Abstract
Objective
AIMS65 is a score designed to predict in-hospital mortality, length of stay, and costs of gastrointestinal bleeding. Our aims were to revalidate AIMS65 as predictor of inpatient mortality and to compare AIMS65’s performance with that of Glasgow–Blatchford (GBS) and Rockall scores (RS) with regard to mortality, and the secondary outcomes of a composite endpoint of severity, transfusion requirements, rebleeding, delayed (6-month) mortality, and length of stay.
Methods
The study included 309 patients. Clinical and biochemical data, transfusion requirements, endoscopic, surgical, or radiological treatments, and outcomes for 6 months after admission were collected. Clinical outcomes were in-hospital mortality, delayed mortality, rebleeding, composite endpoint, blood transfusions, and length of stay.
Results
In receiver-operating characteristic curve analyses, AIMS65, GBS, and RS were similar when predicting inpatient mortality (0.76 vs. 0.78 vs. 0.78). Regarding endoscopic intervention, AIMS65 and GBS were identical (0.62 vs. 0.62). AIMS65 was useless when predicting rebleeding compared to GBS or RS (0.56 vs. 0.70 vs. 0.71). GBS was better at predicting the need for transfusions. No patient with AIMS65 = 0, GBS ≤ 6, or RS ≤ 4 died. Considering the composite endpoint, an AIMS65 of 0 did not exclude high risk patients, but a GBS ≤ 1 or RS ≤ 2 did. The three scores were similar in predicting prolonged in-hospital stay. Delayed mortality was better predicted by AIMS65.
Conclusion
AIMS65 is comparable to GBS and RS in essential endpoints such as inpatient mortality, the need for endoscopic intervention and length of stay. GBS is a better score predicting rebleeding and the need for transfusion, but AIMS65 shows a better performance predicting delayed mortality.
Keywords: Upper gastrointestinal bleeding, AIMS65, Glasgow–Blatchford score, Rockall score
Introduction
Gastrointestinal (GI) bleeding results in 5% of admissions to an emergency department,1 with mortality rates ranging from 2 to 15%.2 Factors found to be predictors of mortality include advanced age, low hemoglobin level, low blood pressure, severe comorbidities, worsening health status, rebleeding, hypoalbuminemia, elevated creatinine, elevated serum aminotransferase levels, onset of bleeding while inpatient, and active bleeding or stigmata of recent hemorrhage at the time of endoscopy.3–9
Multiple scoring systems have been developed to predict the outcomes of these patients.6–8,10–12 The most common one, designed to predict in-hospital death, is the Rockall score (RS), application of which in clinical practice is complex because it includes many variables. The search for a pre-endoscopic clinically applicable score to predict high- and low-risk patients has led to the development of other scores, such as the Glasgow–Blatchford score (GBS) and,10 recently, the AIMS65 score.13
The GBS is based on clinical and laboratory parameters. It was originally developed to predict the need for clinical interventions.10,14–17 Nevertheless, further studies have shown the utility of the GBS in predicting death, blood transfusion, endoscopic intervention, and surgery.14–18
The main drawback of the GBS and the RS is their difficult day to day application, which has reduced their role to research protocols. Thus, the AIMS65 has recently been validated to predict in-hospital mortality, length of stay, and costs.13 AIMS65 has some important advantages: first, it has been developed from a large database and is not weighted, which makes it easy to remember in everyday practice; second, it does not rely on patient’s medical history, but on laboratory values in addition to the patient’s mental status. From its development, some papers have compared the score with the GBS, showing that it was almost equivalent to the GBS.19–22 Nevertheless, the only prospective study did not compare AIMS65 with the RS and it was specially focused on detection of low-risk patients who could benefit from an early discharge.
Moreover, all the previous studies have focused on in-hospital or 30 days mortality, but the clinical deterioration that an acute episode of GI hemorrhage provokes to patients might cause delayed mortality. Indeed, changes in medication, mobility, and habits associated with hospital admissions might unbalance an otherwise difficult equilibrium in patients in which GI hemorrhage might be just an event, part of the outcome of a systemic disease, for which severity could be predicted.
To our best knowledge, the three systems have not been compared, nor has the impact on delayed 6 month mortality been explored in a prospective series.
Our aim was to revalidate AIMS65 as predictor of inpatient and 6-months mortality in a European population. Our secondary goal was to compare the AIMS65 score’s performance with that of the GBS and RS with regards to the secondary outcomes of (a) a composite clinical endpoint of severity; (b) blood transfusion requirements; (c) rebleeding; (d) in-hospital and delayed mortality; and (e) hospital length of stay.
Methods
Study design and population
This was a prospective study on consecutive patients admitted to the “Virgen de las Nieves” University Hospital for the management of upper GI hemorrhage over 24 months (January 2013–January 2015). Upper GI hemorrhage was defined as bleeding from the upper GI tract as manifested as hematemesis and/or melena. Rebleeding was defined by the presence of fresh hematemesis and/or melena associated with the development of shock or a reduction in hemoglobin concentration greater than 2 g/dL over 24 h. Rebleeding included also cases requiring repeated endoscopy, surgery or any interventional radiology procedure.
Patients who refused endoscopy or who refused to sign the informed consent were excluded. Patients were followed during hospitalization and six months after discharge. All of them underwent endoscopy. The timing of endoscopy and the need for endoscopic therapy were determined by the on-call gastroenterologist. The need for transfusion was determined by the treating physicians, who per protocol followed strict criteria previously published.23 These criteria were different when active variceal bleeding was suspected. In those patients a lower threshold of 8 g/dL in hemoglobin levels was established. All patients received high-dose acid suppression therapy (pantoprazole 80 mg intravenously as an initial bolus followed by a continuous infusion of 120 mg for the first 24 hours). The study protocol was approved by the local Human Research Ethics Committee.
Data collection
Information regarding patients’ demographic data, comorbidities, current medications, clinical presentation, laboratory tests, and endoscopic findings was collected. Interventions were recorded, including the need for blood transfusion and the number of packed red cells units per patient, endoscopic therapy, radiologically guided hemostasis, and surgery.
A patient was considered to have a change in mental status if the Glasgow Coma Scale score on presentation was less than 14.
Clinical outcomes documented were in-hospital mortality, delayed 6-months mortality, and rebleeding. As secondary outcomes we studied (a) a composite endpoint of inpatient mortality, in-hospital rebleeding, and endoscopic, radiologic, or surgical intervention, (b) transfusion requirements, and (c) length of stay.
The collected data were used to calculate the GBS, the full RS, and the AIMS65 score. The methods for calculating the scores were as described previously.5,6,13
Data analysis
Statistical analysis was carried out using the software PAWS Statistics 17.0 (SPSS Inc, Chicago, IL, USA).Comparison between the different groups were performed by using the Fisher exact test or the t-student test as appropriate. Correlations between variables were assessed with the Spearman test. The area under the receiver-operating characteristic curve (AUROC) was calculated for each score and binomial outcome, with binomial intervals. AUROCs were tested for equality by means of the Delong χ2 test. All p values were two-sided with the value of 0.05 considered to be the threshold for statistical significance.
Results
Patient characteristics
309 patients (214 men; aged 64.6 ± 16.7 years) were admitted with a diagnosis of upper GI hemorrhage over 24 months. Presenting symptoms were melena (72.2%), hematemesis (48.9%), and hematochezia (8.1%). Endoscopy was performed in all of them, normally within a maximum of 8 hours after admission. A total of 126 patients (40.8%) received endoscopic therapy, which consisted in adrenaline and a sclerosing agent (polidocanol) injection in 88, argon plasma coagulation in 8, hemostatic clipping in 19, variceal banding in 31, balloon tamponade in 3, cyanoacrylate glue injection (Glubran2®; GEM Srl, Viareggio, Italy) in 1, and haemostatic powder application (Hemospray™; Cook Medical, Winston-Salem, NC, USA) in 6. Blood transfusion was needed in 192 patients (62.1%). Overall in-hospital mortality was 9.4% (n = 29) but the GI hemorrhage specific mortality rate was n = 20 (6.4%). The main endoscopic findings were duodenal ulcer (26.9%), esophageal varices (21.4%) gastric ulcer (17.5%), acute gastric erosions (16.2%), esophagitis (11.7%), Mallory–Weiss tears and esophageal ulcers (6.5%), angiodysplasia (5.2%), neoplasms (4.1%), and unidentified source (14.2%) (Table 1). We observed rebleeding in 14 patients, and bleeding persistence in 48. All of them had a repeated endoscopy, but 7 underwent surgery and 2 had an embolization. Two patients with no endoscopic treatment rebled. However, 18 patients with persistent bleeding died (37.5%), but only three received surgery or embolization, because most of them were patients in a poor clinical condition. Regarding the composite endpoint, 135 patients (43.7%) were included in the high risk group. In this group, 29 patients died in the admission (21.5%) 106 patients (78.5%) required transfusion, 125 (92.6%) underwent endoscopic intervention, and 15 (11%) had a delayed mortality within six months after discharge.
Table 1.
Patient characteristics
| Male | 214 | 69.3% |
|---|---|---|
| Medical history | ||
| Cirrhosis | 85 | 27.5% |
| Chronic lung diseases | 32 | 10.4% |
| Chronic renal disease | 31 | 10% |
| Heart failure | 41 | 13.3% |
| Myocardial infarction | 40 | 12.9% |
| Atrial fibrillation | 48 | 15.5% |
| Previous stroke | 29 | 9.4% |
| Previous GI bleeding events | 79 | 25.6% |
| Hypertension | 110 | 35.6% |
| Diabetes | 75 | 24.3% |
| Peripheral vascular disease | 16 | 5.2% |
| Neoplasm | 37 | 12% |
| Smoking habit | 68 | 22% |
| Alcoholic habit | 80 | 25.9% |
| Medication | ||
| NSAIDS | 77 | 24.9% |
| Aspirin | 46 | 14.9% |
| Clopidogrel | 12 | 3.9% |
| Oral anticoagulants | 53 | 17.2% |
| Steroids | 9 | 2.9% |
| Immunosuppressants | 14 | 4.5% |
| Score components | ||
| Age (years) | 65 | 51–78 |
| Albumin (g/dL) | 3.1 | 2.7–3.7 |
| International normalized ratio | 1.48 | 1.1–1.5 |
| Systemic blood pressure | 111 | 95–126 |
| Pulse | 90 | 75–102 |
| Hemoglobin | 9.7 | 7.8–11.6 |
| Urea | 81.31 | 44.5–94.5 |
| Hematemesis | 151 | 48.9% |
| Melena | 223 | 72.2% |
| Hematochezia | 25 | 8.1% |
| Mental status change | 25 | 8.1% |
| Syncope | 45 | 14.6% |
Proportions are presented as percentage. Continuous variables are presented as median (interquartile range).
Performance of the scores
Patients who had endoscopic intervention, received blood transfusion, had inpatient death or in the first 6 months had significantly higher AIMS65, GBS, and RS. GBS and RS were significantly higher in patients who rebled, but not AIMS65 (Table 2).
Table 2.
Comparison of AIMS65, GBS, and RS in different outcome variables
| AIMS65 | GBS | RS | p value | |
|---|---|---|---|---|
| Endoscopic intervention (n = 126) | 1.77 ± 0.1 | 11 ± 0.4 | 6.4 ± 0.2 | |
| No endoscopic intervention (n = 183) | 1.28 ± 0.08 | 9.1 ± 0.33 | 4.1 ± 0.17 | <0.0001 vs. endoscopic intervention |
| Transfusion (n = 192) | 1.8 ± 0.8 | 11.9 ± 0.2 | 5.7 ± 0.2 | |
| No transfusion (n = 117) | 0.97 ± 0.1 | 6.5 ± 0.4 | 3.8 ± 0.2 | <0.0001 vs. transfusion |
| Died in the acute episode (n = 29) | 2.4 ± 0.2 | 13.7 ± 0.6 | 7.1 ± 0.3 | |
| Survived the acute episode (n = 280) | 1.4 ± 0.06 | 9.5 ± 0.2 | 4.8 ± 0.1 | <0.0001 vs. died |
| Died within 6 months (n = 32) | 2.2 ± 0.2 | 11.7 ± 0.7 | 6.3 ± 0.3 | |
| Survived after 6 months (n = 241) | 1.3 ± 0.07 | 9.3 ± 0.3 | 4.6 ± 0.15 | <0.0001 vs. died |
| Rebleeding (n = 13) | 1.85 ± 0.4 | 12.7 ± 0.7 | 6.8 ± 0.5 | |
| No rebleeding (n = 296) | 1.5 ± 0.06** | 9.8 ± 0.3 | 4.9 ± 0.1 | <0.02 vs. rebleeding. **n.s. (p = 0.4) |
GBS, Glasgow–Blatchford score; RS, Rockall score. Values are expressed as mean ± standard deviation.
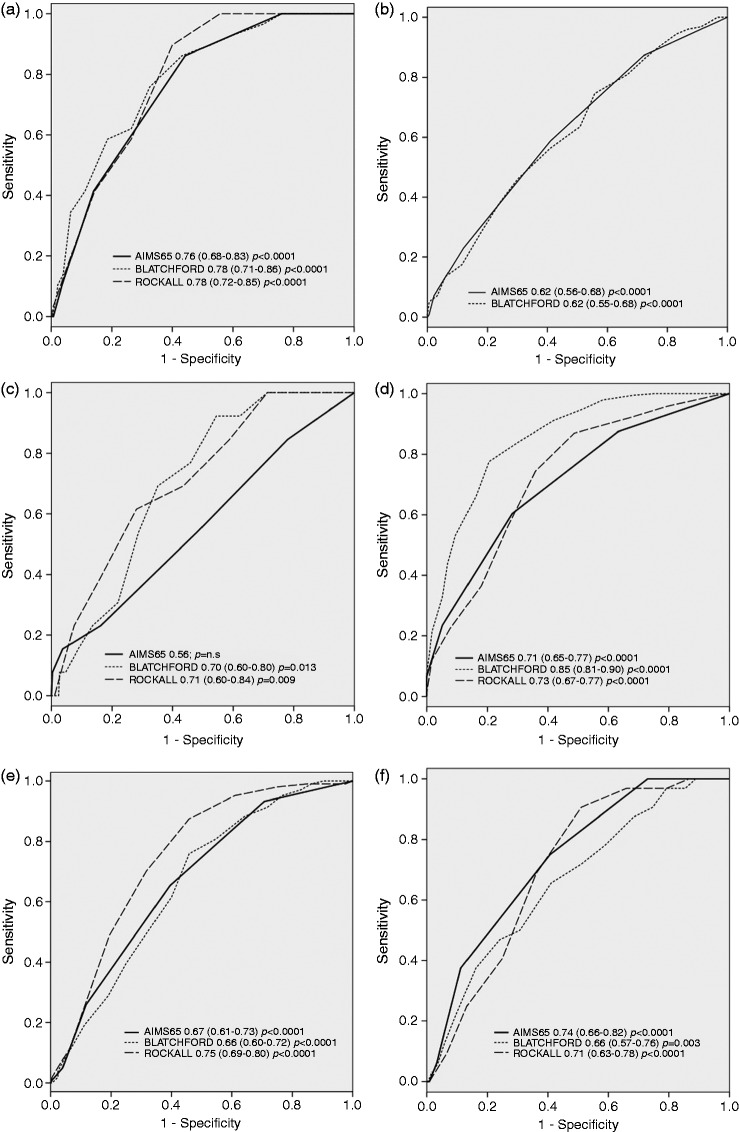
On ROC analyses, AIMS65, GBS, and RS were similar when predicting inpatient mortality (0.76 vs. 0.78 vs. 0.78), finding no differences when comparing the three curves (Figure 1(a)). Regarding endoscopic intervention, AIMS65 and GBS were almost identical (0.62 vs. 0.62) (Figure 1(b)), but in our series AIMS65 proved to be useless when predicting rebleeding compared to GBS or RS (0.56 vs. 0.70 vs. 0.71) (Figure 1(c)). The three scores were very useful when predicting the need for blood transfusions (0.71 vs. 0.85 vs. 0.73), although GBS showed a significantly better performance than the other two scores when the curves were compared (p < 0.03 vs. AIMS65 and p < 0.05 vs. RS) (Figure 1(d)).
Figure 1.
Receiver-operating characteristic curves (ROCs) for the AIMS65, Glasgow–Blatchford, and Rockall risk scores as predictors of (a) inpatient mortality, (b) endoscopic intervention, (c) rebleeding, (d) need for transfusions, (e) length of hospital stay >7 days, and (f) delayed (6 month) mortality.
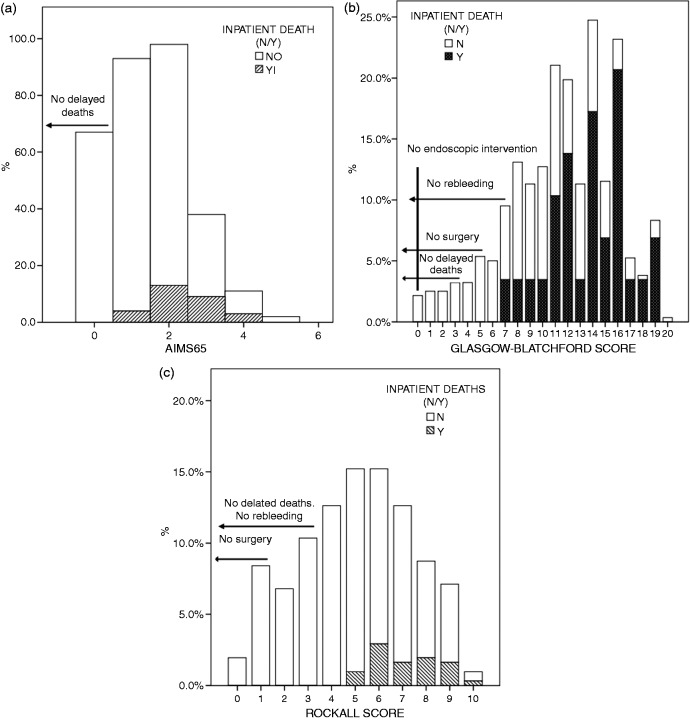
No patient with AIMS65 = 0, GBS ≤ 6, or RS ≤ 4 died. A GBS = 0 warranted no endoscopic therapy, but not AIMS65 = 0. Only a GBS ≤ 3 marked no transfusion requirements, but neither AIMS65 nor RS lower levels warranted no transfusions. No patient with a GBS ≤ 7 or a RS ≤ 2 rebled. GBS ≤ 3 or a RS ≤ 1 were cutoff points under which no surgeries were needed (Figure 2(a), (b), and (c)). When considering the composite endpoint, an AIMS65 = 0 did not excluded patients which were considered high risk, but a GBS ≤ 1 or RS ≤ 2 did.
Figure 2.
Histogram of patients’ (a) AIMS65, with inpatient deaths and delayed deaths, and (b) Glasgow–Blatchford and (c) Rockall scores with inpatient deaths, endoscopic intervention, and delayed deaths.
Mean length of in-hospital stay was 7.7 ± 8.25 days. The three scores predicted length of stay without differences. When considering the prediction of a prolonged in-hospital stay ( >7 days), the three scores had similar AUROCs (Figure 1(e)). We calculated Spearman correlation coefficient finding a higher correlation of RS (r = 0.52; p < 0.0001) with in-hospital stay than GBS (r = 0.35; p > 0.0001) and AIMS65 (r = 0.34; p < 0.0001).
The different cutoff points that maximized the sum of the sensitivity and specificity for each outcome variable are shown in Table 3.
Table 3.
Cutoff points for the different outcome variables
| AIMS65 | GBS | RS | |
|---|---|---|---|
| Inpatient cmortality | 1 (S: 100%; E: 24%; PPV: 12%; NPV: 100%) | 12 (S: 76%; E: 68%; PPV: 19%; NPV: 96%) | 6 (S: 90%; E: 60%; PPV: 19%; NPV: 98%) |
| Endoscopic intervention | 1 (S: 87%; E: 28%; PPV: 45%; NPV: 76%) | 9 (S: 75%; E: 44%; PPV: 48%; NPV: 72%) | − |
| Rebleeding | − | 11 (S: 77%; E: 54%; PPV: 7%; NPV: 98%) | 6 (S: 69%; E: 56%; PPV: 7%; NPV: 98%) |
| Surgery | − | − | − |
| Transfusion | 1 (S: 88%; E: 37%; PPV: 69%; NPV: 64%) | 8 (S: 91%; E: 58%, PPV: 78%, NPV: 80%) | 4 (S: 87%; E: 51%; PPV: 75%; NPV: 71%) |
| Delayed mortality (6 mo) | 2 (S: 38%; E: 89%; PPV: 31%; NPV: 91%) | 10 (S: 72%; E: 49%; PPV: 16%; NPV: 93%) | 6 (S: 69%; E: 64%; PPV: 20%; NPV: 94%) |
| Composite endpoint | 1 (S: 88%; E: 29%; PPV: 49%; NPV: 76%) | 10 (S: 65%; E: 51%; PPV: 51%, NPV: 65%) | 5 (S: 82%; E: 57%; PPV: 60%; NPV: 81%) |
GBS: Glasgow–Blatchford Score; RS: Rockall score; S: sensitivity; E: specificity; PPV: positive predictive value; NPV: negative predictive value
Delayed mortality was accurately predicted by AIMS65 (AUC 0.74, p < 0.0001), better than GBS (AUC 0.66) or RS (AUC 0.71), with significant differences (p < 0.04) (Figure 1(f)). No patient with an AIMS65 = 0, GBS ≤ 3, or RS ≤ 3 had a delayed death (Table 4).
Table 4.
Causes of delayed (6 months) mortality
| N (%) | |
|---|---|
| Advanced neoplasms | 8 (25.1%) |
| Variceal bleeding | 7 (21.1%) |
| Stroke | 4 (12.6%) |
| Congestive heart failure | 3 (9.4%) |
| Recurrent non-variceal GI bleeding | 2 (6.4%) |
| Myocardial infarction | 2 (6.4%) |
| Advanced cirrhosis | 2 (6.4%) |
| Other | 4 (12.6%) |
Discussion
Our results show that AIMS65 is an adequate score for the prediction of inpatient mortality and the need for endoscopic intervention, comparable in these essential end-points to the previously developed scores, the RS and the GBS,4,10 but easier to calculate in daily clinical practice. Considering low-risk patients, an AIMS65 = 0 identified the ones who survived, excluding mortality, as well as a GBS ≤ 3 and a RS ≤ 3. Moreover, AIMS65 performed almost identically to GBS when selecting high-risk patients, when predicting mortality, length of stay, and the need for an endoscopic intervention. Despite the better performance of the GBS, its easy clinical application makes the AIMS65 a good option for some of the clinical outcomes to be predicted in clinical practice.
In this study, AIMS65 showed an acceptable performance when selecting low risk patients, and patients without the need of an endoscopic intervention, showing a better negative predictive value than the GBS in both items (Table 3). When selecting patients suitable for outpatient management, even without endoscopy, GBS has previously showed a good performance.14 This last aspect is important because early discharge of low-risk patients can avoid unnecessary admissions, reducing patients’ discomfort and hospital costs. Moreover, in centers in which endoscopists are not always available, the score can be useful when making the decision to delay endoscopy, just to perform the procedure during “normal” hours. In our study, an AIMS65 = 0 excluded mortality; however, it was unable to predict rebleeding, and even a score of 0 was not able to exclude the need for endoscopic intervention. On the other hand, GBS = 0 excluded endoscopic intervention. Therefore, AIMS65 is not a perfect score for low-risk patients, especially if the goal is to avoid endoscopy. These results are consistent with what has been reported in a series in which fifteen patients with and AIMS65 = 0 needed intervention.22 Similarly, in our series, 16 patients needed endoscopic therapy even when AIMS65 was 0. This also happened in a previous retrospective study,20 which advised caution when using AIMS65 for an early discharge of patients with low scores. In this last study the exclusion of patients without a peptic etiology could have biased the results. Indeed, when testing the validity of a pre-endoscopic score, the most important factor is whether it allows the correct classification of patients before intervention. Nevertheless, in our population, the exclusion of risks with regards to GBS is 0, which is a lower cutoff value than the suggested by previous authors,19,22,24 but coincidental with others.14,16
Anyway, our practice is to perform upper endoscopy to every patient with a consistent suspicion of upper GI bleeding. So, our concern with a score is its ability to select high risk patients, in which a different management would be needed. For low risk patients’ selection, in centers in which there is no available endoscopists, GBS is more accurate in the prediction of a good outcome.14,16,22,25,26
Regarding high-risk patients, AIMS65 was almost identical to GBS when predicting in-patient mortality as well as predicting the need for endoscopic intervention (Figures 1 and 2, Table 3). When considering rebleeding, both GBS and RS were useful, with AUC of 0.70 and 0.71, respectively, but AIMS65 showed no predictive ability. Regarding the need for transfusion, GBS was the best score; however, AIMS65 and RS also showed a significant predictive ability. The inclusion of hemoglobin levels in GBS, as previously suggested, may explain this difference.15,19,22 Considering the applicability of AIMS65, its good performance in predicting endoscopic intervention, inpatient mortality, and severity of upper GI hemorrhage makes it an alternative tool to GBS. It has an obvious drawback in the prediction of rebleeding, but post-endoscopic scores, such as the RS, can be applied at this stage.
Our results reassure our current practice of performing endoscopy to every patient with upper GI bleeding. Indeed, despite a GBS ≤ 0 ruled out endoscopic intervention, this has not been the case in some previous studies.22,25,26
The reasons to explain the differences in the cutoff values of GBS and AIMS65 compared with previous studies are unclear, and could be explained by some differences among them: population and ethnicity, etiology of bleeding, use of proton pump inhibitors (PPIs), timing of endoscopy, and adherence to guidelines regarding endoscopic therapy. Our cohort was predominantly white, of the same mean age as some of the previous reports.19,20,22,24 However, all of them included patients in which upper endoscopy was not performed because of their low risk profile,20,22,24 or the tendency toward lack of follow-up of low-risk patients.19 This could have prevented the researchers from finding some active bleedings that might have stopped spontaneously, and that we found in endoscopy, provided we performed it in every patient. Consistently, we did not have negative outcomes, such as surgery, mortality, or rebleeding in patients with a GBS ≤ 3 or a RS ≤ 3, but we had rebleeding, need for endoscopic intervention, and surgery with an AIMS65 of 0. Regarding the etiology of bleeding, our results are very similar to what has been previously reported.22,24 We included all of the causes of GI bleeding, even post-sphincterotomy, because we need a clinical score that must be accurate in decision making, regardless of the etiology of bleeding. Indeed, after analyzing our results in the different groups of patients regarding the GI bleeding causation, the score behaved correspondingly, except for variceal bleedings, in which AIMS65 had a worse predictive ability for endoscopic intervention and composite severity endpoint. The timing of endoscopy and adherence to guidelines are also important issues. In our institution a gastroenterologist is always on call, and endoscopy is performed in a mean time of 6–8 hours after admission. In the other reports, when available, endoscopy timing was within the first 12 hours at best,22,27 which could determine, in patients under high PPI doses, somatostatin, or other treatments, the cessation of the bleeding with no endoscopic indication of therapy. The easy access to endoscopy could have made us find more active bleeders, which otherwise would have spontaneously stopped bleeding. Another factor could be the inter-observer agreement regarding endoscopic risk stigmata of bleeding, and therefore the decision as to whether to provide therapy. This variability in the decision to provide endoscopic treatment could contribute to the observed differences.
Importantly, our data indicate that the risk of bleeding requiring endoscopic or surgical therapy, as well as the risk of inpatient death, increases in patients with an AIMS65 ≥ 1. This cut-off point can be selected as the marker of high risk patients, and determine further management, in accordance with the guidelines that advocate early endoscopy in high risk patients, as stratified by validated scoring systems.28,29
Another conclusion of our research is that AIMS65 predicts mortality within 6 months after admission. This topic has only been partially analyzed in previous studies, which considered only 30 days mortality.22,27 We considered such an extended period because upper GI bleeding can challenge the precarious clinical balance of frail patients, such as cirrhotic patients, patients with cardiovascular diseases in which changes in their usual treatments determined by their admission, or a worsening chronic condition that might have led to the bleeding, could be the cause of a delayed death. Indeed, cardiovascular causes accounted 30% of all the delayed deaths (Table 4), neoplasms 25% and GI bleeding only 27%. Therefore, GI bleeding might be considered a red flag for frail patients, but further research is needed to address this issue.
Our study has some limitations. First, it is a single center study; second, endoscopic intervention is always at the discretion of the endoscopist and, although we have very strict protocols, biases could have happened. As noted, we performed early endoscopy to every patient, and we might have find a higher number of active or recent bleedings prompting endoscopic intervention. Another limitation derives from the higher proportion of variceal bleedings compared to some other previous papers. AIMS65 had a better performance on non-variceal GI bleeders and this higher proportion of variceal bleedings, which can be explain because ours is a referral center with a liver transplantation program and an important liver unit in our area, could have biased our results resting predictive ability to AIMS65.
In conclusion, AIMS65 is an easily applicable score, which performance is comparable to GBS and RS in some essential endpoints such as inpatient mortality the need for endoscopic intervention and the selection of high risk patients. GBS was better predicting the need for blood transfusions, but both AIMS65 and RS were also useful in this endpoint. AIMS65 failed in two major endpoints in which GBS, the other pre-endoscopic clinical score, showed its superiority: the prediction of rebleeding and the selection of low risk patients which can be discharged without endoscopy. However, AIMS65 was the best score predicting delayed mortality in our series. Its applicability to daily clinical practice offers an option to the previous clinical scores, which have not been widely applied because of their complexity.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Hearnshaw SA, Logan RF, Lowe D, et al. Acute upper gastrointestinal bleeding in the UK: patient characteristics, diagnoses and outcomes in the 2007 UK audit. Gut 2011; 60: 1327–1335. [DOI] [PubMed] [Google Scholar]
- 2.Van Leerdam ME, Vreeburg EM, Rauws EA, et al. Acute upper GI bleeding: did anything change? Time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000. Am J Gastroenterol 2003; 98: 1494–1499. [DOI] [PubMed] [Google Scholar]
- 3.Sugawa C, Steffes CP, Nakamura R, et al. Upper GI bleeding in an urban hospital. Etiology, recurrence, and prognosis. Ann Surg 1990; 212: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barkun A, Sabbah S, Enns R, et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol 2004; 99: 1238–1246. [DOI] [PubMed] [Google Scholar]
- 5.Jairath V, Kahan BC, Logan RF, et al. Outcomes following acute nonvariceal upper gastrointestinal bleeding in relation to time to endoscopy: results from a nationwide study. Endoscopy 2012; 44: 723–730. [DOI] [PubMed] [Google Scholar]
- 6.Rockall TA, Logan RF, Devlin HB, et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996; 38: 316–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cipolletta L, Bianco MA, Rotondano G, et al. Outpatient management for low-risk nonvariceal upper GI bleeding: a randomized controlled trial. Gastrointest Endosc 2002; 55: 1–5. [DOI] [PubMed] [Google Scholar]
- 8.Longstreth GF, Feitelberg SP. Successful outpatient management of acute upper gastrointestinal hemorrhage: use of practice guidelines in a large patient series. Gastrointest Endosc 1998; 47: 219–222. [DOI] [PubMed] [Google Scholar]
- 9.Vreeburg EM, Terwee CB, Snel P, et al. Validation of the Rockall risk scoring system in upper gastrointestinal bleeding. Gut 1999; 44: 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 2000; 356: 1318–1321. [DOI] [PubMed] [Google Scholar]
- 11.Saeed ZA, Winchester CB, Michaletz PA, et al. A scoring system to predict rebleeding after endoscopic therapy of nonvariceal upper gastrointestinal hemorrhage, with a comparison of heat probe and ethanol injection. Am J Gastroenterol 1993; 88: 1842–1849. [PubMed] [Google Scholar]
- 12.Romagnuolo J, Barkun AN, Enns R, et al. Simple clinical predictors may obviate urgent endoscopy in selected patients with nonvariceal upper gastrointestinal tract bleeding. Arch Intern Med 2007; 167: 265–270. [DOI] [PubMed] [Google Scholar]
- 13.Saltzman JR, Tabak YP, Hyett BH, et al. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc 2011; 74: 1215–1224. [DOI] [PubMed] [Google Scholar]
- 14.Stanley AJ, Ashley D, Dalton HR, et al. Outpatient management of patients with low-risk upper-gastrointestinal haemorrhage: multicentre validation and prospective evaluation. Lancet 2009; 373: 42–47. [DOI] [PubMed] [Google Scholar]
- 15.Stanley AJ, Dalton HR, Blatchford O, et al. Multicentre comparison of the Glasgow–Blatchford and Rockall scores in the prediction of clinical end-points after upper gastrointestinal haemorrhage. Aliment Pharmacol Ther 2011; 34: 470–475. [DOI] [PubMed] [Google Scholar]
- 16.Pang SH, Ching JY, Lau JY, et al. Comparing the Blatchford and pre-endoscopic Rockall score in predicting the need for endoscopic therapy in patients with upper GI hemorrhage. Gastrointest Endosc 2010; 71: 1134–1140. [DOI] [PubMed] [Google Scholar]
- 17.Masaoka T, Suzuki H, Hori S, et al. Blatchford scoring system is a useful scoring system for detecting patients with upper gastrointestinal bleeding who do not need endoscopic intervention. J Gastroenterol Hepatol 2007; 22: 1404–1408. [DOI] [PubMed] [Google Scholar]
- 18.Schiefer M, Aquarius M, Leffers P, et al. Predictive validity of the Glasgow–Blatchford bleeding score in an unselected emergency department population in continental Europe. Eur J Gastroenterol Hepatol 2012; 24: 382–387. [DOI] [PubMed] [Google Scholar]
- 19.Hyett BH, Abougergi MS, Charpentier JP, et al. The AIMS65 score compared with the Glasgow–Blatchford score in predicting outcomes in upper GI bleeding. Gastrointest Endosc 2013; 77: 551–557. [DOI] [PubMed] [Google Scholar]
- 20.Jung SH, Oh JH, Lee HY, et al. Is the AIMS65 score useful in predicting outcomes in peptic ulcer bleeding? World J Gastroenterol 2014; 20: 1846–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura S, Matsumoto T, Sugimori H, et al. Emergency endoscopy for acute gastrointestinal bleeding: prognostic value of endoscopic hemostasis and the AIMS65 score in Japanese patients. Dig Endosc 2014; 26: 369–376. [DOI] [PubMed] [Google Scholar]
- 22.Yaka E, Yılmaz S, Doğan NÖ, et al. Comparison of the Glasgow–Blatchford and AIMS65 scoring systems for risk stratification in upper gastrointestinal bleeding in the emergency department. Acad Emerg Med 2015; 22: 22–30. [DOI] [PubMed] [Google Scholar]
- 23.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013; 368: 11–21. [DOI] [PubMed] [Google Scholar]
- 24.Bryant RV, Kuo P, Williamson K, et al. Performance of the Glasgow–Blatchford score in predicting clinical outcomes and intervention in hospitalized patients with upper GI bleeding. Gastrointest Endosc 2013; 78: 576–583. [DOI] [PubMed] [Google Scholar]
- 25.Meltzer AC, Burnett S, Pinchbeck C, et al. Pre-endoscopic Rockall and Blatchford scores to identify which emergency department patients with suspected gastrointestinal bleed do not need endoscopic hemostasis. J Emerg Med 2013; 44: 1083–1087. [DOI] [PubMed] [Google Scholar]
- 26.Chen IC, Hung MS, Chiu TF, et al. Risk scoring systems to predict need for clinical intervention for patients with nonvariceal upper gastrointestinal tract bleeding. Am J Emerg Med 2007; 25: 774–779. [DOI] [PubMed] [Google Scholar]
- 27.Wang CH, Chen YW, Young YR, et al. A prospective comparison of 3 scoring systems in upper gastrointestinal bleeding. Am J Emerg Med 2013; 31: 775–778. [DOI] [PubMed] [Google Scholar]
- 28.Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2010; 152: 101–113. [DOI] [PubMed] [Google Scholar]
- 29.Lim LG, Ho KY, Chan YH, et al. Urgent endoscopy is associated with lower mortality in high-risk but not low-risk nonvariceal upper gastrointestinal bleeding. Endoscopy 2011; 43: 300–306. [DOI] [PubMed] [Google Scholar]