Abstract
Brain metastasis (BM) is the common complication of non‐small cell lung cancer (NSCLC) with a poor prognosis and dismal survival rate. This update meta‐analysis aimed to derive a more precise estimation of radiotherapy plus epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in NSCLC patients with BM. PubMed, EMBASE, Web of Science, Google Scholar, and Cochrane Library were searched to identify any relevant publications. After screening the literature and undertaking quality assessment and data extraction, the meta‐analysis was performed using STATA Version 12.0. In total, 15 studies involving 1552 participants were included. The results indicated that radiotherapy plus EGFR TKIs was more effective at improving response rate and disease control rate (DCR) (risk ratio (RR) = 1.48, 95% confidence interval [CI]: 1.12–1.96, P = 0.005; RR = 1.29, 95% CI: 1.02–1.60, P = 0.035; respectively) than radiotherapy alone or plus chemotherapy. Moreover, radiotherapy plus EGFR TKIs significantly prolonged the time to central nervous system progression (CNS‐TTP) (HR = 0.56, 95% CI [0.33, 0.80]; P = 0.000) and median overall survival (OS) (HR = 0.58, 95% CI [0.42, 0.74]; P = 0.000) but significantly increased adverse events (any grade) (RR = 1.25, 95% CI [1.01, 1.57]; P = 0.009), especially rash and dry skin. These results suggested that radiotherapy plus EGFR TKIs produced superior response rate and DCR and markedly prolonged the CNS‐TTP and OS of NSCLC patients with BM. However, combined groups had the higher rate of incidence of overall adverse effects, especially rash and dry skin.
Keywords: Brain metastases, EGFR TKI, meta‐analysis, non‐small cell lung cancer, radiotherapy
Introduction
Lung cancer is characterized by a high incidence of central nervous system (CNS) metastases, with approximately 40% of patients developing brain metastases (BM) in the course of their disease 1, 2, 3, 4. In particular, it has also been estimated that 25–30% of newly diagnosed non‐small cell lung cancer (NSCLC) patients, who account for a large percentage of lung cancer cases, would suffer from BM 5. Patients with NSCLC who develop BM often have poor prognoses. The median overall survival (OS) time was 7 months, and 1‐year survival rate was 20% in one large series 6. Other studies reported that the OS for NSCLC patients with BM is less than 3–6 months when left untreated. Current treatment options include surgery, whole brain radiation therapy (WBRT), stereotactic radiosurgery (SRS) alone or in combination with other strategies such as chemotherapy and targeted therapy.
Radiotherapy including WBRT and SRS play a critical role in the current treatment of NSCLC patients with BM. They are the cornerstone treatment for patients with BM with the choice of radiation technique dependent on the prognosis of the patients and tumor characteristics such as number, size, and site of lesions 7, 8. Traditionally, patients with multiple BM are treated with WBRT to decrease and delay symptoms of increased intracranial pressure as well as to prevent neurological sequelae. In patients with limited number of BM, usually up to three to four lesions, local treatment (SRS or surgery) should be strongly considered.
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) are the standard therapy for advanced NSCLC patients with EGFR‐activated mutations based on some famous phase III trials 9, 10, 11. Recent preclinical studies demonstrated that EGFR TKIs might have synergistic effect in combination with radiotherapy on tumor control 12, 13. Erlotinib has been shown to cross the blood–brain barrier (BBB) and may be used to improve the effects of WBRT 14. Some studies indicated that radiotherapy plus EGFR TKIs is more suitable to treat multiple brain lesions of metastatic NSCLC than radiotherapy alone or radiotherapy plus chemotherapy, and showed favorable efficacy and safety 15, 16, 17. However, other studies reported that radiotherapy plus EGFR TKIs showed no advantage in neurological progression‐free survival (PFS) or OS 18. What is worse, some studies suggested that radiotherapy plus EGFR TKIs would lead to poorer survival and much more adverse effects (AEs) than control groups 19.
Whether radiotherapy plus EGFR TKIs has superior efficacy and safety than radiotherapy alone or radiotherapy plus chemotherapy remains controversial. Although there has been a meta‐analysis on this topic, only eight publications were included in that meta‐analysis 20. There have been more than seven papers published since this meta‐analysis was conducted. Moreover, it did not assess some common AEs such as dry skin, anemia, and anorexia in two groups. For these reasons, we performed this update meta‐analysis to derive a more precise estimation of evaluation of radiotherapy plus EGFR TKIs in NSCLC patients with BM.
Materials and Methods
Search strategy
PubMed, EMBASE, Web of Science, Google Scholar, and Cochrane Library were searched to identify relevant trials up to June 2015 without language restrictions. Searches were limited to human studies. The main keywords used for the online search were lung neoplasms, lung tumor, lung cancer, brain metastasis, brain neoplasms, radiotherapy, and tyrosine kinase inhibitors.
Inclusion criteria
All articles which met the following criteria were eligible: (1) prospective or retrospective studies; (2) patients had histologically or cytologically confirmed NSCLC and had been diagnosed with multiple BM using CT or MRI; (3) the trials compared radiotherapy (WBRT/SRS/3D‐CRT alone or in combination) plus EGFR TKIs with conventional chemotherapy plus radiotherapy or radiotherapy or TKIs alone and patients who received prior EGFR TKIs would be excluded; (4) trials did not include patients with chemotherapy contraindications or serious vital organ dysfunction; (5) the analyses included response rate, median survival time (MST), time to CNS progression (CNS‐TTP), PFS, OS, AEs (Grade ≥3), or hematological toxicity (Grade ≥3); (6) response rate was determined using the Response Evaluation Criteria in Solid Tumors or WHO evaluation criteria on solid tumors. Complete remission was defined as tumor completely disappearing for at least 4 weeks without any new lesions. Partial response was defined as more than 50% tumor regression for at least for 4 weeks without new lesions. Progressive disease was defined as an increase in the sum of the longest diameters (LD) of the target lesions by 25% or higher, using as reference the smallest sum LD recorded since treatment started or the appearance of one or more new lesions. Stabilized disease was defined as ≤50% tumor regression or an increase ≤25%. (7) Toxicity was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events.
Study selection
The eligibility assessment was first performed by screening titles and abstracts and subsequently reviewing the full text of articles. According to the inclusion criteria, two reviewers performed the selection of all studies independently. The third reviewer resolved the disagreement on whether an article should be included.
Data extraction
Two reviewers independently conducted data extraction in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analyses statement (PRISMA). The following items were collected from each study: first author's name, published year, type of study, country of origin study, percentage of female, performance status, number of patients, average ages, interventions, outcomes and toxicity or AEs.
Quality assessment
Two reviewers independently assessed the risk of bias of the included studies according to The Cochrane Handbook for Systematic Reviews (Version 5.1.0), based on the following criteria: (1) Random sequence generation; (2) Allocation concealment; (3) Blinding of participants and personnel; (4) Blinding of outcome assessment; (5) Incomplete outcome data; (6) Selective reporting; (7) Other bias. Each trial for bias based on the criteria listed above was marked as “low risk,” “high risk,” or “unclear risk.” Trials were judged as low risk of bias (i.e. A rating) when all criteria were assessed as low risk; trials were judged as moderate risk of bias (i.e. B rating) or high risk of bias (i.e. C rating) when one or more criteria were assessed as unclear risk or high risk, respectively.
Statistical analysis
Statistical analyses were performed using STATA Version 12.0 (Stata Corporation LP, College Station, TX). Chi‐squared and I 2 tests were used to test the heterogeneity of different studies. For time‐to‐event data, the HRs with 95% CIs were directly extracted from the research article or calculated using previously published methods proposed by Tierney et al. 21. The I 2 test was used to test for statistical heterogeneity and the I 2 statistic was used to assess the extent of variability attributable to statistical heterogeneity across trials. P > 0.1 for the I 2 test and I 2 < 25% were interpreted as signifying low‐level heterogeneity. When there was no statistically significant heterogeneity, a pooled effect was calculated with a fixed‐effects model; otherwise, a random‐effects model was used. Response rate, severe hematological toxicity, and adverse events were analyzed using dichotomous variables. PFS and OS were calculated using effect variables.
Results
Selection of studies
Totally, we identified 2547 studies that met our selection criteria after searching the relevant databases; 683 of these studies were excluded due to duplications. By verifying related terms in the titles and abstracts, we excluded 1741 irrelevant articles, and another 108 articles were excluded after the full text was read. Finally, 15 studies were selected for the present meta‐analysis 15, 16, 17, 18, 19, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31. A flowchart depicting the study selection is shown in Figure 1.
Figure 1.
Flow chart of studies included in the meta‐analysis.
Characteristics of included studies
There were a total of 1552 NSCLC patients with BM originating from NSCLC in the included studies, with 672 patients having received radiotherapy plus EGFR TKIs. The characteristics of the included trials are summarized in Table 1. Of the 15 studies, four studies were prospective studies including one phase III clinical trial and three phase II trials 17, 18, 19, 22. The analyzed interventions were WBRT/SRS plus EGFR TKIs and WBRT/SRS alone or WBRT/SRS plus chemotherapy, except in the case of Sperduto et al. 2013, which compared the combination treatment WBRT, SRS, and erlotinib with WBRT+SRS treatment. Especially, one study analyzed the 3D‐CRT plus TKI and 3D‐CRT plus VM‐26 (teniposide). Among all of the included studies, EGFR TKIs included gefitinib and erlotinib. Conventional chemotherapy drugs included placebo, temozolomide (TMZ), VMP, pemetrexed, gemcitabine, platinum, and other chemotherapy agents. Ten studies reported sufficient data for RR and DCR. Three studies reported CNS‐TTP and eight studies reported OS results. Eight studies reported AE data. Of note, one study recorded the Kaplan–Meier curve of PFS and OS. To avoid selection bias, we did not extract HRs with 95% CIs from the published figures.
Table 1.
Characteristics of the included studies
| Author | Year | Country | Trial phase | Patient no. (T/C) | Median ages (T/C, years) | Female (T/C, %) | EGFR mutation (%) | Treatment group | Treatment pattern | Control group | Outcomes | Study quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pesce | 2012 | Switzerland | II | 16/43 | 57/63 | 44.0/37.0 | NA | WBRT+gefitinib | Concurrent | WBRT+TMZ | mOS | B |
| Fu | 2012 | China | NA | 38/123 | NA | NA | NA | WBRT/SRS+gefitinib | Concurrent | WBRT/SRS | ORR | C |
| Zeng | 2012 | China | NA | 45/45 | 56/52 | 57.8/53.3 | NA | WBRT+gefitinib | Concurrent | Gefitinib | RR, DCR, mTTP, mPFS, mOS | B |
| Wu | 2012 | China | NA | 35/18 | NA | NA | NA | WBRT+gefitinib | Concurrent | WBRT | RR, DCR, mOS | B |
| Cai | 2013 | China | NA | 65/92 | NA | 38.5/31.5 | 27.4 | WBRT+gefitinib/erlotinib | Concurrent | WBRT | RR, DCR, mPFS, mOS | B |
| Sperduto | 2013 | Multicenter | III | 41/44 | 61/64 | NA | NA | WBRT+SRS+erlotinib | Concurrent | WBRT/SRS | CNS‐TTP, mOS | B |
| Zhuang | 2013 | China | II | 23/31 | 60/63 | 57/58 | 20.4 | WBRT+erlotinib | Concurrent | WBRT | ORR, CNS‐TTP, PFS, mOS | B |
| Liu | 2013 | China | NA | 52/52 | 54/51 | 44.2/48.1 | NA | WBRT+SRS+gefitinib/erlotinib | Concurrent | WBRT+SRS | RR, DCR, PFS | C |
| Zhou | 2013 | China | NA | 36/22 | NA | 58.3/50.0 | NA | WBRT+gefitinib/erlotinib | Concurrent | WBRT+chemotherapy | RR, DCR, mOS | C |
| Fan | 2013 | China | NA | 75/111 | 57/57 | 42.7/27.0 | NA | WBRT/SRS+TKI | Sequential | WBRT/SRS+chemotherapy | mOS | B |
| Lee | 2014 | Britain | II | 40/40 | 61/62 | 62.5/47.5 | 2.9 | WBRT+erlotinib | Concurrent | WBRT+placebo | CNS‐TTP, mOS | B |
| Cai | 2014 | China | NA | 104/178 | 65/65 | 40.4/33.7 | 19.50% | WBRT/SRS+TKI | Sequential | WBRT/SRS | CNS‐TTP, mOS | B |
| Wang | 2015 | China | NA | 37/36 | 61/62 | 32.4/36.1 | 12.30% | 3D‐CRT+gefitinib | Concurrent | 3D‐CRT+chemotherapy | ORR, mOS | B |
| Liu | 2015 | China | NA | 35/15 | 46.3/47.5 | 51.4/53.3 | NA | WBRT+gefitinib | Concurrent | WBRT+chemotherapy | RR, DCR, mOS | C |
| Chen | 2015 | China | NA | 30/30 | 64.5/64.3 | 46.7/50.0 | NA | WBRT+gefitinib | Concurrent | WBRT+chemotherapy | RR, DCR, PFS, mOS | B |
T/C, treatment/control; ECOG PS, eastern cooperative oncology group performance status; NA, not applicable; RR, response rate; DCR, disease control rate; TTP, time to progression; PFS, progression‐free survival; OS, overall survival; WBRT, whole brain radiotherapy; SRS, stereotactic radiosurgery; CNS, central nervous system; 3D‐CRT, three‐dimensional conformal radiotherapy; TMZ, temozolomide.
Methodological quality
In accordance with the recommendations of the Cochrane Handbook for Systematic Reviews, we evaluated the eligible studies using the aspects mentioned above. Four studies 16, 18, 19, 22 mentioned the use of random allocation, but only two of them discussed the methods 19, 22. One study performed or reported their allocation concealment and blinding methods 17. None of the trials reported follow‐up information. All of the articles applied the intent‐to‐treat analysis. Eleven of the 15 eligible studies received B quality scores and four received C quality scores, as shown in Table 1.
Response rate and disease control rate
Ten of the included studies reported the RR and DCR of treatment using radiotherapy plus TKIs and radiotherapy alone or radiotherapy plus chemotherapy 16, 17, 22, 23, 24, 27, 28, 29, 30, 31. RR ranged from 13.0% to 77.1% in the radiotherapy plus TKI groups and 13.3–70.7% in control groups. DCR ranged from 52.2% to 97.1% in the radiotherapy plus TKI groups and 36.4–89.1% in control groups. There was no heterogeneity among the 10 studies in both RR and DCR (P = 0.751, I 2 = 0.0%; P = 0.915, I 2 = 0.0%; respectively), and as a result, the fixed effect model was used for the meta‐analysis. The pooled results indicate that radiotherapy plus TKIs resulted in superior RR and DCR when compared with control groups (RR = 1.48, 95% CI: 1.12–1.96, P = 0.005; RR = 1.29, 95% CI: 1.02–1.60, P = 0.035; respectively) (Fig. 2A and B).
Figure 2.
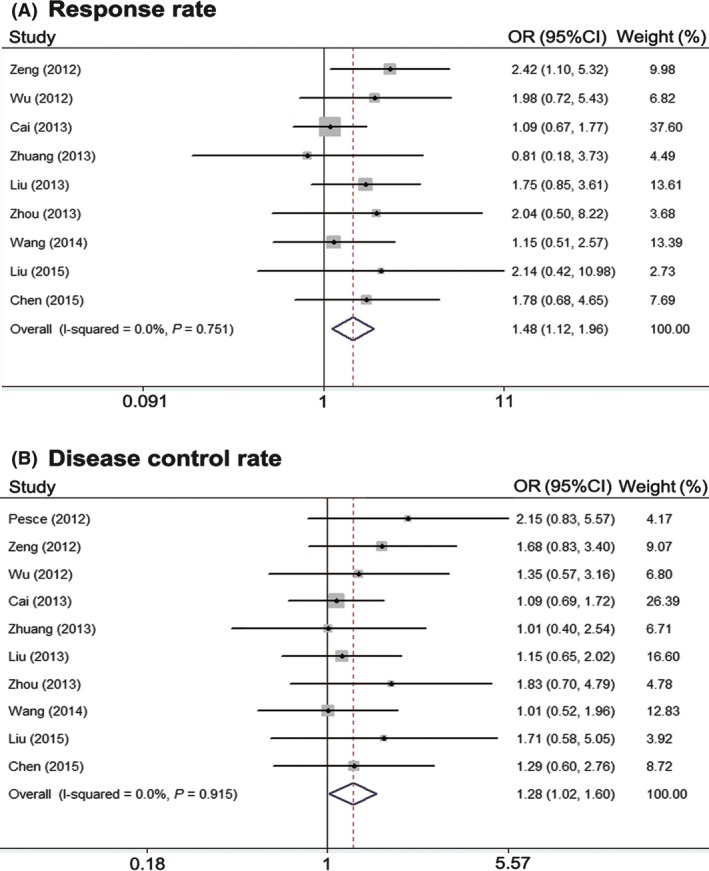
Meta‐analysis of response rate (A) and disease control rate (B).
Survival
Three studies reported CNS‐TTP 15, 17, 18. The studies were heterogeneous (P = 0.133, I 2 = 50.5%). Analysis using a random effect model suggests that in NSCLC patients diagnosed with BM, there was the significantly better CNS‐TTP in radiotherapy plus TKIs groups than in radiotherapy alone or radiotherapy plus chemotherapy (HR = 0.56, 95% CI: 0.33–0.80; P = 0.000) (Fig. 3A). Eight studies reported OS results and there was a significant heterogeneity between them (P = 0.032, I 2 = 54.4%) 15, 16, 17, 18, 19, 22, 23, 25. Accordingly, a random effect model was used for the meta‐analysis of OS. The results suggest that combining radiotherapy with TKIs could prolong OS (HR = 0.58, 95% CI: 0.42–0.74; P = 0.000) (Fig. 3B). In a subgroup analysis, we compared the different results between concurrent and sequential treatment. Only two studies reported the HR of CNS‐TTP and OS of sequential treatment 15, 25. After excluding them, we found that concurrent radiotherapy plus EGFR TKIs could also significantly prolong the CNS‐TTP (HR = 0.55, 95% CI: 0.28–0.82; P = 0.000) and OS (HR = 0.63, 95% CI: 0.45–0.81; P = 0.000) of NSCLC patients with BM (Fig. S1).
Figure 3.
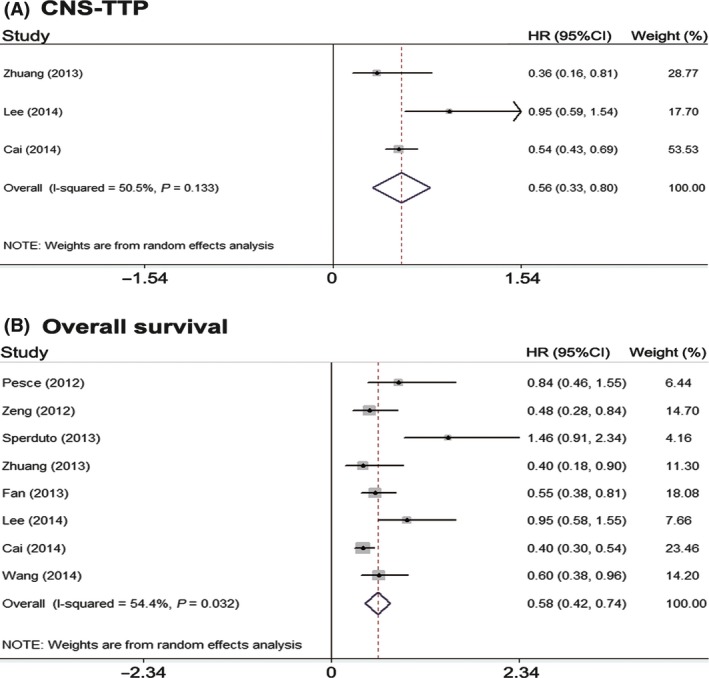
Meta‐analysis of time to central nervous system progression (A) and median overall survival (B).
Adverse effects
Eight enrolled studies had analyzed the treatment‐related toxicity and AEs. A fixed‐effects model was used for the overall AE analysis of these studies based on the heterogeneity values (P = 0.213, I 2 = 12.9%). The results indicate that the incidence of overall AEs was higher in the group treated using radiotherapy plus TKIs (RR = 1.25, 95% CI: 1.01–1.57; P = 0.009). The most common adverse events of TKIs are rash, fatigue, nausea/vomiting, and diarrhea, which are largely mild and fairly tolerable, and pneumonitis rarely occurs. Thus, we performed a subgroup analysis for these AEs as showed in Table 2. Regarding nausea/vomiting, diarrhea, pneumonitis, and other AEs, no difference was observed. However, rashes (RR = 4.97, 95% CI: 2.68–9.21; P = 0.000) and dry skin (RR = 8.44, 95% CI: 1.48–48.28; P = 0.000) were significantly more common in the radiotherapy plus TKI group.
Table 2.
Meta‐analysis of the reported common adverse effects in the included studies
| Adverse effect | Number of studies | Test of association | Test of heterogeneity | ||||
|---|---|---|---|---|---|---|---|
| RR | 95% CI | P | Q | I 2% | P | ||
| Fatigue | 5 | 0.762 | 0.418–1.388 | 0.374 | 1.92 | 0.0 | 0.751 |
| Dyspnea | 2 | 2.287 | 0.193–27.116 | 0.512 | 2.66 | 62.4 | 0.103 |
| Rash | 8 | 4.971 | 2.683–9.208 | 0.000 | 8.33 | 15.9 | 0.305 |
| Anorexia | 2 | 1.634 | 0.677–3.944 | 0.275 | 1.22 | 18.0 | 0.270 |
| Diarrhea | 8 | 1.506 | 0.938–2.417 | 0.090 | 5.00 | 0.0 | 0.660 |
| Headache | 4 | 1.070 | 0.657–1.743 | 0.786 | 2.68 | 0.0 | 0.443 |
| Anemia | 3 | 1.324 | 0.616–2.846 | 0.472 | 2.59 | 22.8 | 0.274 |
| Constipation | 2 | 1.799 | 0.652–4.964 | 0.256 | 0.11 | 0.0 | 0.739 |
| Dry skin | 2 | 8.438 | 1.475–48.278 | 0.017 | 0.50 | 0.0 | 0.477 |
| Nausea | 8 | 0.979 | 0.682–1.407 | 0.910 | 9.54 | 26.6 | 0.216 |
| Pneumonitis | 2 | 1.108 | 0.375–3.273 | 0.852 | 0.64 | 0.0 | 0.425 |
| Total | 8 | 1.247 | 1.012–1.572 | 0.009 | 6.99 | 12.9 | 0.213 |
RR, risk rate; CI, confidence interval.
Discussion
Currently, local radiotherapy remains the standard therapy of NSCLC patients with BM. Several studies have verified that WBRT and/or SRS could palliate the neurological symptoms associated with BM. However, the prognosis for NSCLC patients with BM is poor. Overall, median survival of BM patients is about 2.4–4.8 months after WBRT 32, 33. Recent studies of radiotherapy in combination with conventional chemotherapeutic drugs, such as platinum, paclitaxel, and TMZ, suggest no significant improvement in OS compared with radiotherapy alone owing to their low capacity of penetrating the BBB 34, 35, 36, 37. As the small molecular drugs, TKIs such as gefitinib and erlotinib have the possibility of crossing the BBB. Previous studies demonstrated that gefitinib and erlotinib showed good permeability through the BBB 38, 39, 40. After penetrating into the BBB, TKIs can compete with adenosine triphosphate and provide sufficient radiosensitizing and therapeutic level in the brain to exert their anti‐cancer efficacy 13, 14, 41. Hence radiotherapy plus EGFR TKIs seems to have the promising antitumor effect.
In our study, we demonstrated that radiotherapy plus EGFR TKIs produced superior RR and DCR and markedly prolonged the CNS‐TTP and OS of NSCLC patients with BM. However, combined group increased the incidence of overall AEs, especially rash and dry skin, which may be attributed to TKI therapy. The possible reason about these results may include that the patients in most of the included studies come from East Asia. As is known, East Asian patients with NSCLC have a higher EGFR mutation rate than patients from other ethnicities. In East Asian patients, Matsumoto et al. and Gow et al. have found EGFR mutations in 63% and 44% of BM, respectively 42, 43. This prevalence is similar to that reported in primary tumors of the same population, varying from 30% to 50% 44, 45. In Caucasian cohorts, with a low overall prevalence of EGFR mutations, activating mutations were found in 0–2% of BM 46, 47. In addition, the EGFR mutation rate was 64% and 31% in patients with and without BM, respectively, suggesting that BM would be more frequent in patients with tumors bearing EGFR mutations. However, another two studies with small sample sizes have suggested a discordant EGFR mutation rate between primary and brain metastatic tumors between 0% and 32% 48, 49. Therefore, in future, we need to explore how to precisely select patients with the concordant EGFR mutation between primary lesions and BM. For patients with the discordant EGFR mutation between primary lesions and BM, we need to explore the possible molecular mechanism during the process of BM with the help of advanced techniques such as next generation sequencing.
Considering the effect of WBRT on BBB, which would increase the cerebral concentration of TKIs, we performed subgroup analysis to compare the different results between concurrent and sequential treatment. Our result showed that both concurrent and sequential treatment could significantly prolong the CNS‐TTP and OS of NSCLC patients with BM, possibly due to the synergistic effect of EGFR TKI and WBRT 14. WBRT could increase the penetration of EGFR TKI via disturbing BBB and EGFR TKI could increase the anti‐tumor effect of WBRT in BM 5, 15. Further prospective studies are needed to determine the optimal treatment pattern for NSCLC patients with BM.
The treatment of NSCLC has entered the era of precision medicine based on the different driver gene mutations. The survival of patients with advanced NSCLC has been significantly improved. However, NSCLC patients with BM still suffer from dismal prognosis. The major reasons include the unknown molecular mechanism of the BM process and lack of specific therapeutic targets. Fortunately, several recent studies revealed the potential mechanism of lung cancer metastases to brain. Nguyen et al. demonstrated that a distinct WNT/TCF signaling program through LEF1 and HOXB9 enhances the competence of lung adenocarcinoma cells to colonize the bones and the brain. These findings are useful for achieving a deeper understanding of early metastatic events and the development of improved treatments for lung adenocarcinoma patients at risk of BM 50. Another study showed that brain metastatic cells from lung cancer could express high levels of anti‐plasminogen activator serpins, including neuroserpin and serpin B2, to prevent plasmin generation and its metastasis‐suppressive effects, which may become promising targets for NSCLC patients with BM 51. A more recent study illustrated that a disintegrin and metalloproteinase domain 9 (ADAM9) could regulate lung cancer metastasis to the brain by facilitating tissue plasminogen activator‐mediated cleavage of CDCP1, with potential implications to target this network as a strategy to prevent or treat brain metastatic disease 52. We do believe that with the clear molecular mechanisms revealed, the treatment of BM from lung cancer will make great progress in future years.
Our systematic review with meta‐analysis has some limitations that should be acknowledged. First, the number of included studies was relatively small and some of them are retrospective studies. Thus the quality of evidence supporting the findings is low. Second, it is possible that there may be some degree of publication bias in this area of research. We identified several abstracts describing articles that were not further detailed in standard publications; hence, we could not include these articles in the review. Third, the quality of the data was heterogeneous as several pieces of important information such as prior therapy, performance status, number of BM, and health‐related quality of life outcomes were not consistently reported. Notably, all included articles were not randomized controlled studies except one phase III trial 19, so more robust data with high clinical evidence will be included in future analysis. Last but not least, some previous studies have shown that response to radiotherapy was different according to the type of mutation, and particularly some difference may exist according to KRAS amino acid substitution 53. A recent publication has shown that EGFR TKI could increase, in vitro, the radiosensitivity of tumors harboring a KRAS mutation by limiting chromatin condensation induced by EGFR pathway activation 54. Therefore, it would be interesting to add EGFR TKI to radiotherapy that even if EGFR TKI are known to be not effective on patients with KRAS mutations.
In conclusion, the current evidence suggests that radiotherapy plus EGFR TKIs produced superior RR and DCR and markedly prolonged the CNS‐TTP and OS of NSCLC patients with BM. Meanwhile, combined group increased the incidence of overall AEs, especially rash and dry skin. In future, more high‐quality and large‐scale clinical trials are necessary to confirm the efficacy and safety of radiotherapy plus EGFR TKIs and select the most benefit population in NSCLC patients with BM.
Conflict of interest
The authors have no conflicts of interest to declare.
Supporting information
Figure S1. Meta‐analysis of time to central nervous system progression (A) and median overall survival (B) in concurrent radiotherapy and EGFR TKI group.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81372392). The views expressed in this review are the opinions of the authors. All authors had full access to the data in the study.
Cancer Medicine 2016; 5(6): 1055–1065
References
- 1. Zhang, J. , Yu J., Sun X., and Meng X.. 2014. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of central nerve system metastases from non‐small cell lung cancer. Cancer Lett. 351:6–12. [DOI] [PubMed] [Google Scholar]
- 2. Lombardi, G. , Di Stefano A. L., Farina P., Zagonel V., and Tabouret E.. 2014. Systemic treatments for brain metastases from breast cancer, non‐small cell lung cancer, melanoma and renal cell carcinoma: an overview of the literature. Cancer Treat. Rev. 40:951–959. [DOI] [PubMed] [Google Scholar]
- 3. Adamo, V. , Franchina T., Adamo B., Scandurra G., and Scimone A.. 2006. Brain metastases in patients with non‐small cell lung cancer: focus on the role of chemotherapy. Ann. Oncol. 17(Suppl 2):ii73–ii75. [DOI] [PubMed] [Google Scholar]
- 4. Eichler, A. F. , Chung E., Kodack D. P., Loeffler J. S., Fukumura D., and Jain R. K.. 2011. The biology of brain metastases‐translation to new therapies. Nat. Rev. Clin. Oncol. 8:344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berger, L. A. , Riesenberg H., Bokemeyer C., and Atanackovic D.. 2013. CNS metastases in non‐small‐cell lung cancer: current role of EGFR‐TKI therapy and future perspectives. Lung Cancer 80:242–248. [DOI] [PubMed] [Google Scholar]
- 6. Sperduto, P. W. , Kased N., Roberge D., Xu Z., Shanley R., Luo X., et al. 2012. Summary report on the graded prognostic assessment: an accurate and facile diagnosis‐specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 30:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scoccianti, S. , and Ricardi U.. 2012. Treatment of brain metastases: review of phase III randomized controlled trials. Radiother. Oncol. 102:168–179. [DOI] [PubMed] [Google Scholar]
- 8. Tsao, M. N. , Rades D., Wirth A., Lo S. S., Danielson B. L., Gaspar L. E., et al. 2012. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): an American Society for Radiation Oncology evidence‐based guideline. Pract. Radiat. Oncol. 2:210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mok, T. S. , Wu Y. L., Thongprasert S., Yang C. H., Chu D. T., Saijo N., et al. 2009. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361:947–957. [DOI] [PubMed] [Google Scholar]
- 10. Zhou, C. , Wu Y. L., Chen G., Feng J., Liu X. Q., Wang C., et al. 2011. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 12:735–742. [DOI] [PubMed] [Google Scholar]
- 11. Rosell, R. , Carcereny E., Gervais R., Vergnenegre A., Massuti B., Felip E., et al. 2012. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 13:239–246. [DOI] [PubMed] [Google Scholar]
- 12. Zhuang, H. , Wang J., Zhao L., Yuan Z., and Wang P.. 2013. The theoretical foundation and research progress for WBRT combined with erlotinib for the treatment of multiple brain metastases in patients with lung adenocarcinoma. Int. J. Cancer 133:2277–2283. [DOI] [PubMed] [Google Scholar]
- 13. Chinnaiyan, P. , Huang S., Vallabhaneni G., Armstrong E., Varambally S., Tomlins S. A., et al. 2005. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva). Cancer Res. 65:3328–3335. [DOI] [PubMed] [Google Scholar]
- 14. Weber, B. , Winterdahl M., Memon A., Sorensen B. S., Keiding S., Sorensen L., et al. 2011. Erlotinib accumulation in brain metastases from non‐small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J. Thorac. Oncol. 6:1287–1289. [DOI] [PubMed] [Google Scholar]
- 15. Cai, L. , Zhu J. F., Zhang X. W., Lin S. X., Su X. D., Lin P., et al. 2014. A comparative analysis of EGFR mutation status in association with the efficacy of TKI in combination with WBRT/SRS/surgery plus chemotherapy in brain metastasis from non‐small cell lung cancer. J. Neurooncol. 120:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang, F. , Ning F., Liu C., Hao Y., Li L., Yu Z., et al. 2015. Comparison of Gefitinib versus VMP in the combination with radiotherapy for multiple brain metastases from non‐small cell lung cancer. Cell Biochem. Biophys. 71:1261–1265. [DOI] [PubMed] [Google Scholar]
- 17. Zhuang, H. , Yuan Z., Wang J., Zhao L., Pang Q., and Wang P.. 2013. Phase II study of whole brain radiotherapy with or without erlotinib in patients with multiple brain metastases from lung adenocarcinoma. Drug Des. Devel. Ther. 7:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee, S. M. , Lewanski C. R., Counsell N., Ottensmeier C., Bates A., Patel N., et al. 2014. Randomized trial of erlotinib plus whole‐brain radiotherapy for NSCLC patients with multiple brain metastases. J. Natl Cancer Inst. 106:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sperduto, P. W. , Wang M., Robins H. I., Schell M. C., Werner‐Wasik M., Komaki R., et al. 2013. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non‐small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int. J. Radiat. Oncol. Biol. Phys. 85:1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luo, S. , Chen L., Chen X., and Xie X.. 2015. Evaluation on efficacy and safety of tyrosine kinase inhibitors plus radiotherapy in NSCLC patients with brain metastases. Oncotarget 6:16725–16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tierney, J. F. , Stewart L. A., Ghersi D., Burdett S., and Sydes M. R.. 2007. Practical methods for incorporating summary time‐to event data into meta‐analysis. Eur. J. Cancer 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pesce, G. A. , Klingbiel D., Ribi K., Zouhair A., von Moos R., Schlaeppi M., et al. 2012. Outcome, quality of life and cognitive function of patients with brain metastases from non‐small cell lung cancer treated with whole brain radiotherapy combined with gefitinib or temozolomide. A randomised phase II trial of the Swiss Group for Clinical Cancer Research (SAKK 70/03). Eur. J. Cancer 48:377–384. [DOI] [PubMed] [Google Scholar]
- 23. Zeng, Y. D. , Zhang L., Liao H., Liang Y., Xu F., Liu J. L., et al. 2012. Gefitinib alone or with concomitant whole brain radiotherapy for patients with brain metastasis from non‐small‐cell lung cancer: a retrospective study. Asian Pac. J. Cancer Prev. 13:909–914. [DOI] [PubMed] [Google Scholar]
- 24. Cai, Y. , Wang J. Y., and Liu H.. 2013. Clinical observation of whole brain radiotherapy concomitant with targeted therapy for brain metastasis in non‐small cell lung cancer patients with chemotherapy failure. Asian Pac. J. Cancer Prev. 14:5699–5703. [DOI] [PubMed] [Google Scholar]
- 25. Fan, Y. , Huang Z., Fang L., Miu L., Lin N., Gong L., et al. 2013. Chemotherapy and EGFR tyrosine kinase inhibitors for treatment of brain metastases from non‐small‐cell lung cancer: survival analysis in 210 patients. Onco. Targets Ther. 6:1789–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fu, H. , Zhang X. L., Xiao Y., Liu X. J., Long C., andHu Y. D.. 2012. Evaluation of gefitinib plus radiotherapy in non‐small‐cell lung cancer patients with brain metastases. Zhonghua Yi Xue Za Zhi 92:524–527. [PubMed] [Google Scholar]
- 27. Di Zhou, X. X. , Xie H., Ma X., and Bai Y.. 2013. Therapeutic effects of whole brain radiotherapy with targeted therapy and concomitant chemo‐radiotherapy in treatment of non‐small cell lung cancer with brain metastasis. J. Shanghai Jiaotong University (Medical Science) 33:480–484. [Google Scholar]
- 28. Jingsheng Chen, Y. W. , Chen G., and Zhong H.. 2015. Clinical research of gefitinib plus radiotherapy in lung cancer patients with brain metastasis. Intern. Med. 10:38–40. [Google Scholar]
- 29. Liu, P. 2013. The effect of epidermal growth factor receptor tyrosine kinase inhibitors combined with radiotherapy in non‐small cell lung cancer patients with brain metastasis. J. Chin. Pract. Diagn. Ther. 27:693–694. [Google Scholar]
- 30. Liu, Z. 2015. Effect of whole brain radiotherapy combined with targeted therapy and concurrent chemotherapy in the treatment of non‐small cell lung cancer. Chin. Foreign Med. Res. 13:13–14. [Google Scholar]
- 31. Wu, T. A. , Liu D. R., Wang Z. H., and Peng Y.. 2012. Effects of gefitinib combined with whole brain radiation on brain metastasis from non‐small‐cell lung cancer. Chin. J. Gen. Pract. 10:893–895. [Google Scholar]
- 32. Langer, C. J. , and Mehta M. P.. 2005. Current management of brain metastases, with a focus on systemic options. J. Clin. Oncol. 23:6207–6219. [DOI] [PubMed] [Google Scholar]
- 33. Khuntia, D. , Brown P., Li J., and Mehta M. P.. 2006. Whole‐brain radiotherapy in the management of brain metastasis. J. Clin. Oncol. 24:1295–1304. [DOI] [PubMed] [Google Scholar]
- 34. Chua, D. , Krzakowski M., Chouaid C., Pallotta M. G., Martinez J. I., Gottfried M., et al. 2010. Whole‐brain radiation therapy plus concomitant temozolomide for the treatment of brain metastases from non‐small‐cell lung cancer: a randomized, open‐label phase II study. Clin. Lung Cancer 11:176–181. [DOI] [PubMed] [Google Scholar]
- 35. Guerrieri, M. , Wong K., Ryan G., Millward M., Quong G., and Ball D. L.. 2004. A randomised phase III study of palliative radiation with concomitant carboplatin for brain metastases from non‐small cell carcinoma of the lung. Lung Cancer 46:107–111. [DOI] [PubMed] [Google Scholar]
- 36. Cortes, J. , Rodriguez J., Aramendia J. M., Salgado E., Gurpide A., Garcia‐Foncillas J., et al. 2003. Front‐line paclitaxel/cisplatin‐based chemotherapy in brain metastases from non‐small‐cell lung cancer. Oncology 64:28–35. [DOI] [PubMed] [Google Scholar]
- 37. Verger, E. , Gil M., Yaya R., Vinolas N., Villa S., Pujol T., et al. 2005. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: a phase II randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 61:185–191. [DOI] [PubMed] [Google Scholar]
- 38. Broniscer, A. , Panetta J. C., O'Shaughnessy M., Fraga C., Bai F., Krasin M. J., et al. 2007. Plasma and cerebrospinal fluid pharmacokinetics of erlotinib and its active metabolite OSI‐420. Clin. Cancer Res. 13:1511–1515. [DOI] [PubMed] [Google Scholar]
- 39. Togashi, Y. , Masago K., Fukudo M., Terada T., Fujita S., Irisa K., et al. 2010. Cerebrospinal fluid concentration of erlotinib and its active metabolite OSI‐420 in patients with central nervous system metastases of non‐small cell lung cancer. J. Thorac. Oncol. 5:950–955. [DOI] [PubMed] [Google Scholar]
- 40. Togashi, Y. , Masago K., Fukudo M., Tsuchido Y., Okuda C., Kim Y. H., et al. 2011. Efficacy of increased‐dose erlotinib for central nervous system metastases in non‐small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Chemother. Pharmacol. 68:1089–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Porta, R. , Sanchez‐Torres J. M., Paz‐Ares L., Massuti B., Reguart N., Mayo C., et al. 2011. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur. Respir. J. 37:624–631. [DOI] [PubMed] [Google Scholar]
- 42. Matsumoto, S. , Takahashi K., Iwakawa R., Matsuno Y., Nakanishi Y., Kohno T., et al. 2006. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int. J. Cancer 119:1491–1494. [DOI] [PubMed] [Google Scholar]
- 43. Gow, C. H. , Chang Y. L., Hsu Y. C., Tsai M. F., Wu C. T., Yu C. J., et al. 2009. Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor‐naive non‐small‐cell lung cancer. Ann. Oncol. 20:696–702. [DOI] [PubMed] [Google Scholar]
- 44. Huang, S. F. , Liu H. P., Li L. H., Ku Y. C., Fu Y. N., Tsai H. Y., et al. 2004. High frequency of epidermal growth factor receptor mutations with complex patterns in non‐small cell lung cancers related to gefitinib responsiveness in Taiwan. Clin. Cancer Res. 10:8195–8203. [DOI] [PubMed] [Google Scholar]
- 45. Wu, Y. L. , Zhong W. Z., Li L. Y., Zhang X. T., Zhang L., Zhou C. C., et al. 2007. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non‐small cell lung cancer: a meta‐analysis based on updated individual patient data from six medical centers in mainland China. J. Thorac. Oncol. 2:430–439. [DOI] [PubMed] [Google Scholar]
- 46. Sun, M. , Behrens C., Feng L., Ozburn N., Tang X., Yin G., et al. 2009. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin. Cancer Res. 15:4829–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Daniele, L. , Cassoni P., Bacillo E., Cappia S., Righi L., Volante M., et al. 2009. Epidermal growth factor receptor gene in primary tumor and metastatic sites from non‐small cell lung cancer. J. Thorac. Oncol. 4:684–688. [DOI] [PubMed] [Google Scholar]
- 48. Burel‐Vandenbos, F. , Ambrosetti D., Coutts M., and Pedeutour F.. 2013. EGFR mutation status in brain metastases of non‐small cell lung carcinoma. J. Neurooncol. 111:1–10. [DOI] [PubMed] [Google Scholar]
- 49. Whitsett, T. G. , Inge L. J., Dhruv H. D., Cheung P. Y., Weiss G. J., Bremner R. M., et al. 2013. Molecular determinants of lung cancer metastasis to the central nervous system. Transl. Lung Cancer Res. 2:273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nguyen, D. X. , Chiang A. C., Zhang X. H., Kim J. Y., Kris M. G., Ladanyi M., et al. 2009. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell 138:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Valiente, M. , Obenauf A. C., Jin X., Chen Q., Zhang X. H., Lee D. J., et al. 2014. Serpins promote cancer cell survival and vascular co‐option in brain metastasis. Cell 156:1002–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lin, C. Y. , Chen H. J., Huang C. C., Lai L. C., Lu T. P., Tseng G. C., et al. 2014. ADAM9 promotes lung cancer metastases to brain by a plasminogen activator‐based pathway. Cancer Res. 74:5229–5243. [DOI] [PubMed] [Google Scholar]
- 53. Renaud, S. , Schaeffer M., Voegeli A. C., Legrain M., Guerin E., Meyer N., et al. 2016. Impact of EGFR mutations and KRAS amino acid substitution on the response to radiotherapy for brain metastasis of non‐small‐cell lung cancer. Future Oncol 12:59–70. [DOI] [PubMed] [Google Scholar]
- 54. Wang, M. , Kern A. M., Hulskotter M., Greninger P., Singh A., Pan Y., et al. 2014. EGFR‐mediated chromatin condensation protects KRAS‐mutant cancer cells against ionizing radiation. Cancer Res. 74:2825–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Meta‐analysis of time to central nervous system progression (A) and median overall survival (B) in concurrent radiotherapy and EGFR TKI group.


