Abstract
Background
Increasing attention is focused on polyp-related features that may contribute to the operator-dependent nature of colonoscopy. Few data on polyps are available from high-yield colonoscopies, which may serve as a benchmark for quality control.
Objectives
Describe regional distribution, histology, size and shape of polyps, and the influence of patient age and gender, in colonoscopies performed by a colonoscopist with high lesion detection rate.
Methods
Analysis of 698 consecutive patients with diagnostic, screening or surveillance colonoscopies.
Results
In 704 colonoscopies, 1908 polyps were removed (360 were protruded and 1548 flat; 1313 were hyperplastic, 562 adenomas, 5 serrated adenomas and 8 mixed). There were 232 adenomas in female patients and 343 in male patients; 39% of the adenomas were protruded and 61% were flat. The peak adenoma detection rate (ADR) was 51% in patients beyond age 79 years. Men older than 49 years had a higher ADR than women. In men and women, respectively: 40% and 32% of adenomas were in the right colon, 31% and 22% were in the transverse colon, and 30% and 47% were in the left colon. Beyond age 59 years, the majority of adenomas were in the proximal colon.
Conclusions
An excess of adenomas in the proximal colon started at age 60 and this was more pronounced in men than in women. In all colonic regions, the majority of adenomas had a shape that was flat and smaller than 6 mm.
Keywords: Adenoma, colon cancer, colonoscopy, detection rate, gender differences, hyperplasia, patient age, polyp, quality control, tumor shape
Introduction
Although colonoscopy already has a great impact on public health, there is a need to put more effort into reducing its operator-dependent nature and into overcoming the obstacles that decrease its efficacy for prevention of colonic carcinomas.1–6 The efficacy of cancer prevention can be improved by increasing the adenoma detection rate (ADR) of endoscopists, which not only prevents loss of life-years, but also results in substantial financial saving.7 Each 1% increase in ADR by endoscopists is associated with a 3% decrease in patient cancer risk.8
Efforts to decrease the operator-dependence of colonoscopy have, until recently, focused on setting minimal standards,6,9 thereby focusing on improving on the low end of ADR; however, it has been suggested that the gain in number of life-years saved may even be higher, when training colonoscopists with average ADR to achieve high ADR.7 It remains unclear what the focus of training should be.
Several factors have been identified as influencing ADR and protective effects of colonoscopy, such as the specialty of the endoscopist,10,11 caecal intubation rate,10 quality of bowel preparation,3,12 and the quality of endoscopic equipment.11,13 Polyp-related factors are their size and shape, with decreased detection rates for the flat, depressed or serrated adenomas, or their location in the right colon.1,6,14,15
In order to improve the effect of colonoscopy in protection against colonic cancers, knowledge has to be gained from the experience of colonoscopists with high ADR.1 We report on the results of consecutive colonoscopies performed by a colonoscopist with a high lesion detection rate. These data provide in-depth information on polyp-related factors that may influence ADR, like regional distribution, adenoma size and shape, and the influence of age and gender. Our data may serve as a benchmark for future strategies for quality improvements in colonoscopy, by focusing on improving the detection of the frequently missed flat and right colonic lesions.
Methods
Study design
This is a retrospective analysis of standardized documentation of routine colonoscopies that were performed between 1 January 2010 and 31 December 2013. The analysis was approved by the ethics committee of the Medical University Graz, in Austria (no. 26-166ex13/14).
Clinical setup
Colonoscopies were performed by a gastroenterologist with 20 years of experience performing colonoscopies (author HFH). Colonoscopy reports were standardized according to the hospital requirements for quality control and billing of insurance companies. Reports included photographic documentation of the appendiceal orifice, fecal residue, and photographic and histological documentation of all polyps.
Patients
We included all patients 18 years or older in whom colonoscopies had been performed during the study period. Patients were self-referred or referred by their health care providers for ‘diagnostic’ colonoscopies for the evaluation of symptoms; ‘screening’ according to the Austrian screening program16; or ‘surveillance’ colonoscopies after previous pathology, such as inflammatory bowel disease, adenoma or carcinoma.
Bowel preparation
Standardized preparation was started on the afternoon before the day of colonoscopy. The last meal was breakfast. Thereafter, clear fluids were allowed. At 13:00 hours, 15 mg bisacodyl (3 tablets Dulcolax®, Boehringer Ingelheim, Vienna, Austria) were ingested. One hour later, oral intake of 4 liters of balanced polyethylene glycol (PEG) solution (Golytely, Fresenius-Kabi, Graz, Austria) was started, at a rate not exceeding 1 liter per hour. Patients were offered the addition of lemon or raspberry juice to the PEG solution, and herbal tea.
Patients were informed that if, after 4 liters of the PEG solution, the bowel effluent was not clear (‘chamomile-tea-like stool’), then 1–2 additional liters of the PEG solution should be drunk. We offered to those patients who would not tolerate the PEG solution, a low-volume, high-osmotic bowel preparation (Picoprep®, Ferring, Vienna, Austria). In the large majority of patients, colonoscopy was started in the morning, between 6:30 and 8:30 a.m. If the colonoscopy was scheduled to start after 9:00 a.m., the bowel preparation was split, with 2–3 liters to be drunk on the afternoon of the day before, and 1–2 liters on the morning of the day of colonoscopy, depending on the scheduled time of colonoscopy. In this case, the lavage had to be finished at least 1 hour before starting the colonoscopy.
Colonoscopy
We performed colonoscopies in 1–3 patients, in immediate succession. Patients were monitored (blood pressure, oxygen saturation, heart rate and clinical observation) throughout the procedure, until they woke up. We used for sedation intravenous midazolam (Midazolam Actavis, Salzburg, Austria) and propofol (Propofol 1%, Fresenius Kabi Graz, Austria) as required, to comply with patient requests regarding the depth of sedation and monitoring. Additional intravenous medications were given on demand: In the case of spasms, 20–40 mg scopalamine (Buscapina®, Boehringer Ingelheim, Vienna, Austria); and in the case of arterial hypotension, 500–1000 ml of water-electrolyte solutions (Elomel isoton, Fresenius Kabi, Graz, Austria).
Fujinone colonoscopes (EC 250 or EC 530, Reinhard DiLena, Vienna, Austria) and air insufflation were used. Polyps visualized during insertion of the colonoscope were removed immediately if, because of their size or location, they were judged not to be reliably detectable during withdrawal of the colonoscope. Otherwise, thorough evaluation of the colonic mucosal surface with white light and removal of all polyps was performed during colonoscope withdrawal.
Small polyps were removed by forceps and considerable effort was placed into the retrieval of polyps that had been removed by snare. For histological analysis, polyps larger than 10 mm in diameter were collected individually; smaller polyps were grouped according to the colonic region, except for polyps containing a Paris Type II(c) component,17 which, independent of their diameter, were collected individually for histological analysis. The location of polyps was described according to endoscopic markers of colonic regions; if this was equivocal or if surgical intervention was considered to be possibly required, the distance (in cm) from the anus was noted during retrieval of the colonoscope. The size of small polyps was estimated against the opened biopsy forceps. According to the literature, ‘minute’ polyps are those with a diameter up to 5 mm; ‘small’ polyps were divided into sizes, 6–7 mm and 8–9 mm.18 The shape of the polyps was reported according to the Paris classification.17 Histological samples were analyzed by experienced pathologists (MO and KHP).
Data collection and definitions
We noted the patient age, gender, colonoscopy indication, number of polyps, simplified Paris classification (Type I = protruded and Type II = flat), polyp localization, diameter and histology (adenomatous or hyperplastic).
Colonic regions were described as the caecum, ascending, transverse, descending and sigmoid colon; and the rectum. Colonic regions were grouped into ‘right colon’ (caecum and ascending colon), ‘proximal colon’ (right plus transverse colon), and the ‘left’ or ‘distal’ colon (descending, sigmoid and rectum).19 The oral and aboral legs of the right and left colonic flexures were assigned to the neighboring regions.
Polyp detection rate (PDR) was defined as the proportion of colonoscopies in which a minimum of one polyp was detected, independent of its histology.2 Adenoma detection rate (ADR) was defined as the proportion of colonoscopies in which at least one adenoma was removed.2
Results
General results
We performed 704 colonoscopies in 698 patients (392 female and 306 male). Their mean age was 61.6 years (female patients 61.2 years; and males, 62.2 years). Figure 1(a) shows the number of patients according to gender and grouped in decades of age. Figure 1(b) shows the number of patients in the three groups of indication, grouped by decade of age. Mean age in the patient group with diagnostic colonoscopy was 60.1 years, in the screening group was 60.3 years and in the surveillance group, 65.4 years.
Figure 1.
(a) Total patient number, and number of male and female patients, in the different age groups. (b) Number of patients in the different indication groups.
Visibility at colonoscopy was reduced by fecal residues in 43 patients (6.2%). In 12 patients (1.7%), the caecum was not reached.
The indication for colonoscopy was ambiguous in four patients. In three patients, the description of the location of the polyp, and in five polyps, the diameter of at least one of the polyps, could not be retrieved from the documents. In 13 patients, the resected polyp could not be retrieved for histological analysis. Carcinoma of the sigmoid colon was detected in one patient.
Polyps: size, shape and distribution
We analyzed 1767 polyps for histology, diameter and Paris classification. Table 1 shows the number of polyps according to diameter, histology, Paris classification and location. In 1908 polyps, the Paris classification could be retrieved (360 were Type I; 1548 were Type II). There were 930 polyps in female patients (151 Type I and 779 Type II); and 978 polyps in male patients (209 Type I and 769 Type II).
Table 1.
Polyps: Diameter, histology, Paris classification and colorectal distribution
| Polyp diameter |
|||||||
|---|---|---|---|---|---|---|---|
| minute |
small |
≥10 mm |
|||||
| 1–5 mm | 6–7 mm | 8–9 mm | 10–19 mm | ≥20 mm | |||
| Histology | Adenomas | n | 270 | 189 | 40 | 26 | 5 |
| Same-size polyps (%) | 23.0% | 39.5% | 51.3% | 82% | 100% | ||
| Hyperplasia | n | 903 | 290 | 38 | 6 | 0 | |
| Same-size polyps (%) | 77.0% | 60.5% | 48.7% | 18% | 0% | ||
| Paris Classification | Type I (protruded) | n | 101 | 157 | 47 | 21 | |
| Same-size polyps (%) | 8.6% | 32.7% | 61.8% | 52.9% | |||
| Type II (flat) | n | 1072 | 322 | 31 | 16 | ||
| Same-size polyps (%) | 91.4% | 67.3% | 38.2% | 47.1% | |||
| Location | Rectum | n | 483 | 106 | 13 | 5 | |
| Sigmoid | n | 408 | 165 | 25 | 15 | ||
| Descending | n | 52 | 29 | 6 | 5 | ||
| Transverse | n | 102 | 98 | 21 | 4 | ||
| Ascending | n | 103 | 65 | 8 | 8 | ||
| Caecum | n | 68 | 28 | 6 | 1 | ||
We had 1888 polyps available for histologic analysis: 1313 were hyperplastic, 562 were adenomas, five were serrated adenomas and eight were of mixed histology. Figure 2 shows that the likelihood of a polyp being adenomatous increased with the diameter of the polyp; at each diameter, the Type I polyps had a greater likelihood of being adenomatous than Type II.
Figure 2.
Frequency of adenomatous histology in Paris Type I and Paris Type II polyps.
Table 2 shows the number of Type I and Type II polyps according to age groups, in decades; in each age group, the Type II by far outnumbered the Type I polyps; however, there was an increase in the proportion of all polyps that were Type I, with increasing age.
Table 2.
Polyps: Number and proportion (%) of Paris Type I and Type II, by age group
| Age (years) |
|||||||
|---|---|---|---|---|---|---|---|
| <40 | 40–49 | 50–59 | 60–69 | 70–79 | ≥80 | Total n | |
| Type I polyps (protruded) | 6 (13.6%) | 14 (10.9%) | 76 (16.2%) | 141 (19.8%) | 92 (21.8%) | 31 (23.7%) | 360 |
| Type II polyps (flat) | 38 (86.4%) | 115 (89.2%) | 392 (83.8%) | 573 (80.3%) | 331 (78.3%) | 99 (76.3%) | 1549 |
| Total n | 44 | 129 | 468 | 714 | 423 | 131 | 1909 |
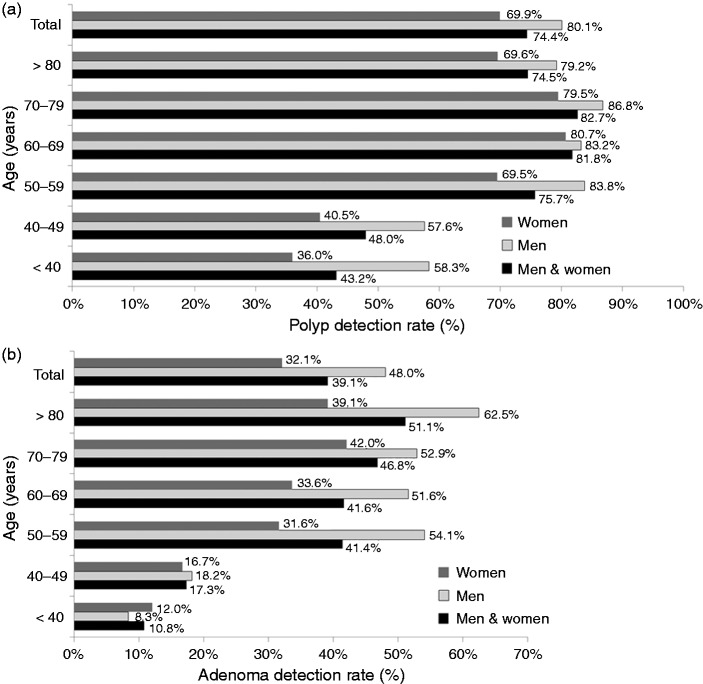
Figure 3(a) (shown in the next section, with 3(b)) shows the PDR in the whole group of patients: They are grouped according to gender and decade of age. There was a marked increase in PDR beyond the age of 49 years, with a peak PDR of 82.7% in patients between 70 and 79 years of age. In all age groups, men had a higher PDR than women.
Figure 3.
(a) PDR in men and women, in the different age groups, and in the whole group of patients. (b) ADR in men and women in the different age groups, and in the whole group of patients.
The mean number of polyps, in patients with at least one polyp, was 3.64 (4.05 in male patients and 3.28 in female patients).
Table 3 shows PDR in diagnostic, screening and surveillance colonoscopies. PDR in the colonic regions was as follows: Right colon 27.0% (male patients 33.0% and female patients 22.3%), transverse colon 21.3% (males 28.2% and females 15.9%) and left colon 60.2% (males 63.8% and females 57.5%).
Table 3.
PDR and ADR in diagnostic, screening and surveillance colonoscopies. Total number of patients and distribution of male and female patients (in %) in the three indication groups
| Colonoscopy indication |
|||
|---|---|---|---|
| Diagnostic | Screening | Surveillance | |
| n | 341 | 184 | 175 |
| Women, n (%) | 221 (56%) | 96 (24%) | 79 (20%) |
| Men, n (%) | 120 (39%) | 88 (29%) | 96 (32%) |
| Mean age, years | 60.1 | 60.3 | 65.4 |
| PDR% | 63.3 | 79.3 | 86.9 |
| ADR% | 32 | 39.7 | 51.4 |
ADR: adenoma detection rate; PDR: polyp detection rate
In 525 of the 704 colonoscopies at least one polyp was detected. In 98 of these 525, the polyps were located in the proximal colon only, with no polyp in the distal colon. In 72 of the 98 colonoscopies with only proximal polyps, one or more of these were adenomas and in 26, they were hyperplastic. Therefore, if endoscopic evaluation would have been stopped at the left colonic flexure, 72 patients with adenomas located only in the proximal colon would have been missed. This accounts for 10.2% of all patients, or 26.4% of patients with adenomas.
Adenomas: size, shape and distribution
We found that 575 polyps were adenomas (232 in female colonoscopy patients and 343 in male patients). Information on the diameter was available for 531 adenomas (Table 1). Out of the 566 adenomas in which both histology and Paris classification was documented, 223 were Type I (39.4%) and 343 were Type II (60.6%).
Figure 3(b) shows the ADR in the whole group of patients, and grouped according to gender and decade of age. There was a marked increase in ADR beyond the age of 49 years, with a peak ADR of 51.1% in patients ≥80 years old. In patients aged 50 years or older, men had a higher ADR than women.
The ADR in the diagnostic colonoscopies was 32.0%, in the screening colonoscopies was 39.7% and in the surveillance colonoscopies was 51.4% (Table 3).
Figure 4 shows the number of Type I and Type II adenomas in the different regions of the colon. The region with the highest number of adenomas was the transverse colon, followed by the ascending and sigmoid colon. In men, there were 126 adenomas (40% of adenomas) located in the right colon, 96 (30.5%) in the transverse colon and 93 (29.5%) in the left colon. In women, 83 adenomas (31.9% of adenomas) were located in the right colon, 56 adenomas (21.5%) in the transverse colon and 121 (46.5%) in the left colon.
Figure 4.
Number of Paris Type I (protruded) and Paris Type II (flat) adenomas in the colonic regions.
A: ascending colon; C: caecum; D: descending colon; R: rectum; S: sigmoid colon and T: transverse colon.
ADR in the colonic regions was as follows: Right colon 19.0% (male patients 25.6% and females 13.9%), transverse colon 12.9% (males 19.4% and females 7.8%) and left colon, 18.5% (males 22.0% and females 15.7%).
Figure 5 shows the mean number of adenomas per patient in the colonic regions and by age group. Below the age of 60 years, there was an even distribution throughout the colon. A shift towards the proximal colon started at 60 years of age, and was most pronounced in the oldest age group.
Figure 5.

Mean number of adenomas per patient.
A: ascending colon; C: caecum; D: descending colon; R: rectum; S: sigmoid colon and T: transverse colon.
Discussion
Our data provide in-depth information on the interrelation between the regional distribution of polyps; their histology, size and shape; and the influence of age and gender. These may serve as bench marks for questions related to strategies on the improvement of quality of colonoscopy. These questions relate to the use of ADR as a quality parameter for colonoscopy,2,4–6,8,9 the use of sigmoidoscopy for screening, the recommended age of onset of screening, the left-to-right shift of colonic adenomas that has been reported in recent years,19,20 the difference in the effects of colonoscopy screening on mortality due to left and right colonic cancer,21 and the influence of the colonoscopy indication on cancer risk.22 Furthermore, our data close a gap between the published ADRs of colonoscopy screening,2,8 and the adenoma detection rates23–25 and colonic distribution23,24,26,27 of adenomas in autopsy studies, some of which were published more than 30 years ago.
The most striking results of our study relate to the regional distribution of adenomas, and the influence of gender and age on this distribution. Comparison of recent publications with older publications on colonoscopy suggested a shift of adenomas from the predominant location in the left, to the right colon.19,20 Within the last decades, the proportion of colonic carcinomas located in the right colon has increased.28 It was proposed that this shift may be due to a change of pathological mechanisms of neoplasia.28
Our data show a 2:1 excess of adenomas in the proximal versus the distal colon, in the whole patient group; and they suggest that male gender and higher age are major factors responsible for this. Right colonic adenomas were predominant in people >60 years old, especially in men.
A recent publication shows that cancers in the proximal colon account for 47% of colorectal cancers in women and for 37% of these cancers in men.29 This is different from the gender predominance in the right colonic location of adenomas in our patients, were men predominated, with 71% of adenomas located in the proximal colon, versus 53% in women. This difference in the influence of gender on the regional distribution of adenomas in our patient group, as compared to the distribution of cancers, may be related to gender differences in the biological behavior of the mucosa of the right and left colon.30 With increasing life expectancy, especially in women, and with improving health of aging people, the future age limits for colonoscopy screening31 may have to be reconsidered.
We suggest that the reported left-to-right shift of colonic adenomas19,20 is partly due to recent improvements in the detection of right colonic adenomas. This is supported by results from autopsy studies, some of them from the 1980s, which demonstrate an excess of adenomas in the right colon,23,24,26,27 some of which presumably were missed by colonoscopy in past decades. Factors which may have contributed to the high detection rate of proximal adenomas in our study are: The excellent preparation for colonoscopy and the high coecal intubation rate. In our patients, 61% of adenomas were flat and therefore, might be difficult to detect if bowel preparation is poor, or endoscopic techniques or equipment are inadequate.14
Throughout this manuscript, the terms ADR and PDR where used for describing the frequency of adenomas and the frequency of polyps. The terms ADR and PDR have been introduced to assess the quality of endoscopists or endoscopic units,2,6 and not for epidemiological studies; however, we have chosen to use these terms in order to facilitate comparisons. High ADR in our patient population is mostly due to detection of small and minute adenomas. According to the literature, ‘minute’ polyps were those with a diameter up to 5 mm; and ‘small’ polyps were between 6–9 mm.18 More than 50% of adenomas were in the minute polyps, and 23% of the minute polyps and 40–50% of the small polyps had adenomatous histology. The clinical significance of small adenomas remains unclear; the negative correlation found between ADR and colon carcinoma mortality8 could be due to removal of small adenomas or to more thorough colonoscopy, with higher detection rates of right-sided advanced but flat lesions.32
Our data extend previous reports11,16,33 showing that ADR is influenced by age, gender and the colonoscopy indication. The group with the lowest ADR is female patients between 50–59 years of age, with an ADR of 32%; men of every age group beyond 50 years of age had an ADR in excess of 50% and increasing to 62.5%, in men >80 years old.
The higher ADR in screening, as compared to diagnostic colonoscopies, at the same mean age in these two patient groups, parallels a publication which shows that the cancer protective effect is higher in screening, than in diagnostic colonoscopies.17 Reasons for this remain unclear and require further study. We confirmed previous reports19 that ADR is highest in surveillance colonoscopies, which was also the oldest group of our patients.
As is shown in Table 3, there were gender differences with regard to the indication for colonoscopy: Whereas women were in the majority referred for evaluation of symptoms, men had no clear predominance in one of the three indication groups. This most likely represents the female preponderance in frequent causes of abdominal symptoms, like irritable bowel syndrome, and the increased incidence of adenomas in men, which after their removal would indicate surveillance. The number of men and women referred for screening was approximately equal and astonishingly low for a country like Austria, which advocates a national colon cancer screening program, the costs of which are fully covered.
In our patient population, 72 patients with one or more adenomas only in the proximal colon would have been missed, if endoscopy would have been limited to the left colon. This accounts for 26% of patients with adenomas. This, as well as the propensity for flat adenomas and carcinomas to develop in the right colon,32 have to be considered by advocates of sigmoidoscopy screening.
Our data close a gap between colonoscopy studies and autopsy studies, some of which have used loupes for mucosal inspection.23,24 This is summarized in Table 4. Vatn and Stalsberg,24 and Williams et al.23 have demonstrated an ADR similar to ours, with ADR in men up to 53% and 52%, and in women up to 43% and 35%, respectively. An excess of adenomas in men, as compared to women, such as we saw in our patients, was reported by Johannsen et al.,26 Williams et al.23 and Coode et al.25; no gender influence was reported by Vatn and Stalsberg,24 and Pendergrass et al.27
Table 4.
Comparison of our colonoscopy study results with autopsy studies
| Author, publication year | n patients, age | Frequency of adenomas (%) |
|||
|---|---|---|---|---|---|
| Men versus women, mean | Age dependency | Right versus left colon | Shape | ||
| This endoscopic study, 2015 | 698 (306 men and 392 women), >18 yrs old | 48% versus 32% | men 8–63% women 12–42% | >60 yrs RC > LC | flat 61%; protruded 39% |
| Autopsy studies with loupe inspection | |||||
| Vatn and Stalsberg, 1982; (autopsy years: 1972–1973) | 445 (264 men and 181 women), >50 yrs | 34% versus 32% | men 6–53% women 7–43% | >70 yrs RC > LC | 36% of adenomas were pedunculated |
| Williams, 1982 | 365 (198 men and 167 women), age: n.d. | 37% versus 29% | men 20–52%; women 15–35% | RC > LC; most in TC | 55% pedunculated, 45% sessile |
| Autopsy studies without loupe inspection | |||||
| Coode et al., 1985 | 200 (146 men and 54 women), >10 yrs | 34% versus 19% | men: 18–49% women: 10–26% | RC > LC | n.d. |
| Johannsen et al., 1989 | 336 (196 men and 140 women), 30–89 yrs | n.d. | men 0–35%, women 0–25% | <60 yrs: LC > RC, ≥60 yrs: RC > LC | n.d. |
| Pendergrass, 2008 | 2991, (gender n.d.) 20–89 yrs, Exclusion if pre-mortem suspicion of IBD, adenoma, or CRC | n.d. | Age 20–49 yrs: men 2.9%, women 2.7% Age 50–89 yrs: men 10.7%, women 10.2% | Whites: LC > RC, increase in RC with age; African-Americans: RC > LC | n.d. |
IBD: irritable bowel disease; CRC: colorectal cancer; LC: left colon, n.d.: not described; RC: right colon; TC: transverse colon, yrs: years
An excess of the proximal over the distal adenomas, as in our patients, was reported by Johannsen et al.26 and Vatn and Stalsberg,24 in older patients, and independent of age, by Williams et al.,23 Coode et al.25 and Pendergrass et al.27 in African Americans. More distal than proximal adenomas have been reported by Pendergrass et al.27 in white Americans and by Vatn and Stalsberg24 in younger patients. An excess of hyperplastic polyps over adenomas, as in our patients, was reported by Williams et al.23; an excess of adenomas over hyperplastic polyps had been reported by Coode et al.,25 Vatn and Stalsberg,24 and Johannsen et al.26 In the loupe autopsy study by Vatn and Stalsberg,24 36% of adenomas protruded, which is close to the 39% of adenomas that were protruding in our study; 55% of adenomas had been described as ‘pedunculated’ by Williams et al.23; however, Williams et al. shows a lower ADR than in our study, which may indicate missing the flat adenomas. The autopsy studies that did not use loupes referred to ‘polyps’ in general, and did not describe whether the adenomas were protruding or flat.
This study is a single-observer and single-institution study. The limitations of our study are its retrospective nature and the selection of patients on the basis of economic welfare. The patients represent an 18% section of the Austrian population, who buy private insurance in addition to obligatory social insurance coverage34; and therefore, have to be considered as representing a group with higher than average economic status. With regard to the underlying pathologies or referral bias, this patient group was not selected, and therefore was representative of a larger section of the population. Our data are unique, due to the specifics of this optional private health care system, with special requirements for documentation and quality control. At the same time, this provided for a very comprehensive data set that was available for retrospective analysis.
In summary, we demonstrate that high resolution colonoscopy can be as good in detecting colorectal adenomas as an autopsy with loupe inspection. An excess of adenomas in the proximal colon begins at age 60 years, and is more pronounced in men than in women. The majority of flat adenomas are in the proximal colon; however, in all regions the majority of adenomas are flat and more than 50% of the adenomas are minute. Our high prevalence of right colonic and flat lesions should lead to a focus on training for the detection of these lesions, which should reduce operator dependence in screening programs, and improve the protective effects of colonoscopy screening.
References
- 1.Rex DK. Can we fix colonoscopy? Yes!. Gastroenterology 2011; 140: 19–31. [DOI] [PubMed] [Google Scholar]
- 2.Leung FW. PDR or ADR as a quality indicator for colonoscopy. Am J Gastroenterol 2013; 108: 1000–1002. [DOI] [PubMed] [Google Scholar]
- 3.Gavin R, Valori RM, Anderson JT, et al. The national colonoscopy audit: A nationwide assessment of the quality and safety of colonoscopy in the UK. Gut 2013; 62: 242–249. [DOI] [PubMed] [Google Scholar]
- 4.Richter JM. Colonoscopy quality improvement: Practice to public health. Gastrointest Endosc 2013; 78: 919–924. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski MF, Regula JR, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancers. New Engl J Med 2010; 362: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann E, Regula J, Ferlitsch M. How can screening colonoscopy be optimized? Dig Dis 2015; 33: 19–27. [DOI] [PubMed] [Google Scholar]
- 7.Hassan C, Rex DK, Zullo A, et al. Efficacy and cost-effectiveness of screening colonoscopy according to the adenoma detection rate. Unit Eur Gastroenterol J 2015; 3: 200–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corley D, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med 2014; 370: 1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rembacken B, Hassan C, Riemann JF, et al. Quality in screening colonoscopy: Position statement of the European Society of Gastrointestinal Endoscopy (ESGE). Endoscopy 2012; 44: 957–968. [DOI] [PubMed] [Google Scholar]
- 10.Baxter NN, Sutradhar R, Forbes SS, et al. Analysis of administrative data finds endoscopist quality measures associated with post-colonoscopy colorectal cancer. Gastroent 2011; 140: 65–72. [DOI] [PubMed] [Google Scholar]
- 11.Adler A, Wegscheider K, Lieberman D, et al. Factors determining the quality of screening colonoscopy: A prospective study on adenoma detection rates, from 12,134 examinations (Berlin Colonocopy Project 3 (BECOP-3)). Gut 2013; 62: 236–241. [DOI] [PubMed] [Google Scholar]
- 12.Froehlich F, Wietlisbach V, Gonvers JJ, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: The European Panel of Appropriateness of Gastrointestinal Endoscopy European Multicenter Study. Gastroint Endosc 2005; 61: 378–384. [DOI] [PubMed] [Google Scholar]
- 13.Banks MR, Haidry R, Butt MA, et al. High resolution colonoscopy in a bowel cancer screening program improves polyp detection. World J Gastroenterol 2011; 1: 4308–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaltenbach T, McGill SK, Kalidindi V, et al. Proficiency in the diagnosis of nonpolypoid colorectal neoplasm yields high adenoma detection rates. Dig Dis Sci 2012; 57: 764–770. [DOI] [PubMed] [Google Scholar]
- 15.Van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rates determined by tandem colonoscopy: A systematic review. Am J Gastroent 2006; 101: 343–350. [DOI] [PubMed] [Google Scholar]
- 16.Ferlitsch M, Reinhart K, Pramhas S, et al. Sex-specific prevalence of adenomas, advanced adenomas and colorectal cancer in individuals undergoing screening colonoscopy. J Am Med Ass 2011; 306: 1352–1358. [DOI] [PubMed] [Google Scholar]
- 17.The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach and colon. Gastroint Endosc 2003; 58: S3–43. [DOI] [PubMed]
- 18.Schoefl R, Ziachehabi A, Wewalka F. Small colorectal polyps. Dig Dis 2015; 33: 38–41. [DOI] [PubMed] [Google Scholar]
- 19.Boroff ES, Gurudu SR, Hentz JG, et al. Polyp and adenoma detection rates in the proximal and distal colon. Am J Gastroent 2013; 108: 993–999. [DOI] [PubMed] [Google Scholar]
- 20.Yamaji Y, Mitsushima T, Ikuma H, et al. Right-side shift of colorectal adenomas with aging. Gastroint Endosc 2006; 63: 453–458. [DOI] [PubMed] [Google Scholar]
- 21.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann Int Med 2011; 154: 22–30. [DOI] [PubMed] [Google Scholar]
- 22.Brenner H, Chang-Claude J, Jansen L, et al. Reduced risk of colorectal cancer up to 10 years after screening, surveillance, or diagnostic colonoscopy. Gastroenterology 2014; 146: 709–717. [DOI] [PubMed] [Google Scholar]
- 23.Williams AR, Balasooriya BAW, Day DW. Polyps and cancer of the large bowel: A necropsy study in Liverpool. Gut 1982; 23: 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: An autopsy study. Cancer 1982; 49: 819–825. [DOI] [PubMed] [Google Scholar]
- 25.Coode PE, Chan KW, Chan YT. Polyps and diverticula of the large intestine: A necropsy survey in Hong Kong. Gut 1985; 26: 1045–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannsen LGK, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. Scand J Gastroenterol 1989; 24: 799–806. [DOI] [PubMed] [Google Scholar]
- 27.Pendergrass CJ, Edelstein DL, Hylind LM, et al. Occurrence of colorectal adenomas in younger adults: An epidemiologic necropsy study. Clin Gastroenterol Hepatol 2008; 6: 1011–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papagiorgis PC, Oikonomakis I, Delaportas D, et al. Proximal shift of colorectal cancer.A persistent phenomenon with multiple causes, patterns and clinical implications. Journal of Balkan Union of Oncology 2014; 19: 605–617. [PubMed] [Google Scholar]
- 29.Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the United States by tumor location and stage, 1992–2008. Cancer Epidemiol Biomarkers Prev 2012; 21: 411–416. [DOI] [PubMed] [Google Scholar]
- 30.Meza R, Jeon J, Renehan AG, et al. Colorectal cancer incidence trends in the United States and United Kingdom: Evidence of right-to-left-sided biological gradients with implications for screening. Cancer Res 2010; 70: 5419–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brenner H, Hoffmeister M, Arndt V, et al. Gender differences in colorectal cancer: Implications for age at the initiation of screening. Brit J Cancer 2007; 96: 828–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hurlstone DP, Cross SS, Adam I, et al. A prospective clinicopathological and endoscopic evaluation of flat and depressed colorectal lesions in the United Kingdom. Am J Gastroent 2003; 98: 2543–2549. [DOI] [PubMed] [Google Scholar]
- 33.Coe SG, Wallace MB. Assessment of adenoma detection rate benchmarks in women versus men. Gastroint Endosc 2013; 77: 631–635. [DOI] [PubMed] [Google Scholar]
- 34.Versicherungsverband Oesterreich. www.vvo.at/informationsblatt-4.html+&cd=1&hl=de&ct=clnk&gl=at (2014, accessed 3 May 2015).