 10.1097/MOP.0b013e32833a46a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160(8):517–25. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Barriers to diabetes management: patient and provider factors. Diabetes Res Clin Pract. 2011;93(1):1–9. doi: 10.1016/j.diabres.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 12.American Foundation for the Blind. [Accessed 15 December 2014];American foundation for the blind launches website to help people with vision loss maintain independence. http://www.afb.org/info/programs-and-services/professional-development/experts-guide/press-release-archive-3641/1235.
- 13. [Accessed 15 December 2014];US adults with diabetes fear blindness or vision loss more than premature death. http://www.redorbit.com/news/health/757152/us_adults_with_diabetes_fear_blindness_or_vision_loss_more/#hWCv8bhsh8bLZgkF.99.
- 14.Salti H, Cavallerano JD, Salti N, et al. Nonmydriatic retinal image review at time of endocrinology visit results in short-term HbA1c reduction in poorly controlled patients with diabetic retinopathy. Telemed J E Health. 2011;17(6):415–9. doi: 10.1089/tmj.2010.0180. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. [Accessed: 11 March 2012];National Diabetes Fact Sheet. 2011 < http://www.cdc.gov/diabetes/pubs/factsheet11.htm>.
- 16.Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18(6):754–60. doi: 10.2337/diacare.18.6.754. [DOI] [PubMed] [Google Scholar]
- 17.Welch GWK, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69–72. doi: 10.1046/j.1464-5491.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 18.Cutrona CDR, editor. Advances in Personal Relationships. Greenwich, CT: JAI Press; 1987. [Google Scholar]; Jones WPD, editor. The provisions of social relationships and adaptation to stress. 1 [Google Scholar]
- 19.La Greca AM, Swales T, Klemp S, Madigan SJS, editors. Adolescents with diabetes: Gender differences in psychosocial functioning and glycemic control. Children’s Health Care. 1995;24 [Google Scholar]
- 20.Weinger K, Butler HA, Welch GW, AM LG. Measuring diabetes self-care: a psychometric analysis of the Self-Care Inventory-Revised with adults. Diabetes Care. 2005;28(6):1346–52. doi: 10.2337/diacare.28.6.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Musen G, Jacobson AM, Ryan CM, et al. Impact of diabetes and its treatment on cognitive function among adolescents who participated in the Diabetes Control and Complications Trial. Diabetes Care. 2008;31(10):2072–6. doi: 10.2337/dc08-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med. 2003;20(1):69–72. doi: 10.1046/j.1464-5491.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Perez LE, Alvarez M, Dilla T, Gil-Guillen V, Orozco-Beltran D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–94. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.