Abstract
Colorectal cancer is one of the leading causes of highly fatal cancer‐related deaths in the whole world. Fast growth is critical characteristic of colorectal cancer, the underlying regulatory mechanism of colorectal cell fast proliferation remains largely unknown. Here, we reported that activation of metabotropic γ‐Aminobutyric acid receptor (GABABR) signaling significantly inhibited the colorectal cell HT29 proliferation by arresting the cell at G1 phase. Inhibition of GABABR activated GSK‐3β by reducing the phosphorylation level of GSK‐3β. Activation of GSK‐3β blocked the function of GABABR signaling on repressing cell proliferation. We further found that GABABR activation inhibited NF‐κB activity. The promotion of cell proliferation caused by downregulation of GABRBR could be blocked by inhibition of NF‐κB activation. Overall, activation of GABABR leaded to inhibition of GSK‐3β activation to repress the NF‐κB function during colorectal cancer cell proliferation. This study revealed critical function of GABABR/GSK‐3β/NF‐κB signaling pathway on regulating proliferation of colorectal cancer cell, which might provide a potential therapeutic target for clinical colorectal cancer treatment.
Keywords: Cell cycle, colorectal cancer, GABABR, GSK‐3β, NF‐κB, proliferation
Introduction
Colorectal cancer (CRC) is a major cause of cancer mortality worldwide with approximately 694,000 recorded deaths from the disease in 2012 1, 2. It is estimated that more than 1 million cases are diagnosed annually and around 600,000 of them are death 3. CRC is an ideal model of research of the molecular pathogenesis of cancer, due to the ease of obtaining biopsy material and the understanding of the development of invasive carcinoma, from normal epithelium to polyps, and carcinoma 4. Colorectal carcinogenesis is a multistep process that requires the accumulation of genetic/epigenetic aberrations in signal transduction pathways 5. However, the regulation of the colorectal cancer cell proliferation process is limited and needs further investigation.
γ‐aminobutyric acid (GABA), the main inhibitory neurotransmitter in the vertebrate brain, acts on ionotropic (GABAA or GABAC) and metabotropic (GABAB) receptors, is an inhibitory neurotransmitter 2. An increasing number of studies have demonstrated the potential roles of neurotransmitter receptors in tumors like GABAB receptors, as a key therapeutic targets in neurological diseases, also can suppress the proliferation of various human tumor cells 2. Wang and his partners have reported that the proliferation of hepatocarcinoma cells Bel‐7402 and Huh‐7 was inhibited by baclofen, an activator of GABABR, in a dose‐dependent manner. Systemic administration of baclofen significantly suppressed the growth of Bel‐7402 xenograft induced in nude mice 6. In addition, Opolski's laboratory found that a remarkable growth inhibition of experimental mammary cancer 16/C was observed in mice treated with baclofen 7. However, the study about the regulation of proliferation and cell cycle in colorectal cancer cell is unknown and still need our further investigation.
Glycogen synthase kinase‐3β (GSK‐3β), a serine/threonine protein kinase, has been regarded as a potential therapeutic target for multiple human cancers 8. Growing evidences showed that GSK‐3β plays an important role in diverse cellular processes including proliferation, differentiation, motility, and survival 9. Recent report had been reported that it can regulate the proliferation of human ovarian cancer cells in vitro (SKOV3 and ES‐2 cells) as well as in vivo 10. Study also showed that the relevance of GSK‐3β as a target for controlling cell cycle progression and proliferative capacity in MCF7, highlighted the cotreatment of breast cancer 11. However, the deep molecular mechanism of the regulation by GSK‐3β on CRC and its signaling pathway worths further investigation.
Lately many reports show that nuclear factor‐κB (NF‐κB) not only plays an important role in the coordination of innate and adaptive immune responses and cell‐cycle regulation, but also proved to have a pivotal role in tumorigenesis 12. NF‐κB is a family of five master transcription factors, including NF‐κB1/p105, NF‐κB2/p100, RelA/p65, RelB, and c‐Rel, which can form various heterodimers or homodimers and bind to consensus DNA sequences at promoter regions of responsive genes 13. NF‐κB is activated in response to various stimuli (cytokines, growth factors, oncoproteins, stress signals) and can follow two distinct pathways: the canonical pathway and the noncanonical pathway 13, 14. Huili Li and his laboratory had demonstrated that inhibition of GSK‐3β could suppress the proliferation of colorectal cells by the downregulation of activity of NF‐κB and NF‐κB‐mediated target genes transcription, which may be of benefit for clinical outcome in patients suffering from colon cancer in future 8. Understanding of how GABABR, GSK‐3β, and NF‐κB participate in the CRC cell proliferation and cell cycles will be helpful in finding deep regulation mechanism of cancer cell in order to facilitate clinical application.
Here, we demonstrated that GABABR played an important role in regulating the CRC cell proliferation. Activation of GABABR leaded to inhibition of GSK‐3β. Repression of GSK‐3β lead to upregulation of cell proliferation could be rescued by inhibition of GABABR. Further, inhibition of GSK‐3β could inhibit NF‐κB so that to suppress the cancer cell proliferation. The downregulation of cell proliferation caused by inhibition of GABABR could be rescued by repression of NF‐κB. These results indicated that the critical function of GABABR/GSK‐3β/NF‐κB signaling in colorectal cancer cell proliferation, and might provide more insight into the specific roles of GABABR to lay the foundation for further clinical application.
Material and Methods
Cell culture
Cells were cultured at 37°C, 5% CO2 in RPMI‐1640 medium (Gibco, New York, USA) with 10% fetal bovine serum (FBS) (Gibco), 100 U/mL of penicillin sodium (Invitrogen, Life Technologies, Carlsbad, California, USA), and 100 mg/mL of streptomycin sulfate (Invitrogen, Life Technologies).
GABAB R1 knockdown: The Plko.1 vector was used to construct shRNA vectors. The shRNA sequences were as follows: shRNA‐GABABR1‐1: 5′‐CCCGAATCTGCTCCAAGTCTTA‐3′; shRNA‐GABABR1‐2: 5′‐ ACCAAGCCACCAAGTACCTATA‐3′.
Proliferation assay
Cells were seeded in the 96‐well plate for the MTS proliferation assay. Cell Titer 96® AQueous One Solution Cell Proliferation Assay kit (Promega, Madison, Wisconsin, USA) was used to perform the MTS proliferation assay following the instruction. MTS is 3‐(4,5‐dimethylthiazol‐2‐yl) ‐5‐(3‐carboxymethoxyphenyl)‐2‐(4‐sul‐fophenyl)‐2H‐tetrazolium. The MTS tetrazolium compound can be bioreduced to be the colored formazan product that is solute in culture medium. The record absorbance at 490 nm was detected by microplate reader.
Bromodeoxyuridine (BrdU) assay
A BrdU Cell Proliferation Assay kit (cat. no. 2752; EMD Millipore, Bedford, MA) was used to detect cell proliferation ability. Cells were seeded in 96 wells (2 × 103 cells/well) in the medium with 10 μL BrdU solution. After incubation, phosphate buffer saline (PBS) solution with 4% paraformaldehyde was used to fix the cells for 15 min. Then, the cells were washed with PBS and treated with DNase for 15 min. The cells were washed with PBS. Then, BrdU antibody(Abcam, Cambridge, Massachusetts, USA)was added and the cells were incubated at 4°C for 8 h. Then, the cells were incubated with secondary antibody at room temperature for 60 min. Cell nucleus were dyed with 4′,6‐diamidino‐2‐phenylindole (DAPI). Then, the BrdU‐positive cells were counted.
FACS assay
Single cell suspension was collected by digesting with trypsin and fixed in 70% ethanol for 12 h at 4°C treatment for fluorescence‐activated cell sorting (FACS) analysis. Then, cells were washed with PBS solution. Then, RNase A was added to the cells for 10 min. Propidium iodide (PI) was added to a final cell suspension with the concentration of 50 μg/mL to stain the chromatin. Cells were analyzed using the flow cytometer.
Quantitative real‐time PCR (qRT‐PCR)
The total RNA was isolated using RNAiso (Takara, Dalian, China). cDNA was subsequently reverse‐transcribed from mRNA by M‐MLV Reverse Transcriptase (Takara). The PCR included 40 cycles of amplification using the Stratagene Mx3000P system with SYBR Green qPCR Mix (BioRad, Hercules, California). Expression of target genes (2−ΔΔCt) was normalized against GAPDH. The sequence of primer used in the qRT‐PCR:
Cyclin D1PF 5′‐ CAATGACCCCGCACGATTTC‐3′.
Cyclin D1 PR 5′‐ CATGGAGGGCGGATTGGAA‐3′.
Rb1 PF 5′‐TTGGATCACAGCGATACAAACTT ‐3′.
Rb1 PR 5′‐ AGCGCACGCCAATAAAGACAT ‐3′.
RBL1 PF 5′‐ ACCACCAAAGTTACCACGAAG ‐3′.
RBL1 PR 5′‐ CCCCAATCATCCGAAAATTACCC ‐3′.
P21 PF 5′‐ TGTCCGTCAGAACCCATGC ‐3′.
P21 PR 5′‐ TGTCCGTCAGAACCCATGC ‐3′.
GAPDH PF: 5′‐ TGTGGGCATCAATGGATTTGG‐3′.
GAPDH PR 5′‐ ACACCATGTATTCCGGGTCAAT‐3′.
Western blotting
SDS lysis buffer (Beyotime, Beijing, China) was used to lyse the cells for protein electrophoresis. Polyvinylidene fluoride (PVDF) membranes were used to transfer the protein and incubate the antibody. The primary antibodies are as followed: p‐GSK‐3β (Signaling way antibody, USA), GSK‐3β (Abcam), p‐IKBα (Abcam), IKBα (Abcam), p‐NF‐κb‐p65 (Abcam), NF‐κb‐p65(Abcam), GAPDH (Santa Cruz, Dallas, Texas, USA) diluted phosphate buffer saline (PBS) with 10% donkey serum albumin. The visualized signaling was performed by enhanced chemiluminescence (ECL)western blotting substrate kit (Thermo, Waltham, Massachusetts, USA).
Statistical analyses
Student's t‐test was used to determined statistical significance. Values were presented as the mean ± SD. *means P < 0.05, **means P < 0.01, ***means P < 0.001, respectively.
Results
Activation of GABABR inhibited the proliferation of colorectal cancer cell
In order to determine the GABABR signaling on regulating the colorectal cancer cell, we performed MTS assay on cell line HT29 and HCT116 to find that the capacity of colorectal cancer cells proliferation was repressed by baclofen (agonist of GABABR, 30 μmol/L−1) (Figs. 1A and S1A). BrdU incorporation assay also showed that the cell proliferation of baclofen group was significantly inhibited (Figs. 1B and S1B). We then used the HT29 to investigate if activation of GABABR can regulate cell cycle by flow cytometry and found that there was a significant increase in the proportion of cells in G1 phase, and a reduction in the proportion of cells in S, and G2/M phases (Fig. 1C). Meanwhile, we detected some cell cycle relative gene expression level by qRT‐PCR. We could find that the G1 phase‐related gene cyclin D1 was downregulated, while the cell cycle inhibitors Rb1, Rbl1, and P21 15 were upregulated (Fig. 1D). In contrast, knockdown of the GABABR significantly promoted the capacity of proliferation (Figs. 1E and F, S1C, S1D). Additionally, the proportion of cells in G1 phase was decreased and increased in the S, G2/M phase by knockdown of the GABABR (Fig. 1G). We also found that cyclin D1 was upregulated and the Rb1,Rbl1, P21 were downregulated(Fig. 1H).
Figure 1.
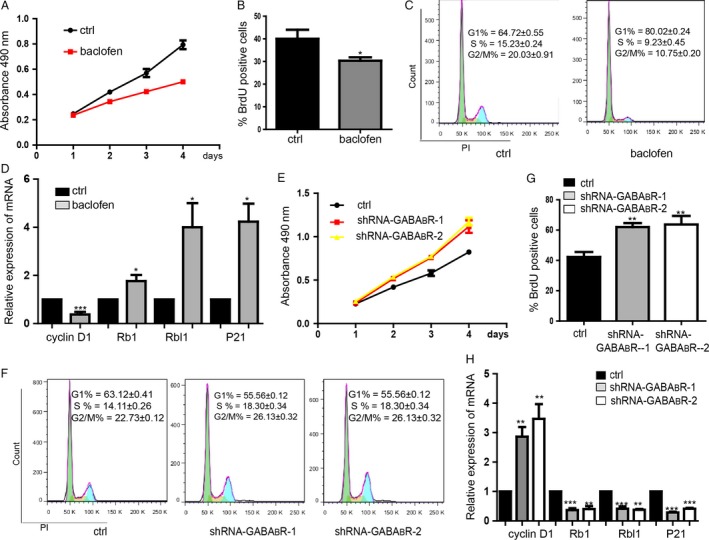
Activation of γ‐Aminobutyric acid receptor (GABABR) inhibited the proliferation of colorectal cancer cell line HT29. (A) Baclofen inhibits HT29 proliferation detected by MTS proliferation assay. Ctrl means the cells treated with physiological saline. Data shown are means ± SD (n = 3). (B) BrdU incorporation assay showed the inhibition of proliferation caused by baclofen. Data shown are means ± SD (n = 4). *P < 0.05 versus the corresponding control. (C) Flow cytometry assay showed the cell cycle arrest at G1 phase by baclofen. Data shown are means ± SD (n = 5). (D) Expression level of mRNA of cell cycle‐related genes. Data shown are means ± SD (n = 4). *P < 0.05, ***P < 0.001 versus the corresponding control. (E) Detection of the proliferation of cells with downregualtion of GABABR. Data shown are means ± SD (n = 5). (F) BrdU incorporation assay of cells with downregualtion of assay GABABR. Data shown are means ± SD (n = 5). **P < 0.01 versus the corresponding control. (G) Cell cycle analysis by flow cytometry assay. Data shown are means ± SD (n = 5). (H) Expression level of mRNA of cell cycle‐related genes. Data shown are means ± SD (n = 4). **P < 0.01, ***P < 0.001 versus the corresponding control.
GABABR signaling repressed colorectal cancer cell proliferation by inhibiting activity of GSK‐3β
To investigate whether GABABR can regulate GSK‐3β to inhibit colorectal cancer cell proliferation and cell cycle, we performed further experiments. Western blot assay showed that activation of GABAR significantly increased the phosphorylation level of GSK‐3β, but could not regulate the expression of GSK‐3β (Fig. 2A). Downregulation of GABABR inhibited the phosphorylation of GSK‐3β, which means the activation of GSK‐3β was increased. (Fig. 2B). In the contrary, knockdown of GABABR could restrain the phosphorylation level of GSK‐3β to promoted GSK‐3β activation (Fig. 2B). Then, we found that wort (wortamannin, 10 μmol/L−1), an agonist of GSK‐3β, could significantly block the proliferation repression caused by GABABR signaling activation (Figs. 2C and D, S2A and B). Activation of GSK‐3β blocked the G1 phase arrest and the influence on cell cycle‐related genes caused by baclofen (Fig. 2E and F).
Figure 2.
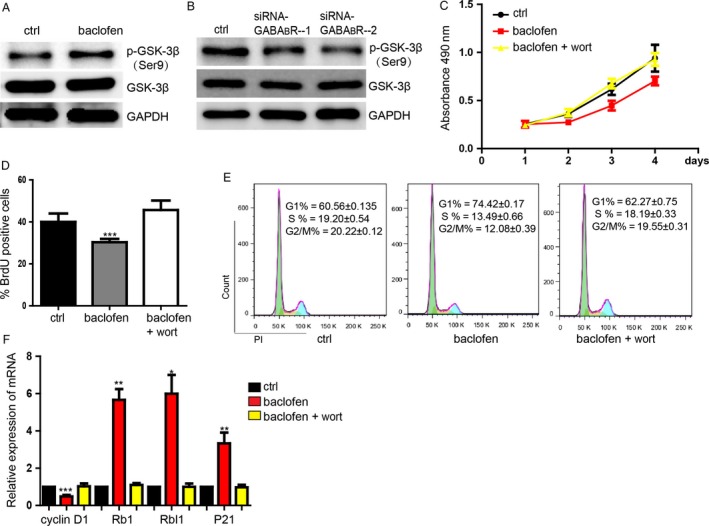
γ‐Aminobutyric acid receptor (GABABR) signaling repressed colorectal cell proliferation by inhibiting activity of GSK‐3β. (A) Representative picture showed the increasing level of phosphorylation level of GSK‐3β caused by activation of GABABR detected by western blot. (B) Downregulation of GABABR activates the GSK‐3β. (C) MTS proliferation assay showed the function of activation of GABABR can be blocked by activation of GSK‐3β by wort. Data shown are means ± SD (n = 4). (D) BrdU incorporation assay of the rescue experiment. Data shown are means ± SD (n = 4). ***P < 0.001 versus the corresponding control. (E) Flow cytometry assay. Data shown are means ± SD (n = 6). (F) The expression level of cell cycle‐related genes detected by qRT‐PCR. Data shown are means ± SD (n = 4). *P < 0.05, **P < 0.01, ***P < 0.001 versus the corresponding control.
GSK‐3β/NF‐κB signaling regulates the colorectal cancer cell proliferation
In order to detect the regulatory mechanism of GSK‐3β on the proliferation, we further found that the repression of cell proliferation caused by inhibition of GSK‐3β by SB216763 could be blocked by Phorbol‐12‐myristate‐13‐acetate (PMA) (200 nmol/L−1) which is the agonist of NF‐κB (Fig. 3A and B, S3A and B). Meanwhile, we found that PMA also could rescue the cell cycle which was arrested at G1 phase caused by SB216763 to be similar with control group (Fig. 3C). Besides, the influence of expression level of cyclin D1 and Rb1, Rbl1, P21 by SB216763 were also restored by PMA to be the similar level of control group (Fig. 3D). These results determined that suppression of GSK‐3β, which lead to restrain colorectal cancer cell proliferation, can be rescued by activation of NF‐κB.
Figure 3.
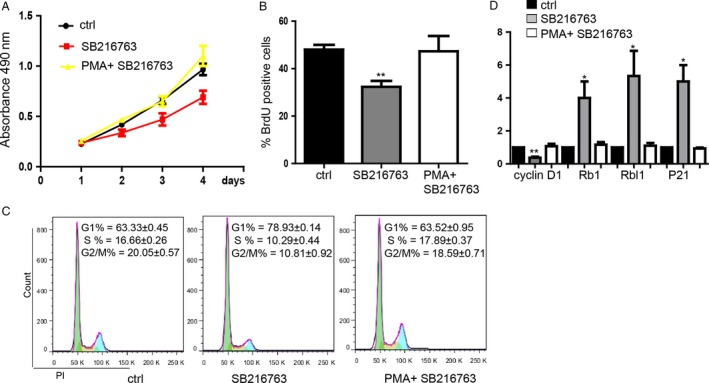
GSK‐3β/NF‐κB signaling regulates the colorectal cancer cell proliferation. (A) MTS proliferation assay showed that the inhibition of cells proliferation caused by SB216763 could be rescued by PMA meanwhile. Data shown are means ± SD (n = 5). (B) BrdU incorporation assay for proliferation analysis. Data shown are means ± SD (n = 5). **P < 0.01 versus the corresponding control. (C) Flow cytometry assay showed that PMA blocked the influence of cell cycle caused by SB216763. Data shown are means ± SD (n = 3). (D) PMA blocked the influence of expression of cell cycle‐related genes caused by SB216763. Data shown are means ± SD (n = 4). *P < 0.05, **P < 0.01 versus the corresponding control.
GABABR/NF‐κB signaling pathway regulates the colorectal cancer cell proliferation
To determine whether GABABR can inhibit NF‐κB to repress the colorectal cancer cell proliferation, we first performed western blot experiment to find that phosphorylation level of p‐IKBα and p‐NF‐κb‐p65 were downregulated compared with GAPDH,while the whole expression of IKBα and NF‐κb‐p65 were not changed significantly, which mean that the NF‐κB was activated (Fig. 4A). Then, we found that PDTC (100 μmol/L−1), an inhibitor of NF‐κB, could rescue the promotion of cell proliferation caused by downregulation of GABABR (Fig. 4B and C, S4A and B). Inhibition of NF‐κB restored the influence on cell cycle arrest at G1 phase caused by downregulation of GABABR (Fig. 4D). Additionally, the influence on expression level of cyclin D1, Rb1, Rbl1, and P21 caused by downregulation of GABABR were also restored by inhibition of NF‐κB (Fig. 4E). Additionally, in order to further detect how GABABR regulates the GSK‐3β/NF‐κB signaling pathway in colon cancer cells, we performed the rescue experiment and found that inhibition of the inactivation of Akt kinase activity by MK‐2206 2HCl (15 μmol/L−1) blocked the repression of proliferation caused by baclofen (Fig. S4C and D). MK‐2206 2HCl also restored the cell proliferation which was repressed by the GSK‐3β signaling inhibitor SB216763 (Fig. S4E and F). So, we hypothesized that Akt might be the mediator of the function of GABABR on regulating GSK‐3β/NF‐κB signaling.
Figure 4.

GABABR/NF‐κB signaling pathway regulates the proliferation. (A) Representative picture showed the detection of the influence of baclofen on NF‐κB signaling. (B) MTS proliferation assay showed that the promotion of cells proliferation caused by downregulation of GABABR could be rescued by PDTC meanwhile. Data shown are means ± SD (n = 3). (C) PDTC blocked the promotion of proliferation caused by downregulation of GABABR. Data shown are means ± SD (n = 3). ***P < 0. 001 versus the corresponding control. (D) PDTC restored the cell cycle influence caused by downregulation of GABABR. Data shown are means ± SD (n = 3). (E) PDTC restored the expression of cell cycle‐related genes influenced by GABABR downregulation. Data shown are means ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001 versus the corresponding control.
Discussion
In summary, we uncovered that the GABABR/GSK‐3β/NF‐κB signaling pathway can regulate the proliferation of colorectal cancer. Upregulation of GABABR significantly inhibited the colorectal cell HT29 proliferation. Further, inhibition of GSK‐3β activation could repress the cell proliferation which can be blocked by downregulation of GABABR. Besides, we further confirmed that the promotion of cell proliferation caused by downregulation of GABRBR could be blocked by inhibition of NF‐κB activation. These results might provide more insight into the specific roles of GABABR/GSK‐3β/NF‐κB signaling pathway in colorectal cancer cell and help us lay the foundation for further clinical application of CRC.
A metabotropic GABAB receptor (GABABR) is one of the GABA family members, which was originally identified as a major inhibitory neurotransmitter in the adult mammalian brain 16. Outside the brain, GABA and its receptors have been found in nonneuronal peripheral tissues, such as the gastrointestinal system 17, lung 18, and liver 19, including the biliary tract system 20. Studies have implicated GABA and its receptors as important players not only in synaptic inhibition, convulsion, pain, depression, and cognition 21, but also in the inhibition of cancer growth and tumor cell migration in, for example, colorectal carcinoma 22, breast cancer 23, and cholangiocarcinoma 16. For example, Zhang D and his lab members found that both the GABA synthetic enzyme (GAD65/67) and GABAB receptor are expressed in mouse and human breast cancer cells. MCF‐7 human breast cancer cells and human breast cancer tissue. Baclofen, a GABABR agonist, significantly promoted 4T1 cells invasion and migration in vitro and metastasis in vivo, an event that was attenuated by GABABR antagonist CGP55845 24. Nevertheless, the mechanisms of GABABR effect were not fully understood. In our study, we found that when GABABR was upregulated by baclofen, the colorectal cancer cell HT29 proliferation was repressed. Activation of GABABR could arrest the cell at G1 phase and reduce cells in S, and G2/M phases, which indicated that activation of GABABR signaling could inhibit the colorectal cell HT29 proliferation by arresting the cell at G1 phase. The G1 phase‐related gene cyclin D1 was downregulated while the cell cycle inhibitor Rb1, Rbl1, and P21 were upregulated by inhibition of GABABR, which helped us determined that activation of GABABR can significantly promoted colorectal cancer cell proliferation by arrested cell at G1 phase.
Furthermore, to investigate how GABABR regulated colorectal cancer cell proliferation and find its downstream moleculars, we considered about GSK‐3β and NF‐κB. The phosphoinositide 3‐kinase/Akt/glycogen synthase kinase‐3β (GSK‐3β) and Wnt/β‐catenin pathways are downregulated in a number of cancers, and these two pathways share a common node protein, GSK‐3β. This protein is responsible for the regulation/degradation of β‐catenin, which reduces β‐catenin's translocation to the nucleus and influences the subsequent transcription of oncogenes 23, 25, 26. GSK‐3β is proved to direct proliferation of breast cancer cells interplayed with histone H3 phosphorylation and DNA methylation. Silencing of GSK‐3β by shRNA prevented histone H3 phosphorylation and reduced DNMT1 expression so that can restrain breast cancer cell proliferation 27. The other, NF‐κB, represents an evolutionarily conserved family of inducible transcription factors that controls a large set of physiological processes ranging from basic inflammatory responses and innate and acquired immunity to the regulation of cell death such as apoptosis, autophagy, and senescence. In addition, NF‐κB coordinates the expression of specific genes that mediates proliferation, cell adhesion, and differentiation 28, 29. Moreover, in several cancers, NF‐κB is constitutively activated and drives tumor cell survival and proliferation 30. For many years the NF‐κB signaling pathway has attracted much interest because of the possibility of targeting it for the treatment of inflammatory diseases and cancer. Therefore, interfering more upstream in the NF‐κB signaling cascade is expected to be much more specific and to cause fewer side effects 31. Signaling upstream of NF‐κB is quite complex and involves multiple protein–protein interactions and posttranslational modifications so there's still more that we can do and we are working on this 32.
Our study revealed that activation of GABABR could increase the phosphorylation level of GSK‐3β which means GABABR could regulate the activity of GSK‐3β. Furthermore, we found that inhibition of GSK‐3β activation, which leads to repression of colorectal cancer cell proliferation, can be rescued by upregulation of NF‐κB activation by PMA. Then, we activated GABABR finding that the activation of NF‐κB was promoted so that the colorectal cancer cell proliferation was inhibited. The promotion of proliferation caused by downregulation of GABABR can be blocked by inhibition of NF‐κB activation which meant that GABABR could regulate cell proliferation though regulating activation of NF‐κB.
These results might provide more insight into the specific roles of GABABR/GSK‐3β/NF‐κB signaling pathway in colorectal cancer cell proliferation to lay the foundation for further clinical application.
Conflict of Interest
None declared.
Supporting information
Figure S1. related to figure 1 GABABR regulates the proliferation of HCT116 cells.
Figure S2. related to figure 2. GABABR signaling repressed HCT116 cell proliferation by inhibiting GSK‐3β activation.
Figure S3. related to figure 3. GSK‐3β/NF‐κB signaling regulates the HCT116 cell proliferation.
Figure S4. related to figure 4. GABABR/NF‐κB signaling pathway regulates the proliferation in HCT116 cells.
Acknowledgment
This research was supported by the Shenzhen commission on innovation and technology(20150303,192210).
Cancer Medicine 2016; 5(6): 1259–1267
Reference
- 1. Siegel, R. , Naishadham D., and Jemal A.. 2013. Cancer statistics, 2013. CA Cancer J. Clin. 63:11–30. [DOI] [PubMed] [Google Scholar]
- 2. Jiang, X. , Su L., Zhang Q., He C., Zhang Z., Yi P., et al. 2012. GABAB receptor complex as a potential target for tumor therapy. J. Histochem. Cytochem. 60:269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jemal, A. , Bray F., Center M. M., Ferlay J., Ward E., and Forman D.. 2011. Global cancer statistics. CA Cancer J. Clin. 61:69–90. [DOI] [PubMed] [Google Scholar]
- 4. Lampropoulos, P. , Zizi‐Sermpetzoglou A., Rizos S., Kostakis A., Nikiteas N., and Papavassiliou A. G.. 2012. TGF‐beta signalling in colon carcinogenesis. Cancer Lett. 314:1–7. [DOI] [PubMed] [Google Scholar]
- 5. Vaiopoulos, A. G. , Kostakis I. D., Koutsilieris M., and Papavassiliou A. G.. 2012. Colorectal cancer stem cells.Stem Cells 30:363–371. [DOI] [PubMed] [Google Scholar]
- 6. Wang, T. , Huang W., and Chen F.. 2008. Baclofen, a GABAB receptor agonist, inhibits human hepatocellular carcinoma cell growth in vitro and in vivo. Life Sci. 82:536–541. [DOI] [PubMed] [Google Scholar]
- 7. Opolski, A. , Mazurkiewicz M., Wietrzyk J., Kleinrok Z., and Radzikowski C.. 2000. The role of GABA‐ergic system in human mammary gland pathology and in growth of transplantable murine mammary cancer. J. Exp. Clin. Cancer Res. 19:383–390. [PubMed] [Google Scholar]
- 8. Li, H. , Huang K., Liu X., Liu J., Lu X., Tao K., et al. 2014. Lithium chloride suppresses colorectal cancer cell survival and proliferation through ROS/GSK‐3beta/NF‐kappaB signaling pathway. Oxid. Med. Cell Longev. 2014:241864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luo, J. 2009. Glycogen synthase kinase 3beta (GSK3beta) in tumorigenesis and cancer chemotherapy. Cancer Lett. 273:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cao, Q. , Lu X., and Feng Y. J.. 2006. Glycogen synthase kinase‐3beta positively regulates the proliferation of human ovarian cancer cells. Cell Res. 16:671–677. [DOI] [PubMed] [Google Scholar]
- 11. Gavilan, E. , Giraldez S., Sanchez‐Aguayo I., Romero F., Ruano D., and Daza P.. 2015. Breast cancer cell line MCF7 escapes from G1/S arrest induced by proteasome inhibition through a GSK‐3beta dependent mechanism. Sci. Rep. 5:10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vaiopoulos, A. G. , Papachroni K. K., and Papavassiliou A. G.. 2010. Colon carcinogenesis: learning from NF‐kappaB and AP‐1. Int. J. Biochem. Cell Biol. 42:1061–1065. [DOI] [PubMed] [Google Scholar]
- 13. Xia, Y. , Shen S., and Verma I. M.. 2014. NF‐kappaB, an active player in human cancers. Cancer Immunol. Res. 2:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smale, S. T. 2011. Hierarchies of NF‐kappaB target‐gene regulation. Nat. Immunol. 12:689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang, Y. , Baskerville S., Shenoy A., Babiarz J. E., Baehner L., and Blelloch R.. 2008. Embryonic stem cell‐specific microRNAs regulate the G1‐S transition and promote rapid proliferation. Nat. Genet. 40:1478–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang, Q. , Zhu C. L., Liu C. H., Xie F., Zhu K., and Hu S. Y.. 2013. Gamma‐aminobutyric acid binds to GABAb receptor to inhibit cholangiocarcinoma cells growth via the JAK/STAT3 pathway. Dig. Dis. Sci. 58:734–743. [DOI] [PubMed] [Google Scholar]
- 17. Maemura, K. , Shiraishi N., Sakagami K., Kawakami K., Inoue T., Murano M., et al. 2009. Proliferative effects of gamma‐aminobutyric acid on the gastric cancer cell line are associated with extracellular signal‐regulated kinase 1/2 activation. J. Gastroenterol. Hepatol. 24:688–696. [DOI] [PubMed] [Google Scholar]
- 18. Liu, Y. , Guo F., Dai M., Wang D., Tong Y., Huang J., et al. 2009. Gammaaminobutyric acid A receptor alpha 3 subunit is overexpressed in lung cancer. Pathol. Oncol. Res. 15:351–358. [DOI] [PubMed] [Google Scholar]
- 19. Minuk, G. Y. , Zhang M., Gong Y., Minuk L., Dienes H., Pettigrew N., et al. 2007. Decreased hepatocyte membrane potential differences and GABAA‐beta3 expression in human hepatocellular carcinoma. Hepatology 45:735–745. [DOI] [PubMed] [Google Scholar]
- 20. Fava, G. , Marucci L., Glaser S., Francis H., De Morrow S., Benedetti A., et al. 2005. Gamma‐Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP‐dependent regulation of the protein kinase A/extracellular signal‐regulated kinase 1/2 pathway. Cancer Res. 65:11437–11446. [DOI] [PubMed] [Google Scholar]
- 21. Watanabe, M. , Maemura K., Kanbara K., Tamayama T., and Hayasaki H.. 2002. GABA and GABA receptors in the central nervous system and other organs. Int. Rev. Cytol. 213:1–47. [DOI] [PubMed] [Google Scholar]
- 22. Thaker, P. H. , Yokoi K., Jennings N. B., Li Y., Rebhun R. B., Rousseau D. L. Jr, et al. 2005. Inhibition of experimental colon cancer metastasis by the GABA‐receptor agonist nembutal. Cancer Biol. Ther. 4:753–758. [DOI] [PubMed] [Google Scholar]
- 23. Grimes, C. A. , and Jope R. S.. 2001. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog. Neurobiol. 65:391–426. [DOI] [PubMed] [Google Scholar]
- 24. Zhang, D. , Li X., Yao Z., Wei C., Ning N., and Li J.. 2014. GABAergic signaling facilitates breast cancer metastasis by promoting ERK1/2‐dependent phosphorylation. Cancer Lett. 348:100–108. [DOI] [PubMed] [Google Scholar]
- 25. Atkins, R. J. , Dimou J., Paradiso L., Morokoff A. P., Kaye A. H., Drummond K. J., et al. 2012. Regulation of glycogen synthase kinase‐3 beta (GSK‐3beta) by the Akt pathway in gliomas. J. Clin. Neurosci. 19:1558–1563. [DOI] [PubMed] [Google Scholar]
- 26. Jope, R. S. 2003. Lithium and GSK‐3: one inhibitor, two inhibitory actions, multiple outcomes. Trends Pharmacol. Sci. 24:441–443. [DOI] [PubMed] [Google Scholar]
- 27. Gupta, C. , Kaur J., and Tikoo K.. 2014. Regulation of MDA‐MB‐231 cell proliferation by GSK‐3beta involves epigenetic modifications under high glucose conditions. Exp. Cell Res. 324:75–83. [DOI] [PubMed] [Google Scholar]
- 28. Karin, M. , and Ben‐Neriah Y.. 2000. Phosphorylation meets ubiquitination: the control of NF‐[kappa]B activity. Annu. Rev. Immunol. 18:621–663. [DOI] [PubMed] [Google Scholar]
- 29. Hayden, M. S. , and Ghosh S.. 2008. Shared principles in NF‐kappaB signaling. Cell 132:344–362. [DOI] [PubMed] [Google Scholar]
- 30. DiDonato, J. A. , Mercurio F., and Karin M.. 2012. NF‐kappaB and the link between inflammation and cancer. Immunol. Rev. 246:379–400. [DOI] [PubMed] [Google Scholar]
- 31. Hinz, M. , and Scheidereit C.. 2014. The IkappaB kinase complex in NF‐kappaB regulation and beyond. EMBO Rep. 15:46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Verstrepen, L. , and Beyaert R.. 2014. Receptor proximal kinases in NF‐kappaB signaling as potential therapeutic targets in cancer and inflammation. Biochem. Pharmacol. 92:519–529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. related to figure 1 GABABR regulates the proliferation of HCT116 cells.
Figure S2. related to figure 2. GABABR signaling repressed HCT116 cell proliferation by inhibiting GSK‐3β activation.
Figure S3. related to figure 3. GSK‐3β/NF‐κB signaling regulates the HCT116 cell proliferation.
Figure S4. related to figure 4. GABABR/NF‐κB signaling pathway regulates the proliferation in HCT116 cells.


