Abstract
Background
Some gastric cancers are Epstein-Barr virus associated.
Aim
To assess the prevalence of Helicobacter pylori and viral co-infection in benign upper digestive diseases.
Methods
One hundred and four outpatients were included in a prospective endoscopic–serologic study. Epstein–Barr virus immunoglobulin G (IgG), immunoglobulin M and viral capsid antigen titres were assayed with an ELISA test. Helicobacter pylori was determined by the modified Giemsa stain and by IgG-chemiluminescence.
Results
The overall prevalence of Helicobacter pylori was 56.7%. Duodenal ulcer patients were infected in 72.5 % of the cases, with the prevalence being 33.3% in functional dyspepsia (p = 0.0008) and 25.8% in reflux patients (p = 0.0001). Epstein–Barr virus IgG was detected in 70.1% of the whole group, 75% of duodenal ulcer patients, 51.2% of functional dyspepsia patients (p = 0.04) and 51.6% of the reflux disease cases (p = 0.04). Co-infection with both agents was detected in 60% of duodenal ulcer patients, 18.1% of functional dyspepsia (p = 0.00014) and 12.9% of reflux disease patients (p = 0.00012). Anti-viral IgG titre displayed a 31.7 ± 3.0 cut-off index in duodenal ulcer, 20.5 ± 3.5 in functional dyspepsia (p = 0.01) and 21.4 ± 3.6 in reflux cases (p = 0.03).
Conclusions
Both Helicobacter pylori and Epstein–Barr virus, and co-infection with these agents, were significantly more prevalent in duodenal ulcer patients than in dyspeptic/reflux patients.
Keywords: Chronic gastritis, duodenal ulcer, Epstein–Barr virus, gastric infections, Helicobacter pylori
Introduction
Epstein–Barr virus (EBV) is one of the most prevalent viral infections worldwide. Infection with this herpesvirus can lead to a wide range of acute and chronic, benign or malignant conditions as listed in Table 1.1–7 It was recently shown that a certain number of gastric carcinomas are associated with EBV infection. The presence of the virus in the lymphocytes around gastric tumours was first demonstrated in 1992. Pathologists revealed that EBV is associated with a lymphoepithelial gastric cancer, located mainly in the gastric corpus. It does not have any distinct clinical features and for now is treated according to the current protocols used in gastric cancer.8,9 Later, the virus was also demonstrated in gastric cancer cells in a large group of patients from the USA.10 Much less is known, however, about the occurrence of EBV infection in benign upper gastrointestinal disorders. The aim of our study was to investigate the prevalence of EBV and Helicobacter pylori (H. pylori) infection as well as their co-infection in benign conditions of the upper gastrointestinal tract.
Table 1.
Conditions associated with Epstein–Barr virus infection
Methods
One hundred and four patients were included in a prospective endoscopic–serologic study. All the patients were given written informed consent and the study was approved by the institutional Ethics and Science Committee, in accordance with the amendments of the Declaration of Helsinki (2008). The inclusion criteria were as follows: aged between 18 and 80 years, benign upper digestive disease confirmed by endoscopy. Patients with malignant, severe cardiopulmonary, liver or renal disease, or Type 1 or 2 diabetes mellitus were excluded. Table 2 contains the demographic data. H. pylori was determined serologically by chemiluminescence (Immulite 2000 analyser, Siemens Healthcare Diagnostics, Erlangen, Germany) and the results were expressed as units/ml: values < 0.9 were considered to be negative, those between 0.9 and 1.0 were in the grey zone and those equal to or above 1.1 were positive. Gastric mucosa was collected endoscopically (Fujinon EG250WR5 videoendoscope, Fujinon Corporation, Japan) and H. pylori was determined from two antral and two corporeal samples by the modified Giemsa stain. The grade of chronic inflammation and gastritis activity was assessed according to the modified Sydney classification.11 EBV immunoglobulin G (IgG), immunoglobulin M (IgM) and viral capsid antigen (VCA) titres were assayed by ELISA and expressed as a cut-off index (BEP 2000 analyser, Siemens Healthcare Diagnostics, Erlangen, Germany). The ELISA methodology was validated in-house. EBV IgGM, IgG and VCA values < 8.5 were considered negative, values between 8.5 and 11.5 were in the grey zone and those over 11.5 were positive. All patients were newly identified dyspeptic cases; the H. pylori positives were naive of previous eradication regimens. No patient with acute mononucleosis was included.
Table 2.
Demographic data of the patients
| Data | Peptic ulcer | Functional dyspepsia | Reflux disease | Total |
|---|---|---|---|---|
| No. of cases | 40 | 33 | 31 | 104 |
| Age (mean + SEM) (years) | 54.1 ± 2.4 | 56.1 ± 2.3 | 50,5 ± 2.19 | 53.7 ± 1.37 |
| M/F ratio (%) | 80/20 | 30/70 | 52/48 | 54/46 |
| Duration of complaints (years) | 5.4 | 3.4 | 3.0 | 3.9 |
| Smoking (%) | 62.5 | 45.4 | 45.1 | 51.9 |
| Alcohol | 40.0 | 18.2 | 32.3 | 30.8 |
Statistics
The prevalence of H. pylori, EBV and any co-infection was calculated for the whole group, as well in duodenal ulcer, functional dyspepsia and gastro-oesophageal reflux disease (GERD) subgroups. The background seroprevalence of EBV and H. pylori was estimated using the 2014 Synlab database: the values of patients in the present study were subtracted from those of the non-dyspeptic general population. Differences in prevalence between the groups were calculated using a chi-square test. Antrum and corpus histologic data were analysed with an ANOVA test. The correlation between the chronic gastritis and activity score and the EBV infection was calculated by linear regression. The statistical calculations were performed by an experienced biomedical statistician, using SigmaStat 3.1. Systat (Software International Ltd 2005, San Jose, California, USA).
Results
The overall prevalence of H. pylori, EBV infection and the co-infection with these agents is presented in Figure 1. According to the laboratory database, the seroprevalence of H. pylori IgG in the general population of our district was 29.4 % (2385 out of 8107 cases) in 2014. EBV IgG was positive in 2254 out of 3578 cases (62.9 %). VCA IgG positivity was noted in 2825 out of 3556 cases (79.4%). Co-infection with H. pylori and EBV was found in 59 out of 211 cases (27.9%).
Figure 1.
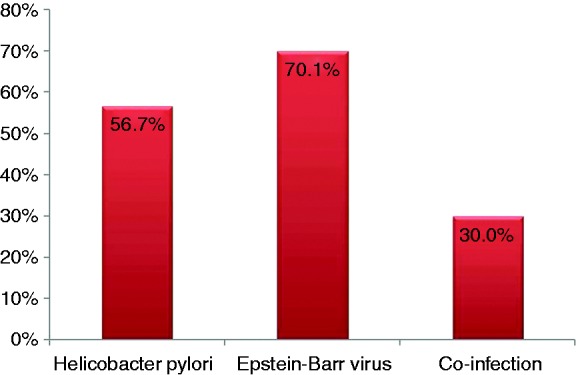
Prevalence of Helicobacter pylori, Epstein-Barr virus and that of co-infection in 104 patients with upper gastrointestinal diseases.
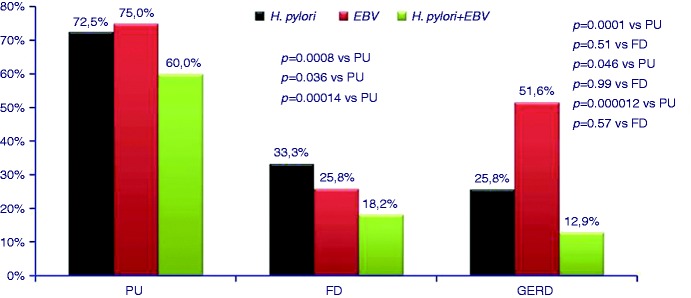
In the subgroup analysis, the prevalence of H. pylori was significantly higher in duodenal ulcer cases versus GERD (p = 0.0001) and functional dyspepsia patients (p = 0.0008) (Figure 2). EBV was also more frequent in duodenal ulcer patients than in GERD (p = 0.046) and functional dyspepsia cases (0.041), although the significance is only marginal (Figure 2).
Figure 2.
Prevalence of Helicobacter pylori, Epstein-Barr virus and co-infection in peptic (duodenal) ulcer (PU), functional dyspepsia (FD) and reflux disease (GERD) patients.
Co-infection with H. pylori and EBV was found in 60.0% of duodenal ulcer patients, 18.18% of GERD and 12.90% of functional dyspepsia cases, the differences being highly significant (duodenal ulcer versus GERD p = 0.000010, duodenal ulcer versus functional dyspepsia p = 0.00014).
The serologic study showed that IgG cut-off levels are significantly higher in H. pylori positive than in negative cases (p = 0.0085), while VCA titres were modestly, but still significantly, elevated in H. pylori positive cases (p = 0.070) (Table 3).
Table 3.
Epstein–Barr virus immunoglobulin G and viral capsid antigen levels in Helicobacter pylori-positive and -negative patients
| No. of cases | H. pylori status | IgG level (mean ± SE) p = 0.0085 | VCA level (mean ± SE) p = 0.070 |
|---|---|---|---|
| 48 | Positive | 30.87 ± 2.98 | 26.18 ± 2.33 |
| 56 | Negative | 20.30 ± 2.56 | 20.21 ± 2.28 |
H. pylori: Helicobacter pylori; IgG: immunoglobulin G; VCA: viral capsid antigen
Subgroup analyses revealed that IgG levels were significantly higher in duodenal ulcer cases than in either GERD (p = 0.037) or functional dyspepsia patients (p = 0.019) (Figure 3). VCA levels were as follows: 26.6 + 2.4 in duodenal ulcer, 21.9 + 3.0 (p = 0.07) and 19.3 + 3.1 cut-off index, respectively (p = 0.5).
Figure 3.
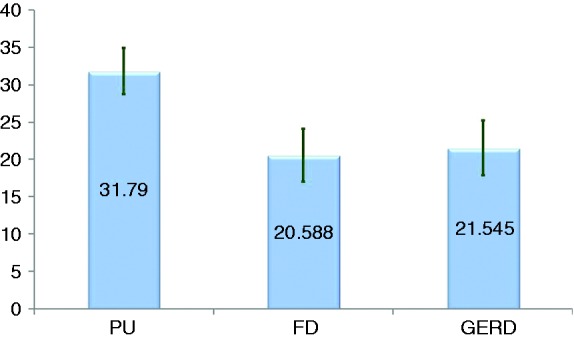
EBV IgG levels (cut-off index) in peptic (duodenal) ulcer (PU), functional dyspepsia (FD) and reflux disease (GERD) patients (mean + SEM).
Histologic analysis showed that chronic inflammation and gastritis activity scores in antrum and corpus were significantly higher in duodenal ulcer patients than in functional dyspepsia and GERD patients (Table 4).
Table 4.
Scores of chronic inflammation and gastritis activity in duodenal ulcer, functional dyspepsia and reflux disease cases (mean ± SEM)
| Feature | Location | DU | FD | GERD |
|---|---|---|---|---|
| Chronic inflammation | Antrum | 2.03 + 1.45a | 0.91 + 0.17 | 1.00 + 0.16 |
| Corpus | 1.15 + 0.13b | 0.55 + 0.1 | 0.66 + 0.17 | |
| Activity score | Antrum | 1.42 + 0.15c | 0.61 + 0.15 | 0.52 + 0.14 |
| Corpus | 0.68 + 0.10d | 0.33 + 0.10 | 0.26 + 0.09 |
p = 0.00012 DU vs. FD, p = 0.00023 DU vs. GERD.
p = 0.0001 DU vs. FD, p = 0.0003 DU vs GERD.
p = 0.0001 DU vs. FD, p = = 0.00034 DU vs. GERD.
p = 0.02 DU vs. FD, p = 0.003 DU vs. GERD.
DU: duodenal ulcer; FD: functional dyspepsia; GERD: gastro-oesophageal reflux disease
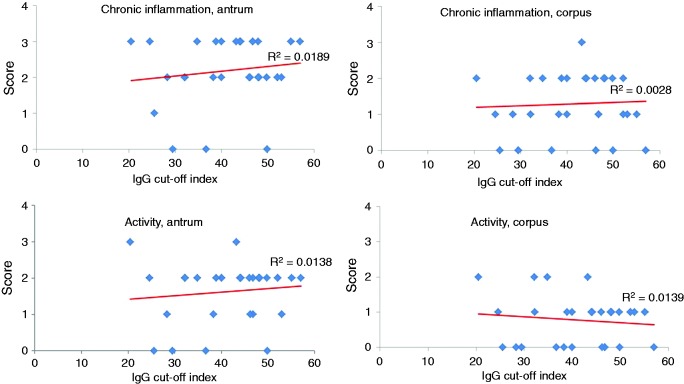
In the whole group, there was no positive correlation between the chronic inflammation and activity score in the antrum and corpus mucosa and the EBV IgG level (R2 = 0.06 and 0.05, respectively). A positive correlation was found in duodenal ulcer patients between the grade of chronic inflammation, the activity score and the EBV IgG level in the antrum, which was absent in the corpus mucosa (Figure 4).
Figure 4.
Correlation between chronic inflammatory score, gastritis activity and EBV IgG levels (cut-off index) in antrum and corpus mucosa of duodenal ulcer patients.
Discussion
The overall prevalence of H. pylori in the general population was 29.4%: this is consistent with our previous study showing that between 1997 and 2012, the prevalence of infection decreased gradually from 71.3% to 32.7%.12 In the groups studied, H. pylori prevalence was higher in duodenal ulcer patients while in functional dyspepsia and reflux cases it was closer to the general prevalence: this, again, is consistent with the epidemiological features of the infection.13
The prevalence of EBV infection in adult dyspeptic patients is somewhat higher than that of the general population. In duodenal ulcer cases the frequency of co-infection with both agents is significantly higher than in GERD or functional dyspepsia cases. Moreover, the level of EBV IgG was also higher in duodenal ulcer patients than in the other two groups.
The presence of EBV in gastric mucosa was demonstrated in 1992: in 175 gastric adenomacarcinoma patients, the in situ hybridisation and polymerase chain reaction detected EBV in 16% of the cases.14 In 1996, the EBV-encoded RNA 1 was detected in malignant epithelial cells and dysplastic epithelium in 12% of the cases.8 Recent studies from the USA,10 Japan,15 Mexico16, Brazil,17 Turkey18 and India19 reported an incidence of 6%–52% of EBV in gastric cancer patients. In meta-analyses from 2009, a pooled prevalence of 8.7% was found in 15,952 cases from 70 studies, which was similar in diffuse (7.6%) and intestinal types (9.5%). No geographical differences were detected in cases from Europe (9.2%), the Americas (9.9%) and Asia (8.3%).20 The most recent systematic review from 2015 reported the prevalence of EBV based on in situ hybridisation, polymerase chain reaction and serology.21 The virus was found in 5.0%–17.9% of cancer cells, but all adjacent non-cancer cells were consistently EBV negative. It was postulated that EBV is present in the mucosa before malignant transformation9 and excessive DNA methylation was proposed as a mechanism.22 Chronic inflammation induced by H. pylori could also lead to polyclonal hypermethylation with an increased risk of gastric cancer.15 Initially, EBV was found only in lymphocytes infiltrating gastric tumours; later its presence was demonstrated in non-atrophic and non-metaplastic mucosa.16
Much less is known about EBV in benign upper gastrointestinal diseases. Lymphocytes are only rarely seen in normal (i.e. non-inflamed) gastroduodenal mucosa. Case reports described that acute EBV infection could cause severe gastritis,23-25 sometimes simulating lymphoma.24 It was also shown that in patients with atrophic gastritis, EBV DNA was detected in 65.7% of the cases and a significant association was found between EBV detection, the severity of inflammation and mucosal atrophy.26 In 2011 it was shown that the EBV nuclear antigen was present in 70% of Indian peptic ulcer cases, as compared with 36% in functional dyspepsia (p < 0.001).27 Similar to our findings, dual infection with EBV and H. pylori was more prevalent in ulcer than dyspeptic patients and the median copy number of EBV DNA was also more elevated in the first group. With the advent of sensitive and specific quantitative polymerase chain reaction, the EBV DNA was undetectable in normal mucosa but was present in 46% of chronic gastritis cases.28 The phenomenon seems to be non-specific, because EBV DNA was also detected in 55% of Crohn’s disease and 64% of ulcerative colitis cases. Finally, in Mexican children with chronic abdominal pain, co-infection with H. pylori and EBV leads to a more severe lymphocytic and polymorphonuclear cell infiltration of the gastric mucosa than for those with H. pylori or EBV infection only. Moreover, CagA+ positive cases have had a stronger inflammation than CagA– patients.29
The positive correlation between chronic inflammation and activity score of chronic gastritis and the EBV IgG level is difficult to explain based on current knowledge: it suggests, nevertheless, some local or general immunologic role of the viral infection which overlaps with H. pylori infection.
The explanation of H. pylori–EBV interplay in the pathogenesis of chronic gastritis and ulcer disease is open to presumptions and speculations: additive inflammatory responses, putative CagA+ and EBV oncogene interaction, activation/increased production of inflammatory mediators such as interleukin IL-8 and IL-1beta were all presumed, and sometimes supported experimentally, but were unproven in humans: the topic warrants further research.
The strength of our study is the demonstration of a significantly higher prevalence of EBV and co-infection with H. pylori + EBV in duodenal ulcer patients, associated with an increased level of immune response as well, expressed by the higher anti-IgG cut-off levels, which could correspond to an increased viral load or a stronger immune response.29 The increased prevalence of these infections does not signify, however, a cause and effect relation.
Recent Mexican work showed, in accordance with our results, that duodenal ulcer is associated with high anti-EBV IgG titres (p = 0.022, odds ratio (OR): 2.5). The authors suggest that the reactivation of EBV in gastric and duodenal mucosa increases the risk of peptic ulcers.30
The weak point of our study is the rather low number of cases; this was a pilot study and cost limitations restricted the inclusion of larger groups of patients. However, in most works the number of cases was between 30 and 100. Secondly, to explore the relation between EBV, H. pylori and duodenal ulcer more thoroughly, the presence of these agents must be demonstrated by appropriate methods (immunochemistry for H. pylori, fluorescence in situ-hybridisation, EBV-encoded RNA, quantitative polymerase chain reaction, etc.31 in the epithelial cells and inflammatory infiltrate in the close vicinity of duodenal ulcers): such investigations are in progress in our department.
Older works suggest that duodenal ulcer offers protection against gastric cancer and this is based on radiologic and endoscopic studies performed decades ago, when the role of EBV was unknown.32 This must be completed and re-evaluated, incorporating the new knowledge on gastroduodenal physiology and infectology.
Conclusion
Both H. pylori and Epstein–Barr virus, and co-infection with these agents, were significantly more prevalent in duodenal ulcer patients than in functional dyspepsia and GERD cases. Anti-viral IgG titre was also higher in H. pylori positive patients than in the negative cases, suggesting an increased viral load or stronger immune response. The possible pathogenetic significance of coexistent H. pylori and EBV infections must be studied further, both in different stages of chronic gastritis and especially in duodenal ulcer patients.
Acknowledgments
GMB is indebted to Mrs Jolán Józan for the statistical work-up of the data, to Miss Anna Szilágyi (Semmelweis University, Department of Physiology, Budapest) for literature searches and to Mr Douglas Arnott (EDMF Language Services Ltd, Budapest, Hungary) for the English proofing of the manuscript. GMB designed the study, performed all endoscopic examinations and wrote the manuscript. JK performed all immunologic examinations and reviewed the manuscript. Both authors have read and agreed with the content of the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Thorley-Lawson DA, Hawkins JB, Tracy SI, et al. The pathogenesis of Epstein–Barr virus persistent infection. Curr Opin Virol 2013; 3: 227–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagneux-Brunon A, Suy F, Pouvaret A, et al. Acute acalculous cholecystitis, a rare complication of Epstein–Barr virus primary infection: Report of two cases and review. J Clin Virol 2014; 61: 173–175. [DOI] [PubMed] [Google Scholar]
- 3.Jerdan K, Aronson I, Hernandez V, et al. Genital ulcers associated with Epstein–Barr virus. Cutis 2013; 91: 273–276. [PubMed] [Google Scholar]
- 4.Attard AA, Praveen P, Dunn PJ, et al. Epstein–Barr virus-positive mucocutaneous ulcer of the oral cavity: The importance of having a detailed clinical history to reach a correct diagnosis. Oral Surg Oral Med Pathol Oral Radiol 2012; 114: e37–e39. [DOI] [PubMed] [Google Scholar]
- 5.Coghill AE, Hildesheim A. Epstein–Barr virus antibodies and the risk of associated malignancies: Review of the literature. Am J Epidemiol 2014; 180: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiman A, Powell JE, Flavell KJ, et al. Seasonal differences in the onset of the EBV-positive and -negative forms of paediatric Hodgkin’s lymphomas. Br J Cancer 2003; 89: 1200–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppieters KT, Wiberg A, Tracy SM, et al. Immunology in the clinic review series: Focus on type 1 diabetes and viruses: The role of viruses in type 1 diabetes: A difficult dilemma. Clin Exp Immunol 2011; 168: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulley ML, Pulitzer DR, Eagan PA, et al. Epstein–Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2-expression and o53 accumulation. Hum Pathol 1996; 27: 20–27. [DOI] [PubMed] [Google Scholar]
- 9.Lugmani YA, Linjawi SO, Shousha S. Detection of Epstein–Barr virus in gastrectomy specimens. Oncol Rep 2001; 8: 995–999. [DOI] [PubMed] [Google Scholar]
- 10.Truong CD, Feng W, Li W, et al. Characteristics of Epstein–Barr virus-associated gastric cancer: A study of 235 cases at a comprehensive cancer center in U.S.A. J Exp Clin Cancer Res 2009; 28: 14–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the histopathology of gastritis, Houston 1994. Am J Surg Pathol 1996; 20: 1161–1181. [DOI] [PubMed] [Google Scholar]
- 12.Buzás GM, Lotz G, Schneider F, et al. Changing prevalence of Helicobacter pylori infection in the 9th district of Budapest. A retrospective endoscopic study, 1997–2012. Orv Hetil 2013; 154: 900–907. [DOI] [PubMed]
- 13.Morgan DM, Crowe SE. Helicobacter pylori infection. In: Feldman M, Friedman LS, Brandt LJ. (eds). Sleisenger and Fordtran’s gastrointestinal and liver disease. pathophysiology/diagnosis/management 2015; Vol. 1 10th edn Philadelphia: Elsevier Saunders, pp. 857–868. [Google Scholar]
- 14.Shibata D, Weiss LM. Epstein–Barr virus-associated gastric adenocarcinoma. Am J Pathol 1992; 140: 769–774. [PMC free article] [PubMed] [Google Scholar]
- 15.Nishikawa J, Yoshiyama H, Iizasa H, et al. Epstein–Barr virus in gastric carcinoma. Cancers 2014; 54: 2259–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-López JLE, Torres J, Camorlinga-Ponce M, et al. Evidence of Epstein–Barr virus association with gastric cancer and non-atrophic gastritis. Viruses 2014; 6: 301–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira de Souza CR, Soares de Oliveira K, Ferraz JJS, et al. Occurrence of Helicobacter pylori and Epstein–Barr virus infection in endoscopic and gastric cancer patients from Northern Brazil. BMC Gastroenterol 2014; 14: 179–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durmaz R, Aydin A, Koroglu M, et al. Investigation of the relationship between Epstein–Barr virus and ordinary gastric carcinoma using the nested polymerase chain reaction. Acta Virol 1998; 42: 359–363. [PubMed] [Google Scholar]
- 19.Rymbai ML, Ramalingam VV, Samarasan I, et al. Frequency of Epstein–Barr virus infection as detected by messenger RNA for EBNA 1 in histologically proven gastric adenocarcinomas in patients presenting to a tertiary care center in South India. Indian J Med Microbiol 2015; 33: 369–373. [DOI] [PubMed] [Google Scholar]
- 20.Murphy G, Pfeiffer R, Camargo C, et al. Meta-analysis show that prevalence of Epstein–Barr virus positive gastric cancer differs based on sex and anatomic location. Gastroenterology 2009; 137: 824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X-Z, Chen H, Castro FA, et al. Epstein–Barr virus infection and gastric cancer. A systematic review. Medicine (Baltimore) 2015; 94: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsusaka K, Funata S, Fuikayama M, et al. DNA methylation in gastric cancer, related to Helicobacter pylori and Epstein–Barr virus. World J Gastroenterol 2014; 20: 3916–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Molot R. Severe gastritis secondary to Epstein–Barr viral infection. Unusual presentation of infectious mononucleosis and associated diffuse lymphoid hyperplasia in gastric mucosa. Arch Pathol Lab Med 2003; 127: 478–480. [DOI] [PubMed] [Google Scholar]
- 24.Chen ZM, Shah R, Zuckerman GR, et al. Epstein–Barr virus: An unrecognized form of severe gastritis simulating gastric lymphoma. Am J Surg Pathol 2007; 31: 1446–1451. [DOI] [PubMed] [Google Scholar]
- 25.Hisamatsu A, Nagai T, Okawara H, et al. Gastritis associated with Epstein–Barr virus infection. Intern Med 2010; 49: 2101–2105. [DOI] [PubMed] [Google Scholar]
- 26.Hirano A, Yanai H, Shimizu N, et al. Evaluation of Epstein–Barr virus DNA load in gastric mucosa with chronic atrophic gastritis using real-time quantitative PCR assay. Int J Gastrointest Cancer 2003; 34: 87–94. [DOI] [PubMed] [Google Scholar]
- 27.Shukla SK, Tripathi A, Prasad KN, et al. Epstein–Barr virus DNA load and its association with Helicobacter pylori infection in gastroduodenal diseases. Braz J Infect Dis 2011; 15: 583–590. [PubMed] [Google Scholar]
- 28.Ran JL, Shen Y-S, Morgan DR, et al. Epstein–Barr virus infection is common in inflamed gastrointestinal mucosa. Dig Dis Sci 2012; 57: 1887–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CárdenaS-Mondragón MG, Carreón-Talavera R, Camorlinga-Ponce M, et al. Epstein–Barr virus and Helicobacter pylori co-infection are positively associated with severe gastritis in pediatric patients. PLOS One 2013; 8: e62580–e62580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cárdenas-Mondragón MG, Torres J, Flores-Luna L, et al. Epstein–Barr virus association with peptic ulcer disease. Anal Cell Pathol (Amst). Epub ahead of print 24 June 2015. DOI: 10.1155/2015/164850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gulley ML, Tang W. Laboratory assays for Epstein–Barr virus-related disease. J Mol Diagn 2008; 10: 279–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ubukata H, Nagata T, Tabuchin T, et al. Why is the coexistence of gastric cancer and duodenal ulcer rare? Examination of factors related to both gastric cancer and duodenal ulcer. Gastric Cancer 2011; 14: 4–112. [DOI] [PubMed] [Google Scholar]