Abstract
Background
Patients with end-stage renal disease (ESRD) receiving dialysis have been reported to have increased risk of cancer. However, contemporary cancer burden estimates in this population are sparse and do not account for the high competing risk of death characteristic of dialysis patients.
Study Design
Retrospective cohort study.
Setting & Participants
US adult patients enrolled in Medicare's ESRD program who received in-center hemodialysis.
Factors
Demographic/clinical characteristics.
Outcomes
For overall and site-specific cancers identified using claims-based definitions, we calculated annual incidence rates (1996-2009). We estimated 5-year cumulative incidence since dialysis therapy initiation using competing-risk methods.
Results
We observed a constant rate of incident cancers for all sites combined, from 3,923 to 3,860 cases per 100,000 person-years (annual percentage change, 0.1; 95% CI, −0.4 to 0.6). Rates for some common site-specific cancers increased (ie, kidney/renal pelvis) and decreased (ie, colon/rectum, lung/bronchus, pancreas, and other sites). Of 482,510 incident hemodialysis patients, cancer was diagnosed in 37,128 within 5 years after dialysis therapy initiation. The 5-year cumulative incidence of any cancer was 9.48% (95% CI, 9.39%-9.57%) and was higher for certain subgroups: older age, males, nonwhites, non-Hispanics, nondiabetes primary ESRD cause, recent dialysis therapy initiation, and history of transplantation evaluation. Among blacks and whites, we observed 35,767 cases compared with 25,194 expected cases if the study population had experienced rates observed in the US general population (standardized incidence ratio [SIR], 1.42; 95% CI, 1.41-1.43). Risk was most elevated for cancers of the kidney/renal pelvis (SIR, 4.03; 95% CI, 3.88-4.19) and bladder (SIR, 1.57; 95% CI, 1.51-1.64).
Limitations
Claims-based cancer definitions have not been validated in the ESRD population. Information for cancer risk factors was not available in our data source.
Conclusions
These results suggest a high burden of cancer in the dialysis population compared to the US general population, with varying patterns of cancer incidence in subgroups.
INDEX WORDS: Hemodialysis, malignancy, carcinoma, tumor, cancer incidence, cancer risk factor, end-stage renal disease (ESRD), chronic kidney failure, US Renal Data System (USRDS), diagnostic code, claims-based cancer definition
Despite reports of increased risk of various cancers in the dialysis population,1-4 the contemporary cancer burden has not been characterized adequately among patients with end-stage renal disease (ESRD) receiving dialysis. In the past 3 decades, several national population-based analyses that identified cancer cases using statutory notifications or billing codes have yielded estimates of cancer incidence in the ESRD population.1,2,4-13 However, population-based estimates of cumulative incidence or annual incidence rates are not available. Furthermore, information is sparse for cancer incidence in dialysis patients by subgroups such as age, sex, race, ethnicity, primary cause of ESRD, and dialysis vintage. Current estimates of cancer incidence in the ESRD population are important to inform cancer-related health care policy and planning for the US Medicare program.
The objective of this study was to describe the cancer burden among ESRD patients receiving hemodialysis, including relevant patient subgroups. Using data from the US Renal Data System (USRDS), a national registry including all patients in Medicare's ESRD program, we described temporal trends in cancer incidence rates (1996-2009). In addition, we estimated the cumulative incidence and relative risk of all cancers combined, as well as site-specific cancers, since dialysis therapy initiation. We used competing-risk methods to avoid inflating the estimated risk of cancer by censoring the deaths that occurred prior to cancer diagnosis (ie, the event of interest).14-17
METHODS
Data and Population
We used data from the USRDS, a national registry that includes all patients in Medicare's ESRD program. The study population included patients with ESRD 18 years or older who received in-center hemodialysis within the period from April 1, 1995, through December 31, 2010. The study population was restricted to individuals with Medicare as their primary payer and both Parts A and B coverage in order to ensure collection of complete claims data for patients. Patients were excluded for a history of HIV (human immunodeficiency virus) infection/AIDS or a malignancy-related primary cause of ESRD.
The study population was limited to patients who remained alive without a cancer diagnosis for at least 9 months after dialysis therapy initiation. This period was selected because 3 months after the first service date is the time required to process Medicare eligibility/enrollment forms and to ensure stability in dialysis treatment modality.18 A subsequent 6-month period (ie, the baseline period) was required for assessment of cancer-free status, as done previously.19-21 History of kidney transplantation evaluation was defined as a claim assigned a V code of V72.83 (other specified preoperative examination) during the 6-month baseline period. Sensitivity and specificity of code V72.83 have not been reported.
Outcomes
We identified incident site-specific cancers using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes from inpatient and outpatient Medicare claims. Table S1 (provided as online supplementary material) presents the ICD-9-CM codes used in the site-specific cancer definitions. The claims-based algorithm used to define site-specific cancers required 2 or more ICD-9-CM diagnosis codes within 6 months, adapted from an algorithm (requiring ≥2 ICD-9-CM diagnosis codes within 2 months) validated in a Medicare population for identification of incident cancers, including lung, colorectal, stomach, breast, and lymphoma (sensitivity: range, 56%-77%; specificity: ≥99%; positive predictive value: range, 56%-77%).22 We extended the period to 6 months between cancer diagnosis codes to allow for delays in health care encounters that may occur due to the high severity of illness characteristic of the ESRD population. Date of cancer onset was defined as the first date of a cancer-related diagnosis code in the claims data. In situ carcinomas were included for 2 sites (ie, breast and bladder), in accordance with the Surveillance, Epidemiology, and End Results (SEER) Program.23 Secondary tumors, benign tumors, and nonmelanoma skin cancers were excluded from the analysis. Only the first cancer diagnosis after dialysis therapy initiation was included as an event. Patients with multiple cancer sites diagnosed on the same date were included in each site-specific analysis.
Annual Incidence Rates of Cancer
We used 14 annual cohorts (ie, 1996-2009) of prevalent hemodialysis patients who met eligibility criteria by January 1 to calculate incidence rates of cancer. All patients with ESRD who initiated in-center hemodialysis on or before April 1 of the previous year and remained alive on January 1 of the cohort year were eligible for that yearly cohort. Patients were eligible for multiple cohorts. Patients with cancer during the 6-month baseline period (ie, July 4 to December 31 of the prior year) were excluded from that yearly cohort. Patients were observed from 9 months post–dialysis therapy initiation to the first cancer diagnosis. Patients were censored due to loss of Medicare as primary payer status, change of modality, loss to follow-up, kidney transplantation, death, or end of study (December 31 of the cohort year).
Annual incidence rates, expressed as cancer diagnoses per 100,000 patients per year, were calculated overall and within strata of patient characteristics. To derive cancer rates adjusted for secular trends, we computed a standardized mortality ratio–weighted model using the 2000 population as the standard, as previously described.21 Incidence rates were adjusted for age at dialysis therapy initiation, sex, race, ethnicity, primary cause of ESRD, and dialysis vintage in years. The Joinpoint Regression Program (version 4.0.4; National Cancer Institute) was used to model trends of adjusted annual incidence rates during the study period (1996-2009) and calculate annual percentage change and 95% confidence intervals (CIs).24,25 Further details are described in Item S1.
Cumulative Incidence Estimates of Cancer
We used a retrospective cohort of incident patients who initiated in-center hemodialysis between April 1, 1995, and April 5, 2010, to estimate the cumulative incidence of cancer. The retrospective cohort design spanned the 5 years after dialysis therapy initiation, including a 3-month eligibility period, a subsequent 6-month baseline period, and a follow-up period for up to 5 years after dialysis therapy initiation. Time at risk was measured from 9 months after dialysis therapy initiation to the first of the following: the event of interest (ie, cancer diagnosis) or censoring (ie, cancer diagnosis at another site, renal replacement therapy modality change to peritoneal dialysis or kidney transplantation, end of Medicare as primary payer status, loss to follow-up, 5 years since dialysis therapy initiation, or end of study on December 31, 2010).
We estimated the cumulative incidence of cancer accounting for the competing risk of death. Cumulative incidence is defined as the probability of cancer given that an individual has survived up to time t without cancer or has had a competing event of death prior to time t. The competing-risks model specifies that individuals who experience the competing event remain in the risk set for the event of interest. Thus, the risk set includes 2 distinct groups: those who have not failed from any cause and those who have previously failed from a competing event.26,27 This analysis required a customized SAS program (SAS Institute Inc).28 Item S1 also presents the cumulative incidence analysis ignoring the competing risk of death (ie, censoring death).
In the analysis accounting for the competing risk of death, crude and standardized cumulative incidence estimates were stratified by several patient characteristics. We used inverse probability of exposure weights to standardize each stratum to the total study sample at baseline with respect to age, sex, race, ethnicity, primary cause of ESRD, and calendar year of dialysis therapy initiation, as appropriate. Inverse probability exposure weights were calculated as the marginal proportion of patients receiving the level of exposure they received (ie, the stabilizing factor) divided by the predicted probability of receiving that exposure from the linear logistic model. For each patient characteristic, we fit both a null linear logistic model to calculate the marginal proportion of exposure and a full linear logistic model to calculate the predicted probability of a particular exposure level for each combination of covariates.28 Age was modeled using restricted quadratic splines, with 4 knots placed at the 5th, 35th, 65th, and 95th percentiles of the age distribution in the study sample.29 Table S2 presents descriptive characteristics of the inverse probability exposure weights. We also estimated the inverse probability of censoring weights to account for informative censoring (Item S1).28 We obtained 95% CIs as a measure of uncertainty due to sampling error using a nonparametric bootstrap. Specifically, we resampled 482,510 patients at random with replacement with equal probability 200 times. The standard deviation of the 200 bootstrap resamples was used as an estimate of the standard error.
Standardized Incidence Ratios
To estimate the risk of cancer in the study population of incident hemodialysis patients relative to that of the US general population, we calculated the standardized incidence ratio (SIR) by dividing the number of observed cancer cases by the number of expected cancer cases from 1996 through 2009. We calculated 95% CIs under the assumption that the number of observed cancer cases followed a Poisson distribution. To estimate the number of expected cancer cases, we multiplied the age-, sex-, and race-specific incidence rates from the US general population (SEER 13 registry data via SEER*Stat, version 8.1.5) by the number of person-years at risk for each age, sex, and race stratum in the study population. Analysis was limited to whites and blacks due to limited availability of rate estimates for other race in the SEER general population. SAS software, version 9.3 (SAS Institute Inc), was used for all analyses.
RESULTS
Annual Incidence Rates of Cancer
Table 1 lists characteristics of the study population of prevalent dialysis patients for selected years of the study period. The number of patients per annual cohort increased each year, from 88,676 in 1996 to 164,214 in 2009. Patients were more likely to be male, be white, or have diabetes as the primary cause of ESRD in recent cohorts. Mean age and mean dialysis vintage increased during the study period.
Table 1.
Demographic and Clinical Characteristics of Selected Annual Study Cohorts at Risk for Cancer (1996, 2000, 2004, and 2009)
| Characteristics | 1996 | 2000 | 2004 | 2009 |
|---|---|---|---|---|
| No. of patients | 88,676 | 110,897 | 142,142 | 164,214 |
| Age at dialysis initiation (y) | 61.3 ± 14.8 | 62.1 ± 14.7 | 62.7 ± 14.6 | 62.5 ± 14.5 |
| Age category at dialysis initiation | ||||
| 18-44 y | 13,322 (15.0) | 15,235 (13.7) | 17,351 (12.2) | 19,398 (11.8) |
| 45-64 y | 31,700 (35.8) | 39,928 (36.0) | 53,421 (37.6) | 66,352 (40.4) |
| 65-74 y | 26,869 (30.3) | 31,698 (28.6) | 37,951 (26.7) | 41,136 (25.1) |
| ≥75 y | 16,785 (18.9) | 24,036 (21.7) | 33,419 (23.5) | 37,328 (22.7) |
| Male sex | 43,934 (49.5) | 55,735 (50.3) | 73,131 (51.5) | 86,406 (52.6) |
| White race | 45,558 (51.4) | 56,951 (51.4) | 74,470 (52.4) | 87,086 (53.0) |
| Reported cause of ESRD | ||||
| Diabetes | 31,411 (35.4) | 46,862 (42.3) | 65,594 (46.2) | 79,446 (48.4) |
| Hypertension | 28,600 (32.3) | 33,840 (30.5) | 16,464 (30.0) | 48,583 (29.6) |
| Glomerulonephritis | 11,649 (13.1) | 14,393 (13.0) | 42,591 (11.6) | 16,945 (10.3) |
| Other | 17,016 (19.2) | 15,802 (14.2) | 17,493 (12.3) | 19,240 (11.7) |
| Dialysis vintage (y) | 2.0 [1.0-4.0] | 3.0 [1.0-5.0] | 3.0 [1.0-5.0] | 3.0 [1.0-5.0] |
| Dialysis vintage category | ||||
| <2 y | 27,238 (30.7) | 33,489 (30.2) | 40,212 (28.3) | 41,247 (25.1) |
| 2-5 y | 45,480 (51.3) | 55,761 (50.3) | 73,137 (51.5) | 84,289 (51.3) |
| >5 y | 15,958 (18.0) | 21,647 (19.5) | 28,793 (20.3) | 38,678 (23.6) |
| History of kidney transplant evaluation | ||||
| No | 88,676 (100.0) | 106,174 (95.7) | 132,563 (93.3) | 147,731 (90.0) |
| Yes | 0 (0.0) | 4,723 (4.3) | 9,579 (6.7) | 16,483 (10.0) |
Note: Values for categorical variables are given as number (percentage); values for continuous variables are given as mean ± standard deviation or median [interquartile range]. Each column represents a separate annual study cohort of prevalent hemodialysis patients that met eligibility criteria by January 1 of the respective year. Patients were eligible for multiple cohorts. For brevity, characteristics are presented for only 4 cohorts (ie, 1996, 2000, 2004, and 2009) and are not presented for 10 cohorts (ie, 1997, 1998, 1999, 2001, 2002, 2003, 2005, 2006, 2007, and 2008).
Abbreviation: ESRD, end-stage renal disease.
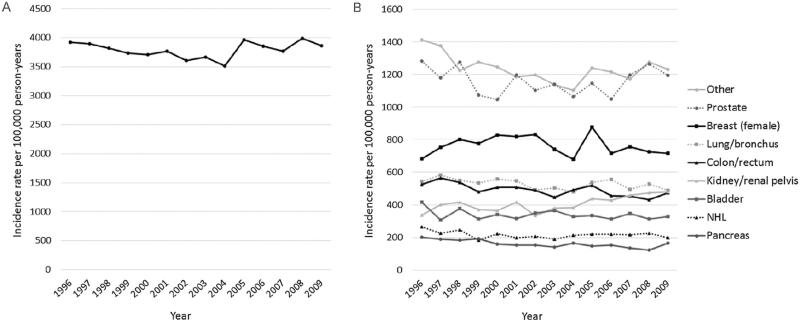
Adjusted annual incidence rates of cancer are presented in Fig 1. We observed a constant rate of incident cancer diagnoses for all sites for 1996 to 2009, from 3,923 to 3,860 cases per 100,000 person-years (annual percentage change, 0.1%; 95% CI, −0.4% to 0.6%; Table 2). From 1996 through 2009, incidence rates increased for cancers of the kidney/renal pelvis; decreased for cancers of the colon/rectum, lung/bronchus, pancreas, and other sites (1996-2003 only); and remained constant for cancers of the prostate, female breast, bladder, non-Hodgkin lymphoma, and other sites (2003-2009 only; Table 2). Across all calendar years, cancers of the prostate and female breast were the most commonly diagnosed, representing 1,195 and 718 cases per 100,000 person-years in 2009, respectively.
Figure 1.
Adjusted annual incidence rates of cancer diagnoses from 1996 through 2009 for: (A) all sites and (B) site-specific cancers. Rates were adjusted for age, sex, race, cause of end-stage renal disease, and dialysis vintage in years. Incident cases were defined as the first cancer diagnosis of the year among patients without a history of cancer in the last 6 months of the previous calendar year. Other cancers were defined as all other site-specific cancers (eg, cancers of the esophagus, stomach, and liver). The sum of the site-specific cancer rates exceeds the rate for any cancer due to patients diagnosed with multiple cancer sites on the same date. Abbreviation: NHL, non-Hodgkin lymphoma.
Table 2.
Annual Percent Change of Cancer Incidence by Cancer Site, 1996-2009
| Cancer Site | APC (95% CI) |
|---|---|
| All sitesa | 0.1% (–0.4% to 0.6%) |
| Kidney/renal pelvis | 2.0% (0.9% to 3.2%) |
| Bladder | –1.1% (–2.3% to 0.1%) |
| Breast, female | –0.3% (–1.5% to 0.9%) |
| Non-Hodgkin lymphoma | –1.1% (–2.4% to 0.2%) |
| Lung/bronchus | –0.8% (–1.5% to –0.1%) |
| Colon/rectum | –1.4% (–2.0% to –0.8%) |
| Pancreas | –2.5% (–3.8% to –1.3%) |
| Prostate | –0.2% (–1.2% to 0.8%) |
| Other sites | |
| 1996–2003 | –2.8% (–4.3% to –1.3%) |
| 2003–2009 | 1.7% (–0.5% to 3.9%) |
Abbreviations: APC, annual percent change; CI, confidence interval.
The category for all sites includes all cancer sites diagnosed during the study period, including sites not listed in the table (eg, liver, stomach, and esophagus).
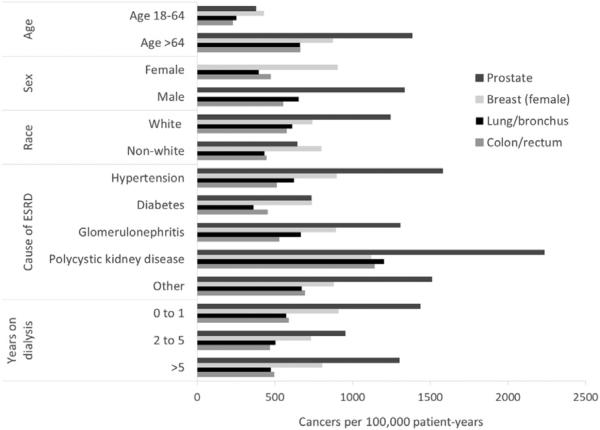
Figure 2 presents adjusted incidence rates for the 4 most frequently diagnosed cancer sites stratified by subgroups. Adjusted incidence rates were much higher among older patients and males and less common among patients with diabetes as the primary cause of ESRD. Prostate cancer had the highest incidence rate across all except 4 strata (ie, age < 65 years, female, nonwhite, or diabetes as primary cause of ESRD).
Figure 2.
Adjusted cancer incidence rates by strata for the top 4 most prevalent cancer sites in years 1996 to 2009. Incident cases were defined as the first cancer diagnosis of the year among patients without a history of cancer in the last 6 months of the previous calendar year. Rates were adjusted for age, sex, race, cause of end-stage renal disease (ESRD), and years on dialysis therapy. Age adjustment was performed for 4 strata (18-44, 45-64, 65-74, and ≥75 years), although only 2 categories are presented due to limited case numbers in the 18- to 44-year age group. The subgroup of interest was omitted from the adjustment for each respective subgroup category.
Cumulative Incidence Estimates of Cancer
Of 482,510 patients who met study eligibility requirements (Fig S1; Table S3), 48.4% were female, 62.7% were white, 32.0% were African American, and 13.1% were Hispanic. The most common reported causes of ESRD were diabetes (50.4%) and hypertension (30.5%). Median age at dialysis therapy initiation was 67 years (Table S4). Median follow-up after dialysis therapy initiation was 2.5 years.
During 988,395 person-years of follow-up between 9 months and 5 years after dialysis therapy initiation, 37,128 patients were given a diagnosis of cancer (including 667 patients with multiple cancer sites diagnosed on the same date). A total of 217,773 (45.1%) patients died prior to receipt of a cancer diagnosis. Twelve percent (n = 57,345) of the 482,510 patients were censored alive and cancer free before 5 years of follow-up or December 31, 2010.
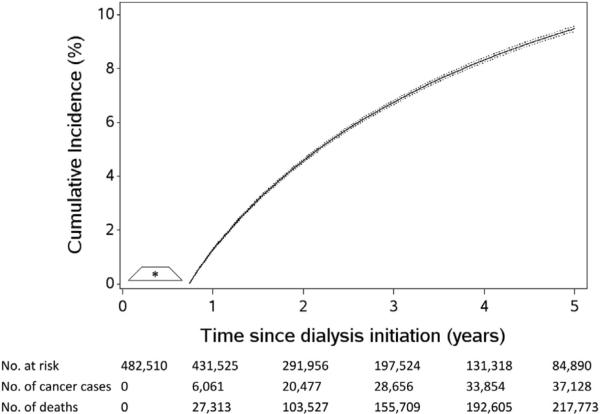
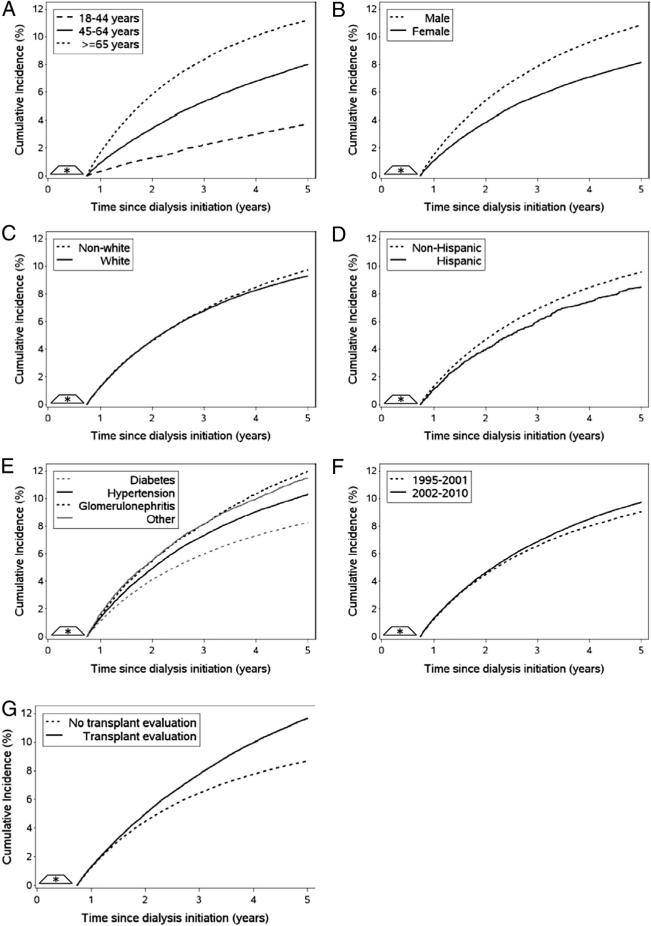
The 5-year crude cumulative incidence of any cancer accounting for death as a competing risk was 9.48% (95% CI, 9.39%-9.57%; Fig 3). Table 3 presents crude and standardized 5-year cumulative incidence estimates of any cancer accounting for death as a competing risk, stratified by patient characteristics. Figs 4 and S2 present standardized and crude cumulative incidence estimates accounting for death as a competing risk, by time from dialysis therapy initiation. Results were not altered meaningfully when drop out was not accounted for in the analysis (~1% change; results not shown). After accounting for case-mix characteristics measured at baseline and the competing risk of death, the 5-year cumulative incidence of any cancer was higher among the following patient subgroups: persons 65 years or older at dialysis therapy initiation (11.28%), males (10.93%), nonwhites (9.79%), non-Hispanics (9.65%), primary ESRD cause other than diabetes (hypertension, 10.39%; other, 11.54%; glomerulonephritis, 12.01%), dialysis therapy initiation in 2003 to 2010 (9.75%), and history of kidney transplantation evaluation (11.67%). Figure S3 presents crude cumulative incidence estimates that censored deaths, stratified by patient characteristics.
Figure 3.
The cumulative incidence of any cancer among patients with end-stage renal disease undergoing hemodialysis, accounting for death as a competing event. The cumulative incidence is denoted by a solid line and the 95% confidence intervals are denoted by a dotted line. Time at risk for a cancer diagnosis began at 9 months after dialysis therapy initiation.
Table 3.
Crude and Standardized 5-Year Cumulative Incidence Estimates of Any Cancer, Accounting for Competing Risk of Death
| Crude | Standardizeda | |
|---|---|---|
| Age at dialysis initiation | ||
| 18-44 y | 3.91% (3.70%-4.11%) | 3.81% (3.55%-4.06%) |
| 45-64 y | 7.83% (7.65%-8.00%) | 8.07% (7.89%-8.25%) |
| ≥65 y | 11.17% (11.05%-11.29%) | 11.28% (11.15%-11.42%) |
| Sex | ||
| Male | 10.65% (10.53%-10.77%) | 10.93% (10.80%-11.05%) |
| Female | 8.27% (8.14%-8.40%) | 8.20% (8.06%-8.33%) |
| Race | ||
| White | 9.75% (9.62%-9.87%) | 9.36% (9.23%-9.48%) |
| Nonwhite | 9.05% (8.91%-9.19%) | 9.79% (9.57%-10.00%) |
| Ethnicity | ||
| Hispanic | 7.17% (6.92%-7.41%) | 8.54% (7.81%-9.27%) |
| Non-Hispanic | 9.83% (9.73%-9.93%) | 9.65% (9.55%-9.76%) |
| Reported cause of ESRD | ||
| Diabetes | 8.14% (8.02%-8.26%) | 8.30% (8.18%-8.42%) |
| Hypertension | 10.78% (10.60%-10.97%) | 10.39% (10.19%-10.59%) |
| Glomerulonephritis | 10.52% (10.18%-10.87%) | 12.01% (11.59%-12.43%) |
| Other | 11.36% (11.05%-11.67%) | 11.54% (11.20%-11.89%) |
| Calendar period of dialysis initiation | ||
| 1995-2002 | 9.26% (9.15%-9.37%) | 9.14% (9.02%-9.25%) |
| 2003-2010 | 9.65% (9.51%-9.79%) | 9.75% (9.60%-9.89%) |
| History of kidney transplant evaluation | ||
| No | 8.90% (8.79%-9.00%) | 8.72% (8.62%-8.82%) |
| Yes | 10.93% (10.74%-11.12%) | 11.67% (11.46%-11.88%) |
Note: Values are given as 5-year cumulative incidence (95% confidence interval).
Abbreviation: ESRD, end-stage renal disease.
Standardized cumulative incidence estimates account for case-mix characteristics (age at dialysis therapy initiation, sex, race, ethnicity, primary cause of ESRD, and year of dialysis therapy initiation) measured at baseline using inverse-probability weights.
Figure 4.
The standardized cumulative incidence of any cancer among patients with end-stage renal disease (ESRD) undergoing hemodialysis, accounting for the competing risk of death. Time at risk for a cancer diagnosis began at 9 months after dialysis therapy initiation. Figures were stratified by patient characteristics, including: (A) age at dialysis therapy initiation, (B) sex, (C) race, (D) ethnicity, (E) primary cause of ESRD, (F) year of dialysis therapy initiation, and (G) history of kidney transplantation evaluation. Cumulative incidence functions were standardized to the total study population by combining the cumulative incidence function with inverse probability of exposure weights that account for case-mix characteristics (age at dialysis therapy initiation, sex, race, ethnicity, primary cause of ESRD, and year of dialysis therapy initiation) measured at baseline.
Tables 4 and S4 present results from more and less common site-specific cancer analyses (ie, ≥150 and <150 site-specific cancer cases during the study period). The most frequently diagnosed cancer sites were prostate (n = 5,396), lung/bronchus (n = 4,969), colon/rectum (n = 4,360), female breast (n = 3,688), kidney/renal pelvis (n = 2,805), bladder (n = 2,216), non-Hodgkin lymphoma (n = 1,284), leukemia (n = 1,077), myeloma (n = 1,024), and pancreas (n = 928). Results from the cumulative incidence analysis treating death as a censoring event are available in Table S5.
Table 4.
The 5-Year Cumulative Incidence of Most Frequent Site-Specific Cancers Among ESRD Patients Undergoing Hemodialysis, Accounting for Competing Risk of Death
| Cancer Sitea | No. of Patients | 5-y Cumulative Incidence (95% CI) |
|---|---|---|
| Lip, oral cavity, and pharynx | ||
| Tongue | 156 | 0.04% (0.04%-0.05%) |
| Mouth | 191 | 0.05% (0.04%-0.06%) |
| Pharynx | 183 | 0.05% (0.05%-0.06%) |
| Digestive system | ||
| Esophagus | 369 | 0.10% (0.09%-0.11%) |
| Stomach | 590 | 0.16% (0.15%-0.18%) |
| Colon/rectum | 4,360 | 1.18% (1.15%-1.22%) |
| Liver | 865 | 0.24% (0.22%-0.26%) |
| Gallbladder | 202 | 0.06% (0.05%-0.06%) |
| Pancreas | 928 | 0.26% (0.24%-0.28%) |
| Respiratory system | ||
| Larynx | 318 | 0.09% (0.08%-0.10%) |
| Lung/bronchus | 4,969 | 1.36% (1.32%-1.39%) |
| Bone and cartilage | 206 | 0.06% (0.05%-0.07%) |
| Skin/connective tissueb | ||
| Melanoma | 872 | 0.24% (0.22%-0.26%) |
| Connective & other soft tissue | 285 | 0.08% (0.07%-0.09%) |
| Reproductive & genitourinary | ||
| Breast (female)c | 3,688 | 1.00% (0.97%-1.03%) |
| Cervix uteri | 345 | 0.09% (0.08%-0.10%) |
| Corpus and uterus | 551 | 0.15% (0.14%-0.16%) |
| Ovary | 341 | 0.09% (0.08%-0.10%) |
| Prostate | 5,396 | 1.41% (1.37%-1.44%) |
| Bladderc | 2,216 | 0.59% (0.56%-0.61%) |
| Kidney/renal pelvis | 2,805 | 0.76% (0.73%-0.79%) |
| Neurologic | ||
| Brain & other nervous system | 560 | 0.15% (0.14%-0.17%) |
| Endocrine | ||
| Thyroid | 337 | 0.09% (0.08%-0.10%) |
| Hematologic | ||
| Non-Hodgkin lymphoma | 1,284 | 0.35% (0.33%-0.37%) |
| Myeloma | 1,024 | 0.27% (0.26%-0.29%) |
| Leukemia | 1,077 | 0.29% (0.27%-0.31%) |
| Ill-defined and unspecified | 1,941 | 0.53% (0.51%-0.56%) |
Note: Data presented for cancer sites with 150 or more cases during the study period. Table S4 presents results for cancer sites with fewer than 150 cases.
Abbreviations: CI, confidence interval; ESRD, end-stage renal disease.
Table S1 presents the International Classification of Diseases, Ninth Revision, Clinical Modification codes used in the site-specific cancer definitions.
Excludes nonmelanoma skin cancer.
Malignant and carcinoma in situ.
Standardized Incidence Ratios
From 1996 through 2009, a total of 35,767 cancer cases were observed in the study population of black and white dialysis patients compared with 25,194 expected cases if the study population had experienced the rates observed in the US general population (SIR, 1.42; 95% CI, 1.41-1.43). Relative risk was elevated for all 8 of the most common incident site-specific cancers diagnosed in the study population. Risk was most elevated for cancers of the kidney/renal pelvis (SIR, 4.03; 95% CI, 3.88-4.19) and bladder (SIR, 1.57; 95% CI, 1.51-1.64; Table 5).
Table 5.
SIRs of Cancer Among Hemodialysis Patients by Cancer Type, Restricted to Patients of White and Black Race, 1996-2009
| Cancer Site | No. of Observed Cancer Cases | SIR (95% CI)a |
|---|---|---|
| All sitesb | 35,767 | 1.42 (1.41-1.43) |
| Kidney/renal pelvis | 2,712 | 4.03 (3.88-4.19) |
| Bladder | 2,165 | 1.57 (1.51-1.64) |
| Breast (female) | 3,552 | 1.42 (1.38-1.47) |
| Non-Hodgkin lymphoma | 1,251 | 1.37 (1.30-1.45) |
| Lung/bronchus | 4,806 | 1.28 (1.25-1.32) |
| Colon/rectum | 4,181 | 1.27 (1.23-1.30) |
| Pancreas | 891 | 1.08 (1.01-1.15) |
| Prostate | 5,248 | 1.06 (1.03-1.09) |
Abbreviations: CI, confidence interval; SEER, Surveillance, Epidemiology, and End Results; SIR, standardized incidence ratio.
Indirect standardization was performed by applying background cancer rates from the 2000 US population (SEER 13 registry data by SEER*Stat, version 8.1.5) to the age, sex, and race distribution of the study population. Annual age-, sex-, and race-specific rates were averaged over the 14-year study period (1996-2009). Age was categorized in 5-year groups. Analysis was limited to whites and blacks due to limited availability of rate estimates for other race in the SEER general population.
The category for all sites includes all cancer sites diagnosed during the study period, including sites not listed in the table (eg, liver, stomach, and esophagus).
DISCUSSION
We conducted a large national study of patients with ESRD undergoing hemodialysis to describe the incidence of cancer in this population. After accounting for the substantial competing risk of death in the ESRD population undergoing dialysis, we observed a high cumulative incidence of cancer, with >9% of the ESRD population being diagnosed with cancer during a 5-year period after initiating dialysis therapy. From 1996 through 2009, we observed constant rates of incident cancer diagnoses for all sites combined. For certain site-specific cancers, there were trends of increasing and decreasing incidence rates. In addition, our results demonstrate varying patterns of cancer incidence in subgroups after accounting for measured patient characteristics. To our knowledge, there are no previous population-based estimates of cumulative incidence or annual incidence rates of cancer in the dialysis population. Therefore these estimates provide novel information on the cancer burden in this unique population.
The 5-year cumulative incidence estimate of 9.48% depends on the risk of both cancer and the competing event of death that precludes development of cancer. The competing-risk approach provides an estimate of the total amount of cancer diagnoses that will occur in the population, which may provide estimates with greater accuracy and precision for health care policy and planning in the dialysis population characterized by very high mortality.30 Despite the higher cancer risk previously documented among transplant recipients compared with dialysis patients, our 5-year cumulative incidence estimate is much higher than the 4.4% estimate among US transplant recipients reported by Hall et al.2,31 This discrepancy can be explained largely by the substantially younger age of the transplantation population compared to our dialysis population (ie, aged ≤60 years: 84% vs 32%) and is supported by the steep increase in cumulative incidence of cancer with increasing age at transplantation.31
We observed an increased risk of cancer in the dialysis population compared to the risk expected if the study population had experienced the rates observed in the US general population. These results suggest that patients with ESRD are uniquely at risk for developing cancer while receiving hemodialysis treatment. Our estimates are similar yet slightly higher than most estimates from previous population-based studies of cancer in the dialysis population, which have reported increased risk for any cancer (SIR range, 1.1-1.8) compared to the general population.1,2,4 One exception is a recent SEER-Medicare study restricted to patients older than 65 years, which reported no increased overall risk of cancer in patients with ESRD compared to the general population.8 However, this discrepancy may be due to inadvertent inclusion of non-ESRD patients with less severe kidney disease, with reportedly lower risk of cancer than patients with ESRD receiving dialysis.2
For some cancer sites (ie, prostate, female breast, lung/bronchus, colon/rectum, and non-Hodgkin lymphoma), our analyses yielded slightly higher cancer burden estimates than most previous population-based studies of cancer in the dialysis population.1,2,4,8 The most notable result is the excess risk for prostate cancer (SIR, 1.06; 95% CI, 1.03-1.09), which contrasts with most previous reports of diminished risk (SIR range, 0.4-1.2).1,2,4,8 There are several explanations for the higher cancer burden estimates yielded by our analyses. First, our cancer definition captured cancer diagnoses occurring in the outpatient setting, whereas the cancer definition used in a previous study with the same USRDS population captured only cancer-related hospitalizations.1 Several cancer sites, including prostate, are increasingly screened, diagnosed, and treated exclusively in the outpatient setting. Thus, requiring hospital admission could underestimate the true cancer burden in the dialysis population. Second, our higher cancer estimates could be due to improved survival in the dialysis population. In our analysis that censored deaths, we observed that 5-year cumulative incidence was similar by era of dialysis therapy initiation. In contrast, the competing-risks approach (which allowed patients who died to remain in the denominator of patients at risk) yielded a higher 5-year cumulative incidence estimate among patients who initiated dialysis therapy in 2003 to 2010 versus 1995 to 2002. This suggests that the higher cancer incidence in the most recent era is due to improved survival in the dialysis population. Thus, differences in mortality by era could contribute to lower cancer burden estimates in older studies.
We observed a similar pattern in the comparison between patients with and without a kidney transplantation evaluation, in which patients with an evaluation had a higher cancer incidence due to longer survival. Another explanation for a higher 5-year cumulative incidence of cancer among patients who received a kidney transplantation evaluation, despite a healthier profile than patients who did not receive an evaluation, is unexpected cancer diagnoses yielded by intensive medical workup involving comprehensive cancer screening.32 Our finding should be interpreted with caution due to the unknown validity and reliability of the code used to define kidney transplantation evaluation.
Several explanations for increased cancer incidence in the dialysis population have been suggested, including ESRD-associated immunodeficiency and nutritional abnormalities.33-41 Excess cancer risk also may be due to the interaction of uremic and dialysis-induced immune dysfunction with established risk factors (eg, UV radiation, tobacco, or alcohol).2 Recently, there has been a focus on the potential role of erythropoietin-stimulating agents, commonly used to manage anemia; in carcinogenesis, they are known to activate erythropoietin receptors on the surface of cancer cells. Additionally, erythropoietin-induced angiogenesis may promote tumor growth.42,43 However, we did not observe temporal trends in overall cancer incidence that correlate with the documented rise and fall of erythropoietin-stimulating agent use and dose.44 Instead, we observed constant incidence rates over the study period, which suggests that erythropoietin-stimulating agent therapy is not related to increased cancer incidence.
Our findings are subject to several limitations. First, our results may not be generalizable to patients with non–dialysis-dependent chronic kidney disease, patients treated by peritoneal dialysis, patients with a non-Medicare primary payer, or patients who died within 9 months of dialysis therapy initiation. Second, claims-based cancer definitions commonly are used in cancer research, but have not been validated in the ESRD population. We adapted our claims-based cancer definition from an algorithm that has high specificity (ie, it minimizes false-positives) for several incident cancers in a Medicare population.22 Third, the possibility of overestimating cancer incidence due to misclassification of prevalent cases as incident cases cannot be excluded. However, identification of prevalent cases of cancer during the 6-month baseline period minimized the possibility of misclassification. Fourth, use of claims-based definitions of cancer made it impossible to determine whether cancers identified as incident cases were truly new primary cancers, metastases, or histories of cancer miscoded as new primary cancers. Last, information on cancer risk factors was absent from the USRDS data.
Strengths of our study include more than a decade of data for the large representative population of US patients with ESRD on hemodialysis therapy. The large sample size allowed characterization of cancer incidence within subgroups. Additionally, competing-risks methodology is an innovative approach appropriate for the inherent competing-risks problem in the dialysis population due to high annual mortality.
There currently are no standard recommendations for cancer screening in the dialysis population (ie, NKF-KDOQI [National Kidney Foundation–Kidney Disease Outcomes Quality Initiative] or KDIGO [Kidney Disease: Improving Global Outcomes] guidelines). However, our results may help inform clinical decisions regarding screening. Our study demonstrates that overall risk of cancer among dialysis patients is higher than that among the general population. However, life-expectancy of individuals receiving dialysis is lower than that of the general population, and previous cost-effectiveness analyses have suggested that general cancer screening would add minimal days of life saved per person.45-47 In practice, cancer screening in dialysis patients has been given with an individualized patient-focused approach based on the patient's cancer risk factors, expected survival, and transplantation status.47 Our findings of differential cancer incidence among certain subgroups highlight the need to potentially reevaluate targeted cancer screening practices. Furthermore, targeted screening for certain cancer types should be considered.
In conclusion, we reported a high and constant overall burden of cancer among patients with ESRD receiving hemodialysis, with certain subgroups of the population exhibiting a particularly elevated cancer risk.
Supplementary Material
ACKNOWLEDGEMENTS
Some of the data reported in this study have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
Support: This research was supported by the National Institutes of Health through contracts from the National Institute of Diabetes and Digestive and Kidney Diseases (2 T32 DK007750-15), the National Cancer Institute (5 T32 CA009330-30), the National Institute of Aging (R01 AG042845 and R01 AG023178), and the National Institute of Child Health and Human Development (R21 HD080214) and through contracts with the Agency for Healthcare Research and Quality's DEcIDE (Developing Evidence to Inform Decisions About Effectiveness) program and the Patient-Centered Outcomes Research Institute.
Footnotes
Contributions: Conception and study design: AMB, AFO, AVK, JKE, MEN, SBW, MAB; statistical analysis: AMB; critical review of results: AMB, AFO, AVK, JKE, MEN, SBW, MAB; supervision and mentorship: AFO, AVK, MAB. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. AMB takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Financial Disclosure: Dr Brookhart reports receiving grant support from Amgen; serving on scientific advisory boards for Pfizer, Amgen, and Merck (honoraria declined, donated, or received by institution); and serving as a consultant to RxAnte and World Health Information Consultants. Dr Kshirsagar reports serving on an advisory board for Fresenius. The other authors declare that they have no other relevant financial interests.
SUPPLEMENTARY MATERIAL
Table S1: ICD-9-CM diagnosis codes used to identify site-specific cancer cases from Medicare claims.
Table S2: Descriptive characteristics of inverse probability of exposure weights by patient characteristic.
Table S3: Demographic and clinical characteristics of patients who initiated dialysis, 1995-2010.
Table S4: 5-y cumulative incidence of less frequent site-specific cancers in HD patients, accounting for competing risk of death.
Table S5: 5-y cumulative incidence of site-specific cancers in HD patients, treating death as censoring event.
Figure S1: Flow diagram of eligibility criteria for incident HD population.
Figure S2: Crude cumulative incidence of any cancer in HD patients, accounting for competing risk of death.
Figure S3: Crude cumulative incidence estimate of any cancer in HD patients, censoring deaths.
Item S1: Detailed methods.
Note: The supplementary material accompanying this article (http://dx.doi.org/10.1053/j.ajkd.2014.12.013) is available at www.ajkd.org
REFERENCES
- 1.Maisonneuve P, Agodoa L, Gellert R, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354(9173):93–99. doi: 10.1016/s0140-6736(99)06154-1. [DOI] [PubMed] [Google Scholar]
- 2.Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. JAMA. 2006;296(23):2823–2831. doi: 10.1001/jama.296.23.2823. [DOI] [PubMed] [Google Scholar]
- 3.Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol. 2010;23(3):253–262. [PMC free article] [PubMed] [Google Scholar]
- 4.Lin HF, Li YH, Wang CH, Chou CL, Kuo DJ, Fang TC. Increased risk of cancer in chronic dialysis patients: a population-based cohort study in Taiwan. Nephrol Dial Transplant. 2012;27(4):1585–1590. doi: 10.1093/ndt/gfr464. [DOI] [PubMed] [Google Scholar]
- 5.Hurst FP, Jindal RM, Fletcher JJ, et al. Incidence, predictors and associated outcomes of renal cell carcinoma in long-term dialysis patients. Urology. 2011;77(6):1271–1276. doi: 10.1016/j.urology.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Chung CJ, Huang CY, Tsai HB, et al. Sex differences in the development of malignancies among end-stage renal disease patients: a nationwide population-based follow-up study in Taiwan. PLoS One. 2012;7(9):e44675. doi: 10.1371/journal.pone.0044675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hwang JC, Weng SF, Weng RH. High incidence of hepatocellular carcinoma in ESRD patients: caused by high hepatitis rate or ’uremia’? A population-based study. Jpn J Clin Oncol. 2012;42(9):780–786. doi: 10.1093/jjco/hys100. [DOI] [PubMed] [Google Scholar]
- 8.Shebl FM, Warren JL, Eggers PW, Engels EA. Cancer risk among elderly persons with end-stage renal disease: a population-based case-control study. BMC Nephrol. 2012;13:65. doi: 10.1186/1471-2369-13-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart JH, Vajdic CM, van Leeuwen MT, et al. The pattern of excess cancer in dialysis and transplantation. Nephrol Dial Transplant. 2009;24(10):3225–3231. doi: 10.1093/ndt/gfp331. [DOI] [PubMed] [Google Scholar]
- 10.Stewart JH, Buccianti G, Agodoa L, et al. Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol. 2003;14(1):197–207. doi: 10.1097/01.asn.0000039608.81046.81. [DOI] [PubMed] [Google Scholar]
- 11.Birkeland SA, Lokkegaard H, Storm HH. Cancer risk in patients on dialysis and after renal transplantation. Lancet. 2000;355(9218):1886–1887. doi: 10.1016/s0140-6736(00)02298-4. [DOI] [PubMed] [Google Scholar]
- 12.Fairley CK, Sheil AG, McNeil JJ, et al. The risk of anogenital malignancies in dialysis and transplant patients. Clin Nephrol. 1994;41(2):101–105. [PubMed] [Google Scholar]
- 13.Kantor AF, Hoover RN, Kinlen LJ, McMullan MR, Fraumenti JF., Jr Cancer in patients receiving long-term dialysis treatment. Am J Epidemiol. 1987;126(3):370–376. doi: 10.1093/oxfordjournals.aje.a114668. [DOI] [PubMed] [Google Scholar]
- 14.Gaynor JJ, Feuer EJ, Tan CC, et al. On the use of cause-specific failure and conditional failure probabilities: examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 15.Korn EL, Dorey FJ. Applications of crude incidence curves. Stat Med. 1992;11(6):813–829. doi: 10.1002/sim.4780110611. [DOI] [PubMed] [Google Scholar]
- 16.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16(8):901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Pepe MS, Mori M. Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med. 1993;12(8):737–751. doi: 10.1002/sim.4780120803. [DOI] [PubMed] [Google Scholar]
- 18.US Renal Data System . Researcher's Guide to the USRDS Database: 2013 ADR Edition. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013. [Google Scholar]
- 19.Kshirsagar AV, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Brookhart MA. The comparative short-term effectiveness of iron dosing and formulations in US hemodialysis patients. Am J Med. 2013;126(6):541.e1–541.e14. doi: 10.1016/j.amjmed.2012.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brookhart MA, Freburger JK, Ellis AR, Wang L, Winkelmayer WC, Kshirsagar AV. Infection risk with bolus versus maintenance iron supplementation in hemodialysis patients. J Am Soc Nephrol. 2013;24(7):1151–1158. doi: 10.1681/ASN.2012121164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaubrun AC, Kilpatrick RD, Freburger JK, Bradbury BD, Wang L, Brookhart MA. Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol. 2013;24(9):1461–1469. doi: 10.1681/ASN.2012090916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hemato-logic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control. 2007;18(5):561–569. doi: 10.1007/s10552-007-0131-1. [DOI] [PubMed] [Google Scholar]
- 23.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010. National Cancer Institute; Bethesda, MD: Apr, 2013. [Google Scholar]
- 24.Joinpoint Regression Program, Version 4.0.4 [computer program] Statistical Research and Applications Branch, National Cancer Institute; Rockville, MD: May, 2013. [Google Scholar]
- 25.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 27.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole SR, Lau B, Eron JJ, Jr, et al. Estimation of the standardized risk difference and ratio in a competing risks framework: application to injection drug use and progression to AIDS after initiation of antiretroviral therapy [published online ahead of print June 24, 2014]. Am J Epidemiol. doi: 10.1093/aje/kwu122. http://dx.doi.org/10.1093/aje/kwu122:1-8. [DOI] [PMC free article] [PubMed]
- 29.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–875. doi: 10.1097/EDE.0b013e31823029dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collins AJ, Foley RN, Chavers B, et al. US Renal Data System 2013 annual data report. Am J Kidney Dis. 2014;63((1)(suppl 1)):e1–e420. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Hall EC, Pfeiffer RM, Segev DL, Engels EA. Cumulative incidence of cancer after solid organ transplantation. Cancer. 2013;119(12):2300–2308. doi: 10.1002/cncr.28043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kasiske BL, Ramos EL, Gaston RS, et al. The evaluation of renal transplant candidates: clinical practice guidelines. Patient Care and Education Committee of the American Society of Transplant Physicians. J Am Soc Nephrol. 1995;6(1):1–34. doi: 10.1681/ASN.V611. [DOI] [PubMed] [Google Scholar]
- 33.Avissar N, Ornt DB, Yagil Y, et al. Human kidney proximal tubules are the main source of plasma glutathione peroxidase. Am J Physiol. 1994;266(2, pt 1):C367–C375. doi: 10.1152/ajpcell.1994.266.2.C367. [DOI] [PubMed] [Google Scholar]
- 34.Descamps-Latscha B. The immune system in end-stage renal disease. Curr Opin Nephrol Hypertens. 1993;2(6):883–891. doi: 10.1097/00041552-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Kato S, Chmielewski M, Honda H, et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol. 2008;3(5):1526–1533. doi: 10.2215/CJN.00950208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonomini M, Forster S, De Risio F, et al. Effects of selenium supplementation on immune parameters in chronic uraemic patients on haemodialysis. Nephrol Dial Transplant. 1995;10(9):1654–1661. [PubMed] [Google Scholar]
- 37.Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol. 2004;24(5):503–510. doi: 10.1159/000081023. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura S, Suemizu H, Nomoto Y, et al. Plasma glutathione peroxidase deficiency caused by renal dysfunction. Nephron. 1996;73(2):207–211. doi: 10.1159/000189042. [DOI] [PubMed] [Google Scholar]
- 39.Connelly-Frost A, Poole C, Satia JA, Kupper LL, Millikan RC, Sandler RS. Selenium, folate, and colon cancer. Nutr Cancer. 2009;61(2):165–178. doi: 10.1080/01635580802404188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lange JH. Reanalysis of epidemiological data for selenium anti-cancer activity. Toxicol Ind Health. 1991;7(4):319–325. doi: 10.1177/074823379100700407. [DOI] [PubMed] [Google Scholar]
- 41.Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, Giovannucci EL. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99(14):1120–1129. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 42.Oster HS, Neumann D, Hoffman M, Mittelman M. Erythropoietin: the swinging pendulum. Leuk Res. 2012;36(8):939–944. doi: 10.1016/j.leukres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Szenajch J, Wcislo G, Jeong JY, Szczylik C, Feldman L. The role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells From clinic to bench— a critical review. Biochim Biophys Acta. 2010;1806(1):82–95. doi: 10.1016/j.bbcan.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 44.Freburger JK, Ng LJ, Bradbury BD, Kshirsagar AV, Brookhart MA. Changing patterns of anemia management in US Hemodialysis patients. Am J Med. 2012;125(9):906–914. e909. doi: 10.1016/j.amjmed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2012 annual data report. Am J Kidney Dis. 2013;61((1)(suppl 1)):e1–e480. doi: 10.1053/j.ajkd.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 46.Holley JL. Do dialysis patients need screening colonos-copies and mammograms? Semin Dial. 2011;24(4):364–365. doi: 10.1111/j.1525-139X.2011.00891.x. [DOI] [PubMed] [Google Scholar]
- 47.Holley JL. Screening, diagnosis, and treatment of cancer in long-term dialysis patients. Clin J Am Soc Nephrol. 2007;2(3):604–610. doi: 10.2215/CJN.03931106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.