Abstract
Background:
Single Widal agglutination test rather than blood culture, is commonly employed to diagnose typhoid fever in Nigeria. We took another look at the Widal agglutination test as a preferred option for diagnosis of typhoid fever by determining the specificity and sensitivity of Widal agglutination test in febrile adult patients.
Materials and Methods:
Two hundred and seventy-one blood samples from consecutive adults (>18 years) with febrile illness attending the General Practice Clinic of the University of Benin Teaching Hospital were tested using the Widal agglutination test, blood culture, and malaria parasite test on each sample to establish the diagnosis of typhoid fever.
Results:
Of the 271 blood samples 124 (45.76%) were positive following a Widal agglutination test, 60 (22.10%) blood samples grew Salmonella organisms on blood culture while 55 (20.29%) blood samples showed a co-infection of typhoid fever and malaria. A sensitivity of 35%, specificity of 51%, positive predictive value of 17%, and a negative predictive value of 73% were observed for Widal agglutination test as a diagnostic modality for typhoid fever infection.
Conclusion:
A single Widal agglutination test is not a valid diagnostic option for typhoid fever while co-infection with malaria parasite is the preponderant microbiological finding in typhoid fever infections. The severity of malaria parasitemia is associated with positive titers on Widal test.
Keywords: Co-infection, febrile illness, malaria, typhoid fever
INTRODUCTION
Fever is a common cause for consultation in most healthcare facilities in the tropics and subtropics,1 with infections accounting for most causes of fever.1,2 In the tropics, malaria and typhoid fever are among the common infectious diseases, with both being endemic in Nigeria.3,4,5
Typhoid fever is a life-threatening systemic infection6 caused by the bacterium, Salmonella typhi,7,8,9,10,11 a Gram-negative, motile, aerobic, nonsporing, intracellular Bacillus.12 It is a global public health problem with an estimated 21.6 million new cases of typhoid fever and 216,500 deaths recorded globally in 2000.7,8 This is especially worse in the developing nations of the world where it is a significant contributor to morbidity and mortality.11
Typhoid fever is spread by the faeco-oral route9,12,13 and commonly presents with nonspecific clinical features such as fever, headache, rigors, joint pain, nausea, vomiting, constipation, and diarrhea which are indistinguishable from other causes of fever such as malaria.1,3,7,14,15,16 The presence of clinical symptoms characteristic of typhoid fever or the detection of a specific antibody response is only suggestive of typhoid fever but not definitive.13,17 Culture and isolation of S. typhi is the gold standard for the diagnosis of typhoid fever.5,8,10,13,18,19,20,21,22,23 Blood culture is the most definitive method of diagnosing typhoid fever, especially in the 1st week of infection.2,5,13,18 About 80–90% of patients are likely to have positive blood cultures during the period of established disease, especially during the 1st week of illness.5,24
In most parts of Africa, Nigeria inclusive, the Widal agglutination test (despite its limitations) is the most common diagnostic tool employed in the diagnosis of typhoid fever because of its relatively cheap cost and the fact that it is easy to perform and requires minimal training and equipment.25,26 However, a number of medical practitioners have often raised alarm on the apparently “high rate” of typhoid fever diagnosed in healthcare facilities in Nigeria and other parts of Africa.3,5,24,25
This apparently “high rate” of typhoid fever may be due to the use and poor interpretation of the Widal test in making a diagnosis of typhoid fever.27,28,29
In view of the apparently “high rate” of typhoid fever, the controversial nature of the Widal agglutination test, and the dearth of studies on the validity of the Widal agglutination test as a diagnostic tool in the University of Benin Teaching Hospital (UBTH), this study was conducted to evaluate the validity of the Widal agglutination test as a diagnostic tool for typhoid fever by comparing it with blood culture (a standard method for diagnosing typhoid fever).
MATERIALS AND METHODS
This was a descriptive cross-sectional study carried out in the General Practice Clinic of the UBTH, Nigeria, after an ethical approval from the Ethics and Research Committee of the Teaching Hospital. The study population comprised febrile adult patients.
Blood samples were tested using Widal agglutination test. The samples were also cultured for Salmonella organisms while microscopy of thick blood film for malaria parasite was carried out.
Data were collected on a proforma and analyzed using the SPSS version 10 statistical software (SPSS version 10, Chicago, IL). Results were presented in statements and charts. Statistical significance was set at P < 0.05.
RESULTS
Table 1 shows the relationship between Widal agglutination test and blood culture diagnostic method, with the Widal agglutination test having a sensitivity of 0.35 (35%), specificity of 0.51 (51%), positive predictive value (PPV) of 0.17 (17%), and a negative predictive value (NPV) of 0.73 (73%).
Table 1.
Relationship between Widal test and blood culture method of diagnosis of typhoid fever
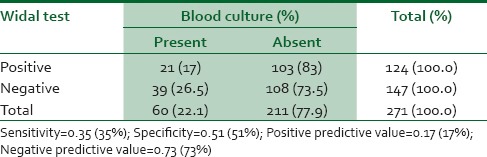
Table 2 shows the relationship between malaria parasite infection and Widal agglutination test results among respondents. With an increase in malaria parasitemia, there was an increase in the proportion of blood samples with significant Salmonella antibody titers using the Widal agglutination test. There was a statistically significant association between the severity of malaria parasitemia and a positive Widal agglutination test (P = 0.006).
Table 2.
Relationship between malaria parasite infection and Widal test among respondents
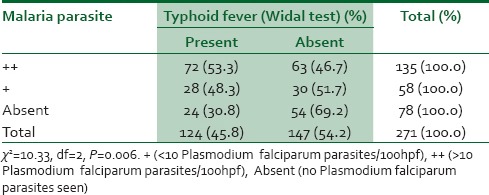
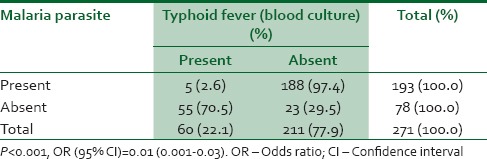
Table 3 shows the relationship between presence of malaria parasite and a positive blood culture for typhoid fever infection. From the data, it would seem that having malaria parasite infection does not presuppose a co-existing typhoid fever infection odds ratio (95% confidence interval [CI]) = 0.01 (0.001–0.03).
Table 3.
Relationship between presence of malaria parasite and typhoid fever infection among respondents
DISCUSSION
This study was aimed at determining the validity of the Widal agglutination test as a preferred option for the diagnosis of typhoid fever infection among adult febrile patients at the Family Medicine Department of the UBTH, Nigeria.
Comparing the Widal agglutination test results with the blood culture results, it was found that out of 124 (45.8%) study subjects that had a positive Widal agglutination test (using a cut-off Salmonella antibody titre of >1:80, in use in the Medical Microbiology Department of UBTH), 21 (17%) had blood culture confirmation of typhoid fever. However, out of 147 (54.2%) study subjects that had negative Widal agglutination test, 39 (26.5%) had blood culture confirmation of typhoid fever.
The calculated sensitivity of Widal test as a diagnostic tool for typhoid fever was 35%, with a 95% CI of 0.2312-0.4836. This shows that Widal agglutination test has a low sensitivity and a 35% ability to correctly identify individuals who actually have typhoid fever.
The calculated specificity of Widal agglutination test was 51%, with a 95% CI of 0.4425–0.5807. This shows that Widal agglutination test has a 51% ability to correctly identify individuals who do not have typhoid fever.
The calculated PPV of Widal agglutination test was 17%, with a 95% CI of 0.1080–0.2469. This shows that Widal agglutination test has a low PPV, with only a 17% ability to accurately determine the proportion of those who are truly positive for typhoid fever.
The calculated NPV of Widal test was 73%, with a 95% CI of 0.6557–0.8037. This shows that Widal test has a high NPV, with a 73% ability to accurately determine the proportion of those who are truly negative for typhoid fever.
The above findings are of great relevance, especially against the backdrop of controversies plaguing the use of Widal test in the diagnosis of typhoid fever.
The validity of a diagnostic tool mainly depends on its sensitivity and PPV. The low (35%) sensitivity and low (17%) PPV of the Widal agglutination test found in this study clearly demonstrate that a single Widal agglutination test is not a valid diagnostic tool for typhoid fever. This is also supported by findings from other studies which cast doubt on the validity and use of the Widal agglutination test.8,13,25,27,30,31
In determining the relationship between Widal agglutination test and malaria parasite infection, it was observed that there was a significant statistical association (P < 0.05) between the presence of malaria parasites and a positive Widal agglutination test. Out of a total of one hundred and ninety-three study subjects that had a positive malaria parasite test, 100 (52.4%) had a positive Widal agglutination test, while only 5 (2.6%) had typhoid infection confirmed by blood culture.
The high prevalence of malaria parasitemia found in this study may imply that the presence of malaria parasites in a patient could produce a positive Widal test result. This is supported by findings from previous studies which documented the fact that the presence of malaria parasite in a patient could produce a false positive Widal test result25,27 on account of the fact that some undefined antigenic determinants possessed by the malaria parasite could cross-react with those of S. typhi, thus producing Salmonella antibodies. It may also explain the apparently “high rate” of typhoid fever diagnosed in private healthcare facilities in Benin City on the basis of a single Widal test result.
In determining the relationship between malaria parasite infection and typhoid fever infection (as confirmed by blood culture), it was found that there was a significant statistical difference (P < 0.05) between those who had only typhoid or malaria infection alone and those who had both typhoid fever and malaria co-infection. Out of 193 study subjects who had a positive malaria parasite test, only 5 (2.6%) had typhoid fever infection (as confirmed by blood culture), while 188 (97.4%) had no blood culture confirmation of typhoid fever infection. Of the 78 respondents (28.8%) that had negative malaria parasite test, 55 (70.5%) of them had blood culture confirmation of typhoid fever infection, while 23 (29.5%) had no blood culture confirmation of typhoid fever infection.
This shows that there is a statistically significant difference between those who had malaria and typhoid fever co-infection and those who had only malaria or typhoid fever infection alone, with most of the individuals likely to have either typhoid fever infection or malaria alone than malaria and typhoid fever co-infection.
The above findings and observations are supported by some previous studies such as the one conducted by Onuigbo24 who found that out of 15 patients clinically diagnosed as typhoid fever, 70% had malaria while the remaining 30% remitted spontaneously. Similarly, in a study to determine the prevalence of typhoid fever in febrile patients with symptoms clinically compatible with typhoid fever in Cameroon, Nsutebu et al.1 diagnosed malaria in 47% of the two hundred patients recruited for the study. In a study conducted in Nigeria, Smith et al.32 found malaria parasites in 64.3% of the patients recruited for the study. However, Tanyigna et al.33 found malaria parasites in 27.4% of study patients. Akinyemi et al.34 in their evaluation of blood collected from clinically diagnosed typhoid fever patients in the metropolis of Lagos, Nigeria, found malaria in 37.2% of the study subjects.
CONCLUSION
This study recommends the need to discourage exclusive reliance on a single Widal agglutination test for the diagnosis of typhoid fever, as it is not evidence based. All febrile patients suspected to have typhoid fever should be properly evaluated and investigated by Physicians with bacteriological culture methods, as bacteriological isolation of S. typhi remains the most accurate means of confirming typhoid fever infection.
There is an overwhelming need for government, professional bodies, media houses, and other stakeholders to organize and promote public health enlightenment programs and campaigns to massively educate and enlighten members of the public on typhoid fever infection. Such programs and campaigns will greatly help to enlighten the general public on the use of valid diagnostic tools in the diagnosis and management of typhoid fever, and thus prevent them from being exploited or exposed to the complications of misdiagnosis or poor management of their febrile illnesses.
Compared with the blood culture as a diagnostic test for typhoid fever infection, this study found that the Widal agglutination test as a diagnostic modality for typhoid fever infection had a low sensitivity (35%) and a low PPV (17%).
The low sensitivity (35%) and low PPV (17%) of the Widal agglutination test, cast doubt on the validity of the Widal agglutination test as a preferred option for the diagnosis of typhoid fever infection.
On account of the above findings, this study concludes that a single Widal agglutination test is not a valid diagnostic option for typhoid fever.
Limitations
This study may be limited by the fact that other bacteriological cultures such as stool, urine, and bone marrow aspirate cultures were not conducted, especially as bone marrow aspirate culture is known to give a high yield of Salmonella even when blood and urine cultures of patients treated with antibiotics are negative.8
In addition, the findings of this study may unlikely be reflective of the findings in the community on account of the fact that it was a hospital based study. Further researches, particularly community-based studies, are therefore recommended.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We wish to acknowledge Dr. Damien D. Uyagu, FMCGP, Dr. Matie Obazee, FWACP, FMCGP, and Dr. (Mrs.) Joan E. Enabulele, FMCDS for their general support.
REFERENCES
- 1.Nsutebu EF, Martins P, Adiogo D. Prevalence of typhoid fever in febrile patients with symptoms clinically compatible with typhoid fever in Cameroon. Trop Med Int Health. 2003;8:575–8. doi: 10.1046/j.1365-3156.2003.01012.x. [DOI] [PubMed] [Google Scholar]
- 2.Falase AO, Akinkugbe OO, editors. A Compendium of Clinical Medicine. 2nd ed. Ibadan: Spectrum Books Limited; 2002. Infections and infestations; p. 799. [Google Scholar]
- 3.Ngwu BA, Agbo JA. Typhoid fever: Clinical diagnosis versus laboratory confirmation. Niger J Med. 2003;12:187–92. [PubMed] [Google Scholar]
- 4.Edino ST, Mohammed AZ, Uba AF, Sheshe AA, Anumah M, Ochicha O, et al. Typhoid enteric perforation in north western Nigeria. Niger J Med. 2004;13:345–9. [PubMed] [Google Scholar]
- 5.Ekenna O. Typhoid fever: Problems of accurate laboratory diagnosis and antimicrobial therapy. Niger Med J. 1992;23:93–9. [Google Scholar]
- 6.Sherwal BL, Dhamija RK, Randhawa VS, Jais M, Kaintura A, Kumar MA. A comparative study of typhidot and widal test in patients of typhoid fever. J Indian Acad Clin Med. 2004;5:244–6. [Google Scholar]
- 7.Parry CM, Hoa NT, Diep TS, Wain J, Chinh NT, Vinh H, et al. Value of a single-tube widal test in diagnosis of typhoid fever in Vietnam. J Clin Microbiol. 1999;37:2882–6. doi: 10.1128/jcm.37.9.2882-2886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhan MK, Bahl R, Bhatnagar S. Typhoid and paratyphoid fever. Lancet. 2005;366:749–62. doi: 10.1016/S0140-6736(05)67181-4. [DOI] [PubMed] [Google Scholar]
- 9.David BH, Herbert LD. Problem pathogens: Extra-intestinal complications of Salmonella enterica serotype typhi infection. Lancet Infect Dis. 2005;5:341–8. doi: 10.1016/S1473-3099(05)70138-9. [DOI] [PubMed] [Google Scholar]
- 10.Willke A, Ergonul O, Bayar B. Widal test in diagnosis of typhoid fever in Turkey. Clin Diagn Lab Immunol. 2002;9:938–41. doi: 10.1128/CDLI.9.4.938-941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centres for Disease Control and Prevention, USA. Typhoid fever: Frequently Asked Questions. [Last accessed on 2015 Dec 28]. Available from: http://www.cdc.gov/ncidod/dbmd/diseaseinfo/files/typhoid_fever_FAQ.pdf .
- 12.Lucas AO, Gilles HM. Communicable diseases: Infections through the gastrointestinal tract. In: Lucas AO, Gilles HM, editors. Short Textbook of Public Health Medicine for the Tropics. 4th rev ed. London: Arnold Publishers; 2003. p. 59. [Google Scholar]
- 13.World Health Organization. WHO/V and B/03.07. Geneva: World Health Organization; 2003. Background Document: The Diagnosis, Treatment and Prevention of Typhoid Fever. [Google Scholar]
- 14.Agbonlahor DE, Aghahowa MO, Idukpaye O, Agbonlahor FE, Ekundayo AO, Emele FE, et al. Baseline typhoidal antibody levels in apparently healthy Nigerians. Niger Q J Hosp Med. 1997;7:242–5. [Google Scholar]
- 15.Gasem MH, Smits HL, Goris MG, Dolmans WM. Evaluation of a simple and rapid dipstick assay for the diagnosis of typhoid fever in Indonesia. J Med Microbiol. 2002;51:173–7. doi: 10.1099/0022-1317-51-2-173. [DOI] [PubMed] [Google Scholar]
- 16.Fadeel MA, Crump JA, Mahoney FJ, Nakhla IA, Mansour AM, Reyad B, et al. Rapid diagnosis of typhoid fever by enzyme-linked immunosorbent assay detection of Salmonella serotype typhi antigens in urine. Am J Trop Med Hyg. 2004;70:323–8. [PubMed] [Google Scholar]
- 17.National Institute for Communicable Diseases. Typhoid fever update. Communicable Diseases Communiqué. 2005;4:1–3. [Google Scholar]
- 18.Singh S. Typhoid fever, pathogenesis and laboratory diagnosis. J Indian Acad Clin Med. 2001;2:17–20. [Google Scholar]
- 19.Jawetz E, Melnick JL, Adelberg EA. Review of Medical Microbiology. 21st ed. New York: McGraw-Hill, Lange Medical Publications; 1998. [Google Scholar]
- 20.House D, Wain J, Ho VA, Diep TS, Chinh NT, Bay PV, et al. Serology of typhoid fever in an area of endemicity and its relevance to diagnosis. J Clin Microbiol. 2001;39:1002–7. doi: 10.1128/JCM.39.3.1002-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhutta ZA, Mansurali N. Rapid serologic diagnosis of pediatric typhoid fever in an endemic area: A prospective comparative evaluation of two dot-enzyme immunoassays and the widal test. Am J Trop Med Hyg. 1999;61:654–7. doi: 10.4269/ajtmh.1999.61.654. [DOI] [PubMed] [Google Scholar]
- 22.Noorbakhsh S, Rimaz S, Rahbarimanesh AA, Mamishi S. Interpretation of the widal test in infected children. Iran J Public Health. 2003;32:35–7. [Google Scholar]
- 23.Olsen SJ, Pruckler J, Bibb W, Nguyen TM, Tran MT, Nguyen TM, et al. Evaluation of rapid diagnostic tests for typhoid fever. J Clin Microbiol. 2004;42:1885–9. doi: 10.1128/JCM.42.5.1885-1889.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onuigbo MC. Typhoid fever epidemic in Nigeria? The abuse of the Widal test and the antibiotic chloramphenicol. Niger Med Practit. 1989;18:23–5. [Google Scholar]
- 25.Nsutebu EF, Ndumbe PM. The Widal test for typhoid fever: Is it useful? Afr Health. 2001;23:5–9. [Google Scholar]
- 26.Pak-Leong L, Frankie CH, Yuet MC, Jegathesan M. One-step 2-minute test to detect typhoid – Specific antibodies based on particle separation in tubes. J Clin Microbiol. 1998;36:2271–8. doi: 10.1128/jcm.36.8.2271-2278.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olopoenia LA, King AL. Widal agglutination test – 100 years later: Still plagued by controversy. Postgrad Med J. 2000;76:80–4. doi: 10.1136/pmj.76.892.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oviasogie FE, Eghafona ON, Agbonlahor DE, Enabulele IO, Orhue PO, Nwobu GO. Use of commercial and locally prepared antigens in the diagnosis of enteric fever. J Appl Basic Sci. 2003;1:23–6. [Google Scholar]
- 29.Isibor JO, Ogbomoiko DO, Nwobu GO, Agwu E, Okogun GRA, Anyanlere M. Serological diagnosis of enteric fever. J Appl Basic Sci. 2003;1:13–6. [Google Scholar]
- 30.Levine MM, Grados O, Gilman RH, Woodward WE, Solis-Plaza R, Waldman W. Diagnostic value of the Widal test in areas endemic for typhoid fever. Am J Trop Med Hyg. 1978;27:795–800. doi: 10.4269/ajtmh.1978.27.795. [DOI] [PubMed] [Google Scholar]
- 31.Alaribe AA, Ejezie GC, Ezedinachi EU. The prevalence of salmonella antibody among malaria patients in Calabar. J Med Lab Sci. 1998;7:34–41. [Google Scholar]
- 32.Smith SI, Odunukwe NN, Niemogha MT, Ahmed AO, Efienemokwu CA, Otuonye MN, et al. Diagnostic methods for typhoid fever in Nigeria. Br J Biomed Sci. 2004;61:179–81. doi: 10.1080/09674845.2004.11732667. [DOI] [PubMed] [Google Scholar]
- 33.Tanyigna KB, Bello CS, Okeke N, Onwukeme KE. Comparison of blood, bone marrow aspirate, stool and urine cultures in the diagnosis of enteric fever. Niger J Med. 2001;10:21–4. [PubMed] [Google Scholar]
- 34.Akinyemi KO, Oyefolu AO, Omonigbehin EO, Akinside KA, Coker AO. Evaluation of blood collected from clinically diagnosed typhoid fever patients in the metropolis of Lagos, Nigeria. J Niger Infect Control Assoc. 2000;3:25–30. [Google Scholar]


